3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2012; 3:107-112. doi:10.7150/jca.4107 This volume Cite
Research Paper
Additively Enhanced Antiproliferative Effect of Interferon Combined with Proanthocyanidin on Bladder Cancer Cells
Department of Urology, New York Medical College, Valhalla, New York, USA.
Received 2012-1-17; Accepted 2012-1-29; Published 2012-3-1
Abstract
Although interferon (IFN) has been often used as immunotherapy for bladder cancer, its efficacy is rather unsatisfactory, demanding further improvement. Combination therapy is one of viable options, and grape seed proanthocyanidin (GSP) could be such an agent to be used with IFN because it has been shown to have anticancer activity. We thus investigated whether combination of IFN and GSP might enhance the overall antiproliferative effect on bladder cancer cells in vitro. Human bladder cancer T24 cells were employed and treated with the varying concentrations of recombinant IFN-α2b (0-100,000 IU/ml), GSP (0-100 μg/ml), or their combinations. IFN-α2b alone led to a ~50% growth reduction at 20,000 (20K) IU/ml, which further declined to ~67% at ≥50K IU/ml. Similarly, GSP alone induced a ~35% and ~100% growth reduction at 25 and ≥50 μg/ml, respectively. When IFN-α2b and GSP were then combined, combination of 50K IU/ml IFN-α2b and 25 μg/ml GSP resulted in a drastic >95% growth reduction. Cell cycle analysis indicated that such an enhanced growth inhibition was accompanied by a G1 cell cycle arrest. This was further confirmed by Western blot analysis revealing that expressions of G1-specific cell cycle regulators (CDK2, CDK4, cyclin E and p27/Kip1) were distinctly modulated with such IFN-α2b/GSP treatment. Therefore, these findings support the notion that combination of IFN-α2b and GSP is capable of additively enhancing antiproliferative effect on T24 cells with a G1 cell cycle arrest, implying an adjuvant therapeutic modality for superficial bladder cancer.
Keywords: interferon, proanthocyanidin, combination therapy, bladder cancer.
Introduction
Bladder cancer is the second most common urologic malignancy next to prostate cancer in the United States, and the majority of bladder cancers present as superficial (80%) with 15% presenting as invasive cancer and 5% as metastatic disease (1). Currently, transitional cell carcinoma (TCC) is the most prevalent primary bladder tumor: 50,000 new cases are diagnosed annually and over 10,000 people die of this disease each year (2). Although endoscopic transurethral resection (TUR) is often performed as a primary therapy, 50%-75% of patients will yet recur in 5 years and about 10% progress to invasive disease (2).
Chemotherapy is another viable option but intravesical administration of bacillus Calmette-Guerin (BCG), an attenuated strain of Mycobacterium bovis, is currently the most effective immunotherapy for high-grade and recurrent superficial bladder cancer and carcinoma in situ (CIS) (3). In randomized studies, BCG has been shown to be superior to both mitomycin C and adriamycin (4). Intravesical BCG following TUR has also been associated with a significant improvement in progression and survival compared to TUR alone (5). In fact, this protocol has become established therapy for superficial bladder cancers, resulting in a ~40% reduction in cancer recurrence (6). However, side effects of BCG therapy are common and limit its use in clinical practice, demanding a safer, more effective therapeutic modality with fewer side effects.
Interferons (IFNs) have been often used as immunotherapy for a variety of urologic malignancies including prostate, bladder, and renal cell carcinomas (7-9). Especially, IFN-α is used as an intravesical agent for treating superficial bladder cancer because it may cause only minor local and systemic toxicity (compared to BCG) (10). However, since its response rate in patients has been shown to be lower than that of BCG therapy (10), the efficacy of IFN-α combined with BCG was assessed in pilot clinical trials and animal studies (11,12), indicating the better, improved outcomes. Thus, these studies would certainly encourage further exploration into other alternative combination therapies, which may lead to the safer, more effective and satisfactory results.
Proanthocyanidins are naturally occurring plant polyphenolic bioflavonoids in fruits, vegetables, nuts, seeds, flowers and bark (13). They are known as natural antioxidants, having biological, pharmacological and chemoprotective properties against oxidative stress or harmful free radicals (13-15). For example, hydrogen peroxide-induced oxidative stress was significantly reduced by proanthocyanidins in cultured macrophage and neuroactive PC-12 cells (14). They have exhibited antibacterial, antiviral, anti-inflammatory, and vasodilatory actions as well (13). Particularly, a unique grape seed proanthocyanidin (GSP) has been extensively characterized: it is a standardized water-ethanol extract from red grape seeds, consisting of oligomeric proanthocyanidins as active components (15). GSP has also demonstrated its anticancer (cytotoxic) effect on several malignancies including breast, lung and gastric cancers in vitro (16).
Accordingly, we investigated whether IFN-α, GSP or their combination might demonstrate the antiproliferative effect on bladder cancer cells in vitro. We also explored the underlying mechanism - how the cancer cell growth might be inhibited with such agents, focusing on the cell cycle regulation. More detailed studies are described and discussed herein.
Materials and Methods
Cell culture
The human bladder cancer T24 cells, derived from a patient with TCC, were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in McCoy's 5a medium containing 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml), and were maintained at 37 ºC in a humidified incubator in an atmosphere of 95% air and 5% CO2. For experiments, cells were seeded in 6-well plates (2 ml per well) or T-75 flasks (10 ml per flask) at the initial cell density of 2 x 105 cells/ml and were cultured with recombinant interferon-α2b (IFN-α2b; Schering Corp., Kenilworth, NJ), grape seed proanthocyanidin (GSP; Dry Creek Nutrition, Inc., Modesto, CA) or their combinations. Cell number/viability was then assessed at specified times using the trypan blue exclusion method.
Cell cycle analysis
A FACScan flow cytometer (Becton-Dickinson, San Jose, CA), equipped with a double discrimination module, was employed for cell cycle analysis. Control or agents-treated cells (~1 x 106 cells per condition) were first resuspended in 500 μl of propidium iodide solution (20 μg/ml propidium iodide, 0.2 mg/ml RNase, 0.2 mg/ml EDTA, 0.5% Nonidet P-40) and incubated for 1 h at room temperature in the dark. Following incubation, ~10,000 nuclei from each sample were analyzed on a flow cytometer, and CellFit software was used to quantify cell cycle compartments to estimate the % of cells distributed in the different cell cycle phases.
Western blot analysis
The procedure essentially followed the protocol described previously (17). Briefly, an equal amount of proteins (7 μg) from control and agent-treated cell lysates was resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The blot was first incubated for 90 min with the primary antibodies against CDK2, CDK4, cyclin D1, cyclin E, or p27/Kip1 (Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with the secondary antibody conjugated with peroxidase for 30 min. The immunoreactive protein bands were detected by chemiluminescence following the manufacturer's protocol (Kirkegaard and Perry Laboratories, Gaithersburg, MD) and quantified using a scan densitometer (Silk Scientific, Oregon, UT).
Statistical analysis
All data were presented as the mean ± SD (standard deviation), and statistical differences between groups were assessed with either the unpaired Student's t test or one-way analysis of variance (ANOVA). Values of p<0.05 were considered to indicate statistical significance.
Results
Effects of interferon-α2b and proanthocyanidin on T24 cell growth
To examine possible effects of interferon-α2b (IFN-α2b) and grape seed proanthocyanidin (GSP) on T24 cell growth, cells were cultured with the varying concentrations of IFN-α2b (0-100,000 = 100K IU/ml) or GSP (0-100 μg/ml) for 72 h. IFN-α2b caused a significant (~50%) growth reduction at 20K IU/ml, which further declined to >67% at ≥50K IU/ml (Fig. 1A). Similarly, a ~35% and >90% growth inhibition were attained with 25 and ≥50 μg/ml of GSP, respectively (Fig. 1B). A drastic growth reduction (~90%) with 50 μg/ml GSP was actually due to a cytotoxic effect, evidenced by ~30% of cells having been dead. These results thus show that IFN-α2b and GSP are capable of inhibiting T24 cell growth but GSP can also induce cytotoxic cell death at the higher (≥50 μg/ml) concentrations.
Effects of IFN-α2b or GSP on T24 cell growth. Cells were cultured with the varying concentrations of either IFN-α2b (0-100,000 IU/ml) or GSP (0-100 μg/ml), and cell numbers in IFN-α2b-treated (A) or GSP-treated (B) cells were determined at 72 h. All data represent mean ± SD (standard deviation) from three independent experiments (*p<0.05; **p<0.01).

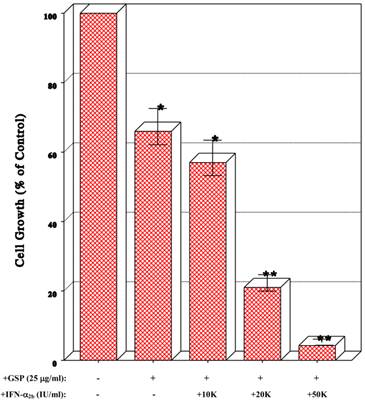
Additive antiproliferative effects of IFN-α2b and GSP
It was tempting to examine whether combinations of IFN-α2b and GSP might improve the antiproliferative effects. As 25 μg/ml of GSP appeared to induce a moderate (~35%) growth inhibition (Fig. 1B), this concentration was used in combination with the varying concentrations of IFN-α2b. Such studies showed that combinations of GSP (25 μg/ml) and IFN-α2b at 10K IU/ml (little effect by itself), 20K IU/ml (a ~50% inhibitory effect), and 50K IU/ml (a ~67% inhibitory effect) led to the improved 43%, 79%, and >95% growth reduction (p<0.05), respectively (Fig. 2). Thus, the IFN-α2b/GSP combinations appear to induce the additive antiproliferative effects on T24 cells.
Effects of combination of IFN-α2b and GSP on cell growth. Cells were treated with combinations of GSP (25 μg/ml) and 10K, 20K, or 50K IU/ml of IFN-α2b for 72 h, and cell growth was assessed by the % of viable cell numbers relative to that in control (100%). The data are mean ± SD from three separate experiments (*p<0.05; **p<0.03).
Effects of IFN-α2b and GSP on cell cycle
To explore the mechanism of such additive effects of the IFN-α2b/GSP combinations, cell cycle analysis was performed using the specific concentrations of IFN-α2b (20K IU/ml) and GSP (25 μg/ml), which seemed to be rather suitable for this study. After T24 cells were treated with IFN-α2b (20K IU/ml), GSP (25 μg/ml), or their combination for 72 h, the results of cell cycle analysis were then summarized in Table 1. Compare to cell numbers of the G1 and S phases in controls, significant changes in those numbers (p<0.05) were seen with IFN-α2b treatment, while GSP alone showed only the marginal effects. In contrast, the IFN-α2b/GSP combination induced a 58% increase and 64% decrease in G1 and S phase cell numbers (p<0.05), respectively. This cell accumulation in the G1 phase is known as a G1 cell cycle arrest (18). Thus, the IFN-α2b/GSP combination may primarily target the G1-S phase transition in the cell cycle, subsequently leading to the growth cessation.
Effects of IFN-α2b and GSP on Cell Cycle Phase Distributions.
| Conditions | % of Cells in Cell Cycle Phases | ||
|---|---|---|---|
| G1 | S | G2/M | |
| Control | 49.3 ± 4.7 | 38.1 ± 2.8 | 12.6 ± 1.4 |
| + IFN-α2b (20K IU/ml) | 61.7 ± 4.6* | 26.7 ± 3.1* | 11.6 ± 1.1 |
| + GSP (25 μg/ml) | 56.7 ± 5.0 | 33.2 ± 2.9 | 10.1 ± 1.6 |
| + IFN-α2b (20K)/GSP (25) | 77.9 ± 4.3* | 13.6 ± 1.4* | 8.5 ± 0.9 |
All data are mean ± SD from three separate experiments.
* p<0.05 (compared to those in Control).
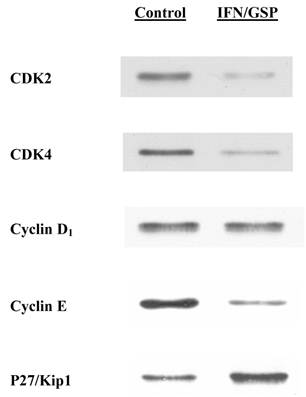
Down-regulation of G1 cell cycle regulators by IFN-α2b/GSP combination
To confirm such an IFN-α2b/GSP-induced G1 cell cycle arrest, we also examined its effects on the specific cell cycle regulators for the G1-S phase transition (18). After cells were treated with or without the IFN-α2b (20K IU/ml)/GSP (25 μg/ml) combination for 72 h, the expressions of CDK2, CDK4, cyclin D1, cyclin E, and p27/Kip1 were analyzed on Western blots. Such analysis revealed that the expressions of CDK2, CDK4 and cyclin E were drastically reduced by ~80% with the IFN-α2b/GSP treatment (compared to those in controls), although no change in cyclin D1 was observed (Fig. 3). In contrast, p27/Kip1 protein, a CDK2 inhibitor, was significantly (~2.7 fold) up-regulated in IFN-α2b/GSP-treated cells (Fig. 3). Thus, altered expressions of these regulators would provide the further evidence for a blockage of G1-S phase transition, confirming that the IFN-α2b/GSP-induced growth inhibition is indeed mediated through a G1 cell cycle arrest.
Western blot analysis on cell cycle regulators. After cells were treated with or without the combination of IFN-α2b (20K IU/ml) and GSP (25 μg/ml) for 72 h, the expressions of several cell cycle regulators were analyzed on Western blots. Autoradiographs of CDK2, CDK4, cyclin D1, cyclin E, and p27/Kip1 in control and IFN-α2b/GSP-treated cells are shown for comparison.
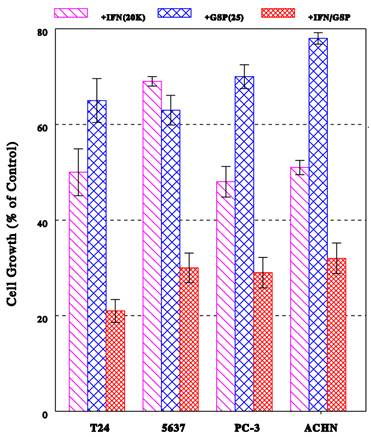
Effects of IFN-α2b/GSP combination on proliferation of other cancer cells
Now, one may raise the question if the IFN-α2b/GSP combination would also demonstrate its enhanced antiproliferative effect on other bladder cancer cells or different cancer cell types. This was tested using another bladder cancer 5637 cells, prostate cancer PC-3 cells, and renal cancer ACHN cells, which had been treated with IFN-α2b (20K IU/ml), GSP (25 μg/ml), or their combination for 72 h. Figure 4 shows the effects of these agents on proliferation of three cancer cell lines. Overall, all cancer cells treated with various agents exhibited altered growth patterns, which were significantly different from their respective control cells (p<0.05). IFN-α2b alone was capable of inducing a ~50% growth inhibition in both PC-3 and ACHN cells but a ~30% inhibition in 5637 cells. GSP alone caused a lesser but 22-37% growth reduction in all these cells. Nevertheless, the IFN-α2b/GSP combination resulted in an enhanced 70%, 71%, and 68% growth inhibition in 5637, PC-3, and ACHN cells, respectively. Thus, these results suggest that the IFN-α2b/GSP combination may commonly demonstrate its potentiated antiproliferative effect on not only T24 cells but also a variety of human cancer cells.
Growth inhibitory effect of IFN-α2b/GSP combination on various cancer cells. Bladder cancer 5637, prostate cancer PC-3, and renal cancer ACHN cells were treated with IFN-α2b (20K IU/ml), GSP (25 μg/ml), or their combination for 72 h, and cell growth was assessed by the % of viable cell number (in each condition) relative to that in respective control cells. The growth profile of T24 cells is also shown for comparison. All data are mean ± SD from three separate experiments, showing statistically significant differences (p<0.05) compared to respective controls (100%).
Discussion
Although IFN-α has been often used as immunotherapy for bladder cancer because of its relatively low toxicity (compared to BCG), its less efficacy has been also inquired for a significant improvement. Additionally, IFN-α therapy has several drawbacks, such as high cost and repeated administration. A standard intravesical IFN-α instillation is often carried out with 50-100 million IU of IFN-α (19), but whether this high dosage would be sufficient to induce optimal immunity is uncertain because of its short retention time inside the bladder (19). Accordingly, to improve the efficacy of such IFN-α monotherapy, clinical trials of combination therapy using IFN-α and BCG (11) have been conducted on patients with bladder cancer. Despite some encouraging outcomes, further studies are yet required for establishing the more potent, safer, and cost-effective treatment modalities.
This interesting issue prompted us to explore an alternative approach using grape seed proanthocyanidin (GSP) in combination with IFN-α2b, because GSP has been shown to be a natural, non-toxic antioxidant with anticancer effect (15,16). Our study showed that IFN-α2b or GSP was capable of individually inducing a significant growth reduction in T24 cells. Interestingly, GSP also exhibited the cytotoxic effect (inducing cell death) at its higher concentrations (≥50 μg/ml). When combinations of IFN-α2b and GSP were tested to further improve the overall efficacy, all combinations resulted in the additively enhanced antiproliferative effect, implying that both IFN-α2b and GSP may share the common growth regulatory pathway. In addition, this IFN-α2b/GSP-enhanced growth inhibition in T24 cells was also demonstrated in another bladder cancer (5637), prostate cancer (PC-3), and renal cancer (ACHN) cells (Fig. 4), suggesting its prevalent potency over bladder cancer as well as various cancer cells.
Our next aim was to probe the antiproliferative mechanism of IFN-α2b/GSP combination, focusing on the cell cycle regulation. Such study revealed that the IFN-α2b/GSP-induced growth inhibition was associated with a 64% reduction in the S-phase cell population, due to a blockage of the cells entering from the G1 to the subsequent S phase (i.e. a G1 cell cycle arrest). This finding was also verified by analyzing G1-specific cell cycle regulators: expressions of CDK2, CDK4, and cyclin E were drastically (~80%) down-regulated while p27/Kip1 was greatly (~2.7 fold) up-regulated in IFN-α2b/GSP-treated cells. Specific modulations of these regulators are indicative of a G1 cell cycle arrest, which is the crucial cellular event leading ultimately to a growth cessation. Yet, it is also important to examine the IFN-mediated signaling pathways to further define the mechanism of IFN-α2b/GSP-induced growth inhibition, since activation of specific IFN-inducible genes by signal transduction (20) is well known to dictate biological actions of IFNs (including IFN-α2b). Such study is currently underway in our laboratory.
It would be worthwhile mentioning the possible clinical relevance of IFN-α2b/GSP-enhanced antiproliferative effect. As a high-dose instillation of IFN-α leads to its high cost (19), it would be more practical if such a high dosage could be somehow reduced without losing, or rather, with improving its efficacy. Our study then showed that the relatively low concentrations of IFN-α2b (compared to its monotherapy) were required to be highly effective when combined with GSP. This suggests that the combination of IFN-α2b and GSP may not only help enhance IFN-α2b activity but also help cut its cost down. However, it is yet required to address how the effective concentrations (e.g., 20K or 50K IU/ml) of IFN-α2b and GSP in this in vitro study would be extrapolated to animals or actual patients. Nevertheless, several studies have already reported antitumor activity of GSP in vivo. For example, GSP was found to inhibit prostate tumor growth and angiogenesis (21) as well as breast cancer metastasis in mice (22) or enhance cytotoxic effect of doxorubicin in mice bearing Sarcorma 180 and Hepatoma 22 (23). No palpable side effects of GSP have been yet reported in these animal studies, and the LD50 of GSP in the rats has been estimated to be >5,000 mg/kg body weight (24), verifying its low toxicity. Moreover, patients with chronic pancreatitis demonstrated the symptomatic improvements, such as the reduction in both pain index and incidence of vomiting (25), with a daily dose of 200-300 mg of GSP. This also implies that GSP may have few side effects and is safe to be used in clinical practice.
In conclusion, IFN-α2b and GSP can individually demonstrate antiproliferative effect on bladder cancer T24 cells. When they were combined, such inhibitory activity would be additively enhanced, resulting in a nearly complete growth cessation. In addition, this additive potentiation can be seen in other cancer cell types as well. The underlying mechanism of IFN-α2b/GSP-enhanced growth inhibition appears to be more likely attributed to a G1 cell cycle arrest. Therefore, specific IFN-α2b/GSP combination may provide alternative, adjuvant intravesical therapy for superficial bladder cancer.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Pow-Sang JM, Seigne JD. Contemporary management of superficial bladder cancer. Cancer Control. 2000;7:335-9
2. Witjes JA, Mulders PF, Debruyne FM. Intravesical therapy in superficial bladder cancer. Urology. 1994;43:2-5
3. Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689-94
4. Lamm DL, Blumenstein BA, Crawford ED. et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacillus Calmette-Guerin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325:1205-9
5. Herr HW, Schwalb DM, Zhang ZF. et al. Intravesical bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J Clin Oncol. 1995;13:1404-8
6. Amling CL. Diagnosis and management of superficial bladder cancer. Curr Probl Cancer. 2001;25:219-78
7. Harris DT, Matyas GR, Gomella LG. et al. Immunologic approaches to the treatment of prostate cancer. Semin Oncol. 2000;26:439-47
8. Glashan RW. A randomized controlled study of intravesical alpha-2b-interferon in carcinoma in situ of the bladder. J Urol. 1990;144:658-61
9. Bukowski RM. Cytokine therapy for metastatic renal cell carcinoma. Semin Urol Oncol. 2001;19:148-54
10. Belldegrun AS, Franklin JR, O'Donnell MA. et al. Superficial bladder cancer: the role of interferon-alpha. J Urol. 1998;159:1793-1801
11. Stricker P, Pryor K, Nicholson T. et al. Bacillus Calmette-Guerin plus intravesical interferon alpha-2b in patients with superficial bladder cancer. Urology. 1996;48:957-61
12. Gan YH, Zhang Y, Khoo HE, Esuvaranathan K. Antitumor immunity of Bacillus Calmette-Guerin and interferon alpha in murine bladder cancer. Eur J Cancer. 1999;35:1123-9
13. Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179-89
14. Bagchi D, Kuszynski CA, Balmoori J, Bagchi M, Stohs SJ. Hydrogen peroxide-induced modulation of intracellular oxidized states in cultured macrophage J774A.1 and neuroactive PC-12 cells, and protection by a novel grape seed proanthocyanidin extract. Phytother Res. 1998;12:568-71
15. Joshi SS, Kuszynski CA, Bagchi M, Bagchi D. Chemopreventive effects of grape seed proanthocyanidin extract on Chang liver cells. Toxicology. 2000;155:83-90
16. Ye X, Krohn RL, Liu W. et al. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol Cell Biochem. 1999;196:99-108
17. Mordente JA, Konno S, Chen Y, Wu JM, Tazaki H, Mallouh C. The effects of brefeldin A (BFA) on cell cycle progression involving the modulation of the retinoblastoma protein (pRB) in PC-3 prostate cancer cells. J Urol. 1998;159:275-9
18. Sherr CJ. The Pezcoller lecture: cancer cell cycles revised. Cancer Res. 2000;60:3689-95
19. Luo Y, Chen X, Han R, O'Donnell MA. Recombinant bacille Calmette-Guerin (BCG) expressing human interferon-alpha 2B demonstrates enhanced immunogenicity. Clin Exp Immunol. 2001;123:264-70
20. Bandyopadhyay SK, Rackley RR, Matin SF, Sadhukhan PC. Interferon-α response and signal transduction pathway in transitional carcinoma cell lines. Adv Exp Med Biol. 2003;539(Pt A):15-32
21. Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733-40
22. Mantena SK, Baliga MA, Katiyar SK. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis. 2006;27:1682-91
23. Zhang XY, Bai DC, Wu YJ, Li WG, Liu NF. Proanthocyanidin from grape seeds enhances anti-tumor effect of doxorubicin both in vitro and in vivo. Pharmazie. 2005;60:533-8
24. Bagchi D, Bagchi M, Stohs SJ. et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology. 2000;148:187-97
25. Banerjee B, Bagchi D. Beneficial effects of a novel IH636 grape seed proanthocyanidin extract in the treatment of chronic pancreatitis. Digestion. 2001;63:203-6
Author contact
![]() Corresponding author: Sensuke Konno, Ph.D., New York Medical College, Department of Urology, Munger pavilion 4th Floor, Valhalla, NY 10595, USA. Phone: 914-594-3745; Fax: 914-594-4428 E-mail: sensuke_konnoedu.
Corresponding author: Sensuke Konno, Ph.D., New York Medical College, Department of Urology, Munger pavilion 4th Floor, Valhalla, NY 10595, USA. Phone: 914-594-3745; Fax: 914-594-4428 E-mail: sensuke_konnoedu.

 Global reach, higher impact
Global reach, higher impact