3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(13):2449-2455. doi:10.7150/jca.17720 This issue Cite
Research Paper
Study on Inhibitory Effect of MaiMenDong Decoction and WeiJing Decoction Combination with Cisplatin on NCI-A549 Xenograft in Nude Mice and Its Mechanism
1. Jiangsu Collaborative Innovation Center of Traditional Chinese Medicine (TCM) Prevention and Treatment of Tumor, Nanjing University of Chinese Medicine, Nanjing, 210023, China;
2. The Second Affiliated Hospital of Soochow University, Suzhou, 215004, China;
3. Department of Medical Oncology, Cancer Hospital of JiangSu Province, Nanjing, 210023, China;
4. Department of Pharmacology and Winship Cancer Institute, Emory University School of Medicine, Atlanta, GA 30322, USA.
Received 2016-9-26; Accepted 2017-5-10; Published 2017-7-23
Abstract
MaiMenDong Decoction and WeiJing Decoction (Jin formula) is a traditional Chinese medication that consists of 8 medicinal plants, which recorded in the classical TCM literature Jin Kui Yao Lue and has been utilized in the treatment of lung diseases for hundreds of years in China. The present study aimed to determine the anti-tumor activity and the underlying mechanisms of Jin formula combined with cisplatin in the treatment of non-small cell lung cancer (NSCLC). Xenograft model of NCI-A549 was established in Balb/c nude mice. Five groups, including normal, MOCK, Jin, cisplatin (DDP), and Jin+DDP were included in the study. We found that Jin formula ameliorated the body weight loss caused by DDP 15 days after drug administration. Moreover, the combination of Jin with DDP enhanced the anti-tumor function of DDP. Microarray analysis showed that Jin suppressed gene expression of certain pathways which regulating cell cycle and apoptosis. Furthermore, DDP mainly decreased the gene expression level of angiogenesis associated factors, such as VEGFA, TGF-β and MMP-1. Moreover, co-treatment with Jin and DDP not only down-regulated Bcl-2 and E2F1, but also decreased the expression of MYC, MET, and MCAM. In addition, co-formula decreased the levels of p-AKT (thr308) and p-PTEN, increased Bax/Bcl-2 value, and resulted in apoptosis of tumor cells. Taken together, Jin+DDP significantly inhibited the growth of A549 cell transplanted solid tumor with slight side effect compared to the treatment by DDP only, and had a better effect than the Jin group. The mechanisms may be mainly associated with inactivation of PI3K/AKT pathway and apoptosis induction.
Keywords: Jin formula, Cisplatin, NSCLC, Apoptosis, PI3K/AKT pathway.
Introduction
Lung cancer is one of the most common cancer types in human beings and occupies the top cancer death world widely [1, 2]. NSCLC accounts for approximately 80% of all incidences [3, 4]. Standard therapeutic approaches for NSCLC are surgical resection and adjuvant chemo and radio-therapy. However, these therapies can frequently lead to side effects [5, 6]. Therefore, new drugs with low-toxic side-effects are absolutely required. Traditional Chinese medicine (TCM) has been used to treat human diseases for a long history due to its safety and efficacy, whereas single herb is rarely used alone, instead, the formula is the essence of TCM [7-9]. MaiMenDong Decoction and WeiJing Decoction (Jin formula) is an ancient herbal formula recorded in the classical TCM literature Jin Kui Yao Lue. TCM believes that lung tumor is associated with Qi and Yin deficiencies, Qi insufficiency in the spleen and lungs, or pathological alterations because of Qi stagnation, blood stasis, and phlegm and toxin accumulation. The Jin formula can refill both Qi and Yin, reinforce the spleen and lungs, and clear the lungs. The Jin formula can also remove phlegm and activate blood circulation to remove stasis. Our study evaluated the effect and the mechanisms of Jin formula combination with chemotherapeutic agent DDP for NSCLC.
Materials and Methods
Cells
Human lung cancer (NCI-A549) cells used in this study were originally from the Cell Line Bank (Shanghai, China) and maintained in media with 10% fetal bovine serum (FBS, Gibco), 100U/ml penicillin and 100mg/ml streptomycin (Amresco). These cells were cultured at 37 °C in 5% CO2.
Drugs
MaiMenDong Decoction and WeiJing Decoction (Jin formala) contain several herbs according to Jin Kui Yao Lue. MaiMenDong Decoction is composed of Ophiopogon japonicus (30 g), prepared Rhizoma pinelliae (15 g), Ginseng radix (30 g), and Glycyrrhiza radix (12 g). WeiJing Decoction is composed of peach kernel (15 g), unprepared Coix lacryma-jobi seeds (30 g), Chinese wax gourd seed (30 g), and Phragmititis caulis (30 g). We assessed the quality of the herbs according to the Chinese pharmacopoeia (People's Medical Publishing House; Beijing: 2010). The decoction was prepared by a trained technician in accordance with standard procedures. One unit of Jin formula contained 1200 ml of decoction. Cisplatin was obtained from Nanjing Pharmaceutical Factory, Ltd.
Mice and xenograft
6-8 weeks old Balb/c female athymic (nude) mice (n=40) that weigh 20 ± 2g were acquired from the Academy of Military Medical Sciences (Beijing, China). In nude mice, the tumor regression model was widely used to assess anti-cancer activity. This model was applied to assess inhibition of solid tumor growth with different drug treatments. Briefly, each mouse was subcutaneously injected with 1×107 A549 cells in the axilla. An apparently visible tumor mass grown was observed 7 days after injection. When the tumor size was about 50mm3, the mice were randomly divided into five groups: Normal group (saline only), MOCK group (saline only), Jin group (0.83g/kg/day Jin formula), DDP group (5mg/kg/day DDP), Co-formula group (Jin formula+DDP, same dosage as above). Eight mice were included in each group. Mice of Jin formula, MOCK, and normal groups were orally administrated, and mice of DDP group was injected i.p. daily for 15 days.
The study was approved by the Jiangsu Animal Care and Use Committee and followed the national and institutional rules of animal experiments.
Anti-tumor activity in vivo
Mice were monitored after drug treatments. Each mouse was weighed every two days. The longest (a) and shortest (b) tumor diameters (mm) were recorded and formula for an ellipsoid sphere (0.52×a×b2) was utilized to determine the volume of tumor. The tumor weight was also determined when the animals were sacrificed. The effect of the anti-tumor treatment was expressed below:
Inhibition rate (%) = (W mock - W treatment)/W mock ×100%
W mock and W treatment are tumor weight of mock and treatment group, respectively. Tumors were preserved in liquid nitrogen for future studies.
PCR microarray
Trizol Reagent (Invitrogen) was used to isolate total RNA from experimental tumor samples and RNeasy®MinElute™ Cleanup Kit (Qiagen) was used to purify RNA following the manufacturer's instructions. The concentration of RNA was determined by UV absorption with a Nanodrop Spectrophotometer (Bio Lab Ltd, USA). cDNA generated from the RNA using the Super Script III Reverse Transcriptase (Invitrogen, USA) was utilized for RT-PCR (Super Array Bioscience Corporation, USA). The names of all genes tested are listed in Table 2A- Table 2C. ΔΔCt method was applied to evaluate data:
a) Calculate the ΔCt in every treatment group for each pathway-focused gene.
ΔCt [group 1(G1)] = average Ct - average of HK genes' Ct for G1 array
ΔCt (G2) = average Ct - average of HK genes' Ct for G2 array
b) Calculate the ΔΔCt for each gene across two PCR Arrays (or groups).
ΔΔCt = ΔCt (G2) - ΔCt (G1)
c) Calculate the fold-change for every gene from G1 to G2 as 2-ΔΔCt.
Immunoblot
Tumors were grinded with subsequent addition of lysis buffer, and then treated with ultra-sonic disrupter on ice for thirty minutes. The homogenate were then centrifuged at 12000rpm/min for thirty minutes. The supernatant was harvested and the samples were separated on a 10% polyacrylamide gel and then transferred to a PVDF membrane, which was incubated with primary antibodies to E2F1, CyclinD3, p-AKT (thr308), P21, Bcl-2, AKT, CDK4, P27, Bax, p-PTEN(ser380), p-AKT (ser473), p-GSK3β (ser9) (all from Cell Signaling Technology, USA) and β-actin (Santa Cruz Biotechnology, USA). Blots were incubated with peroxidase-labeled secondary antibodies (Cell Signaling Technology, USA). Antibody binding was detected using a Gel Imaging System (Bio-Rad, USA).
Statistical analysis
The data was analyzed by SPSS11.5 software and presented as mean±SD (mean ± standard deviation). The differential significance between the mean of mock and treatment groups was evaluated with one-way analysis of variance (ANOVA) followed by a paired t-test and Dunnett's t-test correction. Significant difference was defined at the level of p< 0.05.
Results
Co-administration of Jin and DDP has a synergistic effect on NCI-A549 in mice
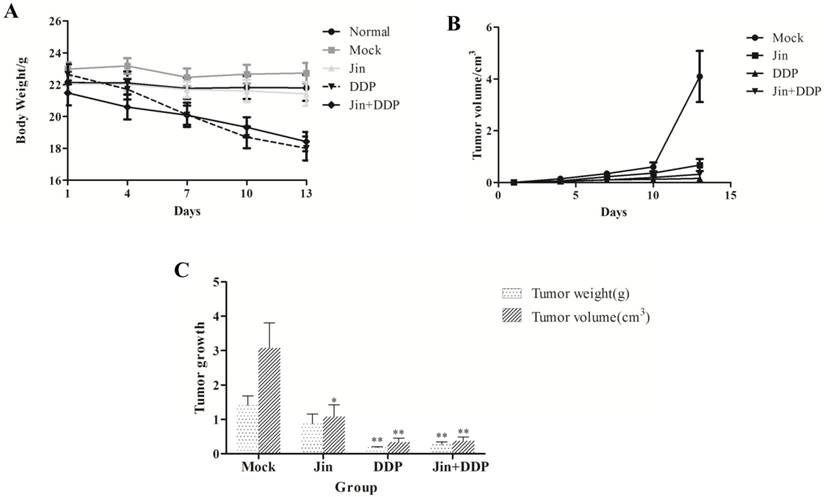
During the treatment, the body weight in DDP group reduced significantly. However, the body weight well-maintained in Jin+DDP group. All of the mice in Jin group remained stable weight with normal activity (Fig. 1A). The growth of the solid tumor was inhibited by Jin, DDP, and Jin+DDP compared to the MOCK group (Fig. 1B). Jin significantly inhibited the tumor volume (p<0.05), whereas no significant difference was found in tumor weight compared to MOCK group (p>0.05). Meanwhile, DDP and Jin+DDP were found significantly inhibited both the tumor size and tumor weight (p<0.01) compared to MOCK group after sacrifice (Fig. 1C). The tumor-inhibition rates of MOCK, DDP, and Jin+DDP groups were 27.4%, 80.5%, and 75.9%, respectively (Table 1).
Effects of the Jin to the tumor volume and weight of NCI-A549 tumor-bearing mic (mean±SD)
| Group | n | Volume/cm3 | Weight/g | Inhibitory Rate/% |
|---|---|---|---|---|
| MOCK | 8 | 2.34±1.96 | 1.08±0.80 | - |
| Jin | 8 | 0.82±0.83* | 0.87±0.71 | 27.4 |
| DDP | 8 | 0.34±0.54** | 0.21±0.13** | 80.5 |
| Jin+DDP | 8 | 0.28±0.27** | 0.26±0.19** | 75.9 |
All data were expressed as mean±SD. * p < 0.05, ** p < 0.01 versus MOCK control
Effects of different treatments on the suppression of NCI-A549 transplanted tumor and survival condition of nude mice. (A) Mice of DDP group lost weight significantly, and mice of Jin+DDP group have markedly improved, and all the mice of Jin group keep stable weight with normal activity. (B) All drugs significantly suppress the solid tumor growth compared with MOCK group. (C) Jin and DDP inhibited both the volume and weight of tumors, and their combination displays a certain synergy. Values are means of three separate experiments ± SD. *p<0.05 and **p<0.01 by Student's paired t-test compared to MOCK group.
Gene expression profiles of tumor in different treatment groups
To elucidate the molecular mechanisms of inhibition of the tumor growth by Jin, PCR Array was utilized to find the gene expression profiles after treatment. We found 6, 6, and 11 putative genes showed a statistically 2-fold difference in expression in tumors of different groups compared to MOCK group. Among the genes, 3, 0, and 3 were up-regulated and 3, 6, and 8 were down-regulated in Jin, DDP, and co-formula groups, respectively (Fig. 2), which are listed and grouped by their functions (Table 2A—2C). The up-regulated genes were involved in TGF-β pathway. In contrast, the genes associated with cell cycle, proliferation, apoptosis, and angiogenesis were down-regulated, which are associated with the PI3K/AKT, MAPK and WNT/β-catenin Pathways, etc.
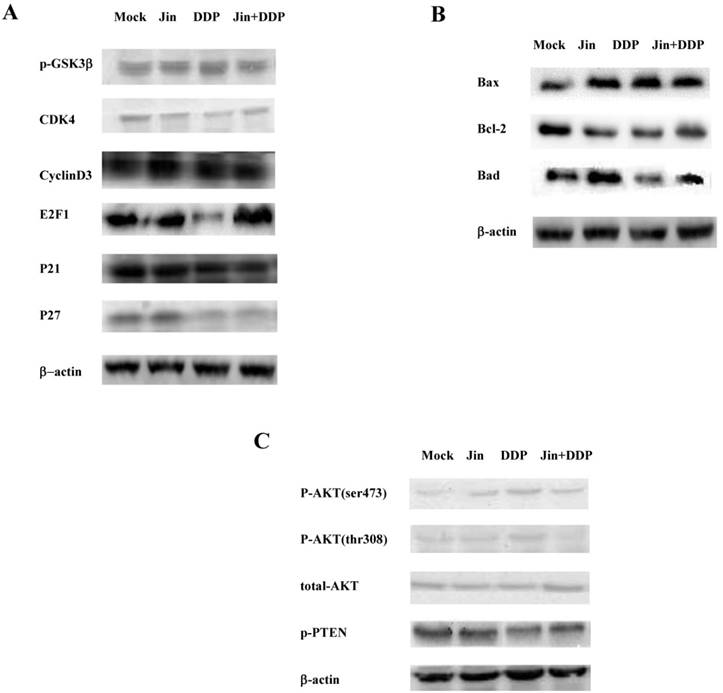
Jin induces cell cycle arrest and apoptotic molecules primarily through PI3K/AKT pathway
To confirm the results of microarray, we applied western blot to detect the cell cycle, apoptosis, and PI3K/AKT pathway related proteins. In terms of the cell cycle, Jin and DDP significantly decreased the expression of CDK4 compared with MOCK group, co-formula also decreased the expression of CDK4 compared with MOCK group, and increased slightly compared with Jin and DDP. The expression of E2F1 significantly decreased in DDP group. However, its expression was markedly elevated in co-formula group. Meanwhile, the expression of CyclinD3, P21 and P27 has no changed (Fig. 3A). Considerable increase in the Bax/Bcl-2 value was observed in all three treatment groups compared to MOCK group, however, the effect of co-formula was not as good as that of Jin or DDP group (Fig. 3B).
In terms of PI3K/AKT pathway, the expression of p-AKT (thr308) and p-AKT (ser473) was increased in Jin and DDP group compared to MOCK group, and co-formula significantly decreased the expression of p-AKT (thr308) compared to MOCK group. The expression of p-PTEN was increased in Jin group. However, the expression was significantly decreased in co-formula group. These results suggest that Jin and DDP have an effect on cell cycle, apoptosis and PI3K/AKT pathway. However, the effect is multidirectional. We found co-formula has synergetic effect on the above aspects.
Putative gene expression after different treatments. (A) Differential gene expression of Jin. (B) Differential gene expression of DDP. (C) Differential e gene expression of Jin+DDP.
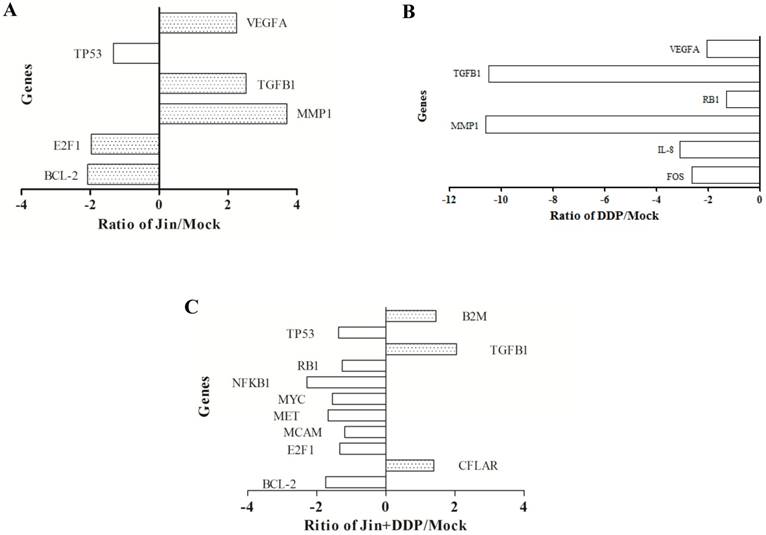
Differential expression gene of Jin compared with MOCK
| Symbol | Description | P-value | Up- or Down- Regulation |
|---|---|---|---|
| TGFβ-1 | Transforming growth factor, beta receptor 1 | 0.030503 | 2.52 |
| VEGFA | Vascular endothelial growth factor A | 0.030110 | 2.24 |
| MMP1 | Matrix metalloproteinase 1 | 0.156316 | 3.70 |
| TP53 | Tumor protein p53 | 0.024884 | -1.32 |
| E2F1 | E2F transcription factor 1 | 0.001859 | -1.96 |
| BCL2 | B-cell CLL/lymphoma 2 | 0.004719 | -2.07 |
Differential expression gene of DDP compared with MOCK
| Symbol | Description | P-value | Up- or Down- Regulation |
|---|---|---|---|
| MMP1 | Matrix metalloproteinase 1 | 0.007014 | -10.61 |
| IL8 | Interleukin 8 | 0.024599 | -3.09 |
| FOS | FBJ osteosarcoma oncogene | 0.015379 | -2.64 |
| VEGFA | Vascular endothelial growth factor A | 0.148721 | -2.07 |
| TGFβ-1 | Transforming growth factor, beta receptor 1 | 0.729707 | -10.49 |
| RB1 | Retinoblastoma 1 | 0.020272 | -1.31 |
Differential expression gene of Jin+DDP compared with MOCK
| Symbol | Description | P-value | Up- or Down- Regulation |
|---|---|---|---|
| B2M | Beta-2-microglobulin | 0.000352 | 1.45 |
| TGFβ-1 | Transforming growth factor, beta receptor 1 | 0.076041 | 2.04 |
| NFkB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | 0.042805 | - 2.28 |
| MYC | V-myc myelocytomatosis viral oncogene | 0.020287 | - 1.55 |
| TP53 | Tumor protein p53 | 0.013766 | - 1.37 |
| RB1 | Retinoblastoma 1 | 0.033653 | - 1.26 |
| MCAM | Melanoma cell adhesion molecule | 0.035749 | - 1.19 |
| MET | Met proto-oncogene | 0.013751 | - 1.67 |
| CFLAR | CASP8 and FADD-like apoptosis regulator | 0.039591 | 1.38 |
| E2F1 | E2F transcription factor 1 | 0.004253 | - 1.33 |
| BCL2 | B-cell CLL/lymphoma 2 | 0.005305 | - 1.74 |
Discussion
For centuries, Chinese medicinal herbs have had a wide range of uses. TCM formulae are rich in potential cancer chemo-preventive and therapeutic agents [10, 11]. TCM believes that lung cancer is caused by the deficiencies of Qi and Yin, Qi insufficiency in the lungs and spleen, or pathological alterations because of Qi stagnation, blood stasis, and phlegm, and toxin accumulation. The Jin formula can refill both Qi and Yin, reinforce the lungs and spleen, and clear the lungs. The Jin formula can also remove phlegm and activate blood circulation to remove stasis.
This study is the first to investigate the mechanism and demonstrate by which Jin formula induce apoptosis and anti-tumor activity in A549 human lung cells in vivo. The results suggest that Jin could ameliorate the side effects of chemotherapeutic agents, such as body weight loss, and co-formula enhanced the anti-tumor function of Jin.
Microarray analysis of signal transduction in cancer cells showed that the three treatments acted on multiple targets in cell cycle, apoptosis, proliferation, PI3K/AKT, MAPK, and WNT/β-catenin Pathways, etc. Jin up-regulate the expression of TGF- β, which plays a dual role in the process of tumor and also could negatively regulate the expression of CDK. Meanwhile, Jin suppresses the gene expression belonging to some pathways, including PI3K/AKT, cell cycle, and apoptosis. DDP mainly decreases the expression of angiogenesis factors, such as VEGFA, TGF-β and MMP-1. The combination of Jin with DDP not only down-regulate the Bcl-2 and E2F1, but also decrease the expression of MYC, MET, and MCAM.
It has been known that PI3K/AKT pathway is critical for the cancer cell survival when being exposed to different kinds stimuli of apoptosis, including osmotic stresses, and oxidative, matrix adhesion, irradiation, chemotherapeutic drugs, and ischemic shock [12-14]. Upon activation, AKT affects cellular functions, such as gene transcription, cell cycle, apoptosis, and protein synthesis through the phosphorylation of downstream substrates, including GSK3β and Bad. It has reported that the AKT gene is up-regulated in numerous cancers including NSCLC and its activity is constitutive. [15-17]. The AKT activation can be blocked by PTEN via inhibition of phosphorylation [18, 19].
The regulation of cell cycle is of great in the control of growth, and therefore, dysregulation of the cell cycle mechanism has been connected to carcinogenesis [20-22]. CDK4/CyclinD complex promotes change of G1 phase to S phase by the retinoblastoma (Rb) protein phosphorylation and release of E2F1 [23, 24]. CDK4 has been found to be up-regulated in numerous tumors, such as NSCLC. The activity of CDK/Cyclin complexes is negatively regulated through binding CKIs and the Cip/Kip family. Among them, P21 is known to induce cell cycle arrest in G0/G1 phase via inhibition of activity of the CDK4/CyclinD complex, and it appears to be an important partner for P53 [27, 28].
Apoptosis is the outcome of a complex interaction between pro- and anti- apoptotic molecules [29]. Proteins of the Bcl-2 family are important regulators of the apoptotic pathway, and Bcl-2 family can be subdivided into two families: one family, including Bcl-2, Bcl-XL, are anti-apoptotic proteins, the other, including Bax, Bad, are pro-apoptotic proteins. Data have demonstrated that many antitumor agents induced cell death by targeting the Bcl-2 family proteins, and the Bax/Bcl-2 value plays a crucial role in determining whether cells will die by apoptosis [30, 31].
The animal experiment showed that all treatment inhibited the tumor growth, and DDP has the best inhibitory effect, and Jin has the weakest. However, the mice of DDP group have severe weight loss, and combination with Jin has greatly alleviated the weight loss, meanwhile, enhanced the inhibitory effect of Jin.
The western blots data demonstrated that Jin significantly decreased the CDK4, and ClyclinD3 expression, meanwhile increased the ratio of Bax/Bcl-2 compared with mock group. DDP could increase Bax/Bcl-2 ratio, and decreased the expression of CDK4, E2F1, and p-PTEN compared with mock group. Co-formula group significantly increased Bax/Bcl-2 expression, and decreased CDK4, p-PTEN and p-AKT (thr308) expression.
Because the complexity of herbal formula, the results of western blot has not exhibit so consistent, and the tumor-inhibition rate is not the highest. However, co-formula significantly reduced the adverse effects of DDP, meanwhile, enhanced the anti-tumor effect of Jin.
Jin formula and combination treatment with DDP induce cell cycle arrest and activate apoptotic proteins via suppress PI3K/AKT pathway. (A) The expression levels of cell cycle regulatory proteins were analyzed by Western blotting. (B) The expression levels of apoptosis-related proteins were analyzed by Western blotting. (C) The expression levels of phosphorylation of AKT and PTEN were analyzed by Western blotting.
In summary, our results demonstrate that Jin+DDP significantly suppresses the growth of the transplanted tumor with slight side effect compared to DDP and have a better effect compared to Jin alone. The mechanism of the suppression of the tumor growth by the co-formula is mainly associated with inactivating PI3K/AKT pathway to induce cancer cell apoptosis. Combination of Jin with DDP will be a good therapy for NSCLC.
Acknowledgements
We thank members of the Fu lab, Emory University and Jiangsu key laboratory for TCM formulae research, Nanjing University of Chinese Medicine for assistance and enlightening discussions. This work was supported in part by National Science & Technology Pillar Program in the 11th Five-year Plan of China 2006BAI11B08-01(to H.F., and X.Z), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions (to X.Z), the Research and Innovation Program of Postgraduates in Jiangsu Province (to M.C.). Technology Pillar Program in the 11th Five-year Plan of China 2006BAI 11B08-01 (to H.F. and X.Z.), the U.S. National Institutes of Health grants P01 CA 116676 (to H.F.), People Program (Marie Curie Actions) of the European Union's Seventh Framework Program FP7/2007-2013/ under REA grant agreement No. PIRSES-GA-2013-612589: CHETCH (China and Europe taking care of healthcare solutions) (to X.Z.), and the National Natural Science Foundation of China (81503374) (to M.C.).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kelly J.J, Lanier AP Schade T. et al. Cancer disparities among Alaska native people, 1970-2011. Prev Chronic Dis. 2014;11:E221
2. MJ Machiela, CA Hsiung, XO Shu. et al. Genetic variants associated with longer telomere length are associated with increased lung cancer risk among never-smoking women in Asia: a report from the female lung cancer consortium in Asia. Int J Cancer. 2015;137:311
3. LF Forrest, J Adams, M White. et al. Factors associated with timeliness of post-primary care referral, diagnosis and treatment for lung cancer: population-based, data-linkage study. Br J Cancer. 2014;111(9):1843-51
4. Kato M, Shukuya T, Takahashi F. et al. Pemetrexed for advanced non-small cell lung cancer patients with interstitial lung disease. BMC Cancer. 2014;14:508
5. Hargrave E. Previously unknown side effect of crizotinib emerges. Pharmacogenomics. 2014;15(3):260
6. HH Strom, RM Bremnes, SH Sundstrøm. et al. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-33
7. Cheng JH, Liu WS Li ZM. et al. A clinical study on global TCM therapy in treating senile advanced non-small cell lung cancer. Chin J Integr Med. 2007;13(4):269-74
8. Li YQ, H. Sun, and D. Xue, A severe dermatologic adverse effect related with gefitinib: case report and review of the literature. J Cancer Res Ther. 2013;9(Suppl 2):S110-3
9. Jiang Y, Zhang Y, Luan J. et al. Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology. 2010;62(6):573-83
10. Zhou ZY, Xu L Li HG. et al. Chemotherapy in conjunction with traditional Chinese medicine for survival of elderly patients with advanced non-small-cell lung cancer: protocol for a randomized double-blind controlled trial. J Integr Med. 2014;12(3):175-81
11. Lan X. and Y. Jiang, The therapeutic effects of the radiotherapy plus TCM treatment observed in senile non-parvicellular lung cancer patients at the late stage. J Tradit Chin Med. 2003;23(1):32-4
12. Nishimura Y, Takiguchi S Ito S. et al. EGFstimulated AKT activation is mediated by EGFR recycling via an early endocytic pathway in a gefitinibresistant human lung cancer cell line. Int J Oncol. 2015;46(4):1721-9
13. Dent P. Crosstalk between ERK, AKT, and cell survival. Cancer Biol Ther. 2014;15(3):245-6
14. Panagiotou I, Tsiambas E Lazaris AC. et al. PTEN expression in non small cell lung carcinoma based on digitized image analysis. J BUON. 2012;17(4):719-23
15. Nagata Y, Takahashi A Ohnishi K. et al. Effect of rapamycin, an mTOR inhibitor, on radiation sensitivity of lung cancer cells having different p53 gene status. Int J Oncol. 2010;37(4):1001-10
16. Scrima M, De Marco C Fabiani F. et al. Signaling networks associated with AKT activation in non-small cell lung cancer (NSCLC): new insights on the role of phosphatydil-inositol-3 kinase. PLoS One. 2012;7(2):e30427
17. Tang J.M, He QY Guo RX. et al. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51(2):181-91
18. Jang H.D, Noh JY Shin JH. et al. PTEN regulation by the Akt/GSK-3beta axis during RANKL signaling. Bone. 2013;55(1):126-31
19. Astle M.V, Hannan KM Ng PY. et al. AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene. 2012;31(15):1949-62
20. Chen L, Wei T Si X. et al. Lysine acetyltransferase GCN5 potentiates the growth of non-small cell lung cancer via promotion of E2F1, cyclin D1, and cyclin E1 expression. J Biol Chem. 2013;288(20):14510-21
21. de Lima A.P, Pereira Fde C, Vilanova-Costa CA. et al. Induction of cell cycle arrest and apoptosis by ruthenium complex cis-(dichloro)tetramineruthenium(III) chloride in human lung carcinoma cells A549. Biol Trace Elem Res. 2012;147(1-3):8-15
22. Jancik S, Drabek J Berkovcova J. et al. A comparison of Direct sequencing, Pyrosequencing, High resolution melting analysis, TheraScreen DxS, and the K-ras StripAssay for detecting KRAS mutations in non small cell lung carcinomas. J Exp Clin Cancer Res. 2012;31:79
23. Capasso S, Alessio N2 Di Bernardo G. et al. Silencing of RB1 and RB2/P130 during adipogenesis of bone marrow stromal cells results in dysregulated differentiation. Cell Cycle. 2014;13(3):482-90
24. Chai Y.S, Yuan ZY Lei F. et al. Inhibition of retinoblastoma mRNA degradation through Poly (A) involved in the neuroprotective effect of berberine against cerebral ischemia. PLoS One. 2014;9(6):e90850
25. Padmanabhan J, Brown KR2 Padilla A. et al. Functional Role of RNA Polymerase II and P70 S6 Kinase in KCl Withdrawal-induced Cerebellar Granule Neuron Apoptosis. J Biol Chem. 2015;290(9):5267-79
26. Zalc A, Hayashi S1 Auradé F. et al. Antagonistic regulation of p57kip2 by Hes/Hey downstream of Notch signaling and muscle regulatory factors regulates skeletal muscle growth arrest. Development. 2014;141(14):2780-90
27. Grabliauskaite K, Hehl AB Seleznik GM. et al. p21(WAF1) (/Cip1) limits senescence and acinar-to-ductal metaplasia formation during pancreatitis. J Pathol. 2015;235(3):502-14
28. Chinzei N, Hayashi S Hashimoto S. et al. Cyclindependent kinase inhibitor p21 does not impact embryonic endochondral ossification in mice. Mol Med Rep. 2015;11(3):1601-8
29. Jung S.K, Lee MH Lim do Y. et al. Isoliquiritigenin induces apoptosis and inhibits xenograft tumor growth of human lung cancer cells by targeting both wild type and L858R/T790M mutant EGFR. J Biol Chem. 2014;289(52):35839-48
30. Alibek K, Irving S2 Sautbayeva Z. et al. Disruption of Bcl-2 and Bcl-xL by viral proteins as a possible cause of cancer. Infect Agent Cancer. 2014;9:44
31. Brenner C. and A. Lemoine, Mitochondrial Proteins (e.g, VDAC, Bcl-2, HK, ANT) as Major Control Points in Oncology. Front Oncol. 2014;4:365
Author contact
![]() Corresponding authors
Corresponding authors

 Global reach, higher impact
Global reach, higher impact