3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(2):310-320. doi:10.7150/jca.22362 This issue Cite
Research Paper
Potent peptide-conjugated silicon phthalocyanines for tumor photodynamic therapy
1. State Key Laboratory of Medicinal Chemical Biology, Tianjin Key Laboratory of Protein Sciences, College of Life Sciences, Nankai University, Tianjin 300071, P. R. China;
2. College of Material Science and Chemical Engineering, Tianjin University of Science and Technology, Tianjin 300457, P. R. China;
3. Tianjin University of Traditional Chinese Medicine, Tianjin 300193, P. R. China;
4. International Medicine Center, Tianjin Hospital, Tianjin 300457, P. R. China.
* These authors contributed equally to this work.
Received 2017-8-14; Accepted 2017-10-15; Published 2018-1-1
Abstract
Phthalocyanines (Pcs) are a group of promising photosensitizers for use in photodynamic therapy (PDT). However, their extremely low solubility and their strong tendency to aggregate in aqueous solution greatly restrict their application. Conjugation of Pc macrocycles with peptide ligands could be a very useful strategy to optimize the physical properties of Pcs not only by increasing their water solubility and reducing their aggregation but also by endowing the conjugates with a tumor-targeting capability. To develop highly potent photosensitizers for tumor PDT, we prepared new peptide-conjugated photosensitizers using silicon Pc (SiPc), which has much higher photodynamic activity than zinc Pcs, as the light activation moiety and the cRGDfK peptide (or simply cRGD) as the peptide moiety. A polyethylene glycol linker and an extra carboxylic acid group were also tested for introduction into the conjugates to optimize the conjugate structure. The conjugates' photophysical and photodynamic behaviors were then carefully evaluated and compared using in vitro and in vivo experiments. One of the prepared conjugates, RGD-(Linker)2-Glu-SiPc, showed excellent physical properties and photodynamic activity, with an EC50 (half maximal effective concentration) of 10-20 nM toward various cancer cells. This conjugate eradicated human glioblastoma U87-MG tumors in a xenograft murine tumor model after only one dose of photodynamic treatment, with no tumor regrowth during observation for up to 35 days. The conjugate RGD-(Linker)2-Glu-SiPc thus showed highly promising potential for use in tumor treatment.
Keywords: photodynamic therapy, silicon phthalocyanine, integrin, photosensitizer, peptide conjugate.
Introduction
Photodynamic therapy (PDT), as a highly localized and tumor-specific treatment modality, has emerged as an important therapeutic modality in the management of cancer [1-4]. Phthalocyanines (Pcs) are a group of promising photosensitizers for use in PDT [5-7] due to their advantageous photophysical properties, including intense light absorption (molar extinction coefficient ε > 1 × 105 L·mol-1·cm-1) in the red to near-infrared region (> 650 nm) and high quantum yields of singlet oxygen formation [8]. However, Pc-based photosensitizers have the significant disadvantages of extremely low solubility and a strong tendency to aggregate in aqueous solution due to the hydrophobic nature of the planar Pc macrocycle structure [9-10], which render Pcs photodynamically inactive in aqueous media [11-12]. Modifications are thus necessary for the biological application of Pcs. Conjugation of Pc macrocycles with peptide ligands could be a very useful strategy to optimize the physical properties of Pcs [13]. The conjugation of Pcs with hydrophilic peptide ligands can markedly increase the water solubility of the whole conjugate and greatly reduce the aggregation of Pcs [14]. Moreover, certain peptide ligands have a receptor-targeting capability, which is helpful for receptor-mediated internalization of Pcs [15]. These dual functions make peptide conjugation a useful strategy for Pc modification.
In the literature, zinc Pcs are the most used Pcs for the design of peptide-conjugated photosensitizers. For example, van Lier et al. synthesized a series of water-soluble zinc Pc-peptide conjugates targeting gastrin-releasing peptide (GRP) and integrin receptors, and these conjugates were screened for their photodynamic efficacy against various cancer cell lines [16]. Vicente et al. synthesized four zinc Pc-peptide conjugates designed to target the epidermal growth factor receptor (EGFR), and several of them showed efficient targeting capabilities, although their photocytotoxicity toward cancer cells was not very potent [17]. Our group also synthesized conjugates of hydrophilic-modified zinc Pcs with an EGFR-binding peptide [18] or folic acid ligand [19], with the aims of increasing the tumor-targeting capability and reducing background accumulation.
However, zinc Pcs have only very low photodynamic activities, with EC50 (half maximal effective concentration) values of approximately 1-10 μM [20-23]. Photodynamic activity is one most important parameters influencing the treatment outcome of photosensitizers. Therefore, to date, very few peptide-Zinc Pc conjugates have been successfully confirmed as promising photosensitizers for in vivo photodynamic cancer therapy. Compared with zinc Pcs, silicon Pcs (SiPcs) consistently have much higher photodynamic activity; the literature has shown that they always have EC50 values in the nanomolar range [24-27]. For example, PC-4, an asymmetric axially substituted SiPc, has an EC50 in the low nanomolar range [28-29]. Therefore, use of SiPcs to construct peptide conjugates may offer a solution to greatly improving the photodynamic activity of Pc-peptide conjugates, although not much relevant work has been reported to date [4, 30-31]. Here, we endeavored to design and synthesize this type of peptide-conjugated photosensitizer using SiPc as the light activation moiety and a short cyclic sequence, Arg-Gly-Asp-d-Phe-Lys (cRGDfK peptide, or simply cRGD), as the peptide moiety, with the goal of developing highly potent photosensitizers for tumor PDT. The cRGD peptide has a high affinity for the ανβ3 integrin receptor, which is widely expressed on tumor blood vessels, but not in normal tissues [32-34]. Conjugation of the cRGD peptide significantly improved the druggability properties of SiPcs, such as their water solubility, aggregation, and tumor-targeting capability.
However, the very strong hydrophobicity and extremely low water solubility of SiPcs may still result in big problems for the design of this type of conjugate. Unmodified SiPcs have extremely low water solubility and thus cannot be dissolved even in dimethylsulfoxide (DMSO). The strong hydrophobicity of SiPcs also greatly affects the affinity of peptide ligands. In the literature, chemical modifications of Pcs through the attachment of hydrophilic substituents, such as sulfonates, carboxylates, quaternized amino groups, carbohydrate or polyhydroxylate, to peripheral positions in the macrocycle are strategies generally used to increase the water solubility of Pcs, and especially zinc Pcs. However, the peripheral modification of SiPcs using hydrophilic groups is very difficult, and to date, no relevant practical methods have been published. To solve the problem of strong hydrophobicity associated with SiPcs, we opted to add a proper length of polyethylene glycol (PEG) linker between the Pc macrocycle and the peptide ligand, with the aims of both increasing the water solubility of the whole conjugate and avoiding the direct connection of these two moieties [35-36]. Moreover, a more strongly hydrophilic carboxylic group was tested for incorporation in the conjugate to further increase the conjugate's water solubility. Here, we report the synthesis and photophysical and biological evaluation of the resultant series of SiPc conjugates. The influence of the PEG linker length and carboxylic acid substitution on the biological behavior of the conjugates and the in vivo tumor treatment potential were carefully evaluated.
Results and discussion
Molecular design of cRGD-conjugated SiPcs
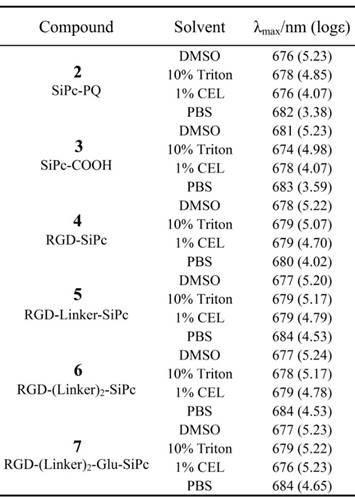
We designed the peptide-conjugated Pc photosensitizers by conjugation of an axially substituted SiPc with the cRGD peptide ligand, with the aim of developing new potent tumor-targeting photosensitizers for PDT (Fig. 1 and Scheme 1). The SiPc with axial substitution of the carboxylic group for peptide ligation, which has relatively strong absorption in the near-infrared region (ca. λ = 681 nm, logε = 5.23) and a high singlet oxygen quantum yield (0.32), was used as the photosensitizer moiety. The cRGD peptide was used for coupling with the Pc for targeting purposes and increased water solubility. The strong hydrophobic property of the SiPc macrocycle may have a significant influence on the receptor affinity of the cRGD ligand; thus, short or long PEG linkers were also introduced into the conjugates to increase the distance between the ligand and the SiPc macrocycle. A glutamic acid residue with a free strongly hydrophilic carboxylic acid group was also added to one conjugate to test if a further increase in hydrophilicity affected the biological function of the conjugates.
Synthesis procedure
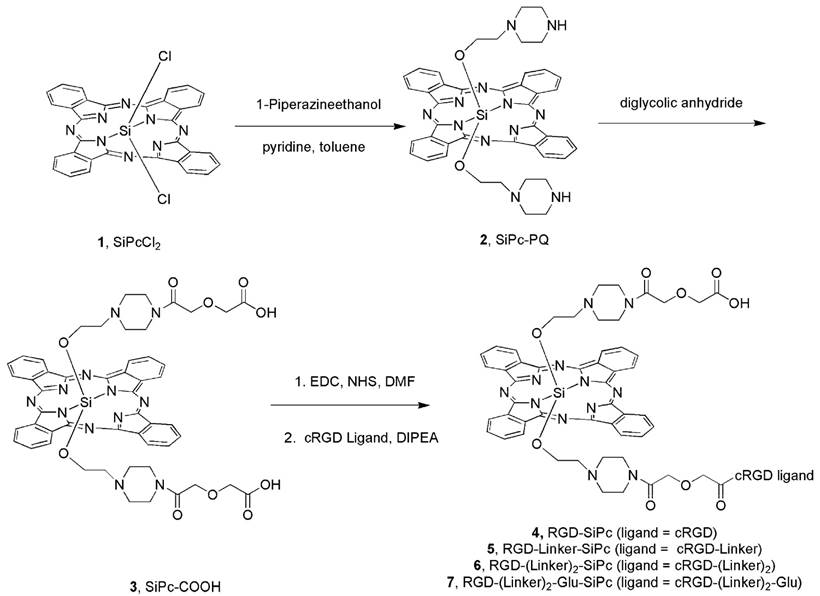
Scheme 1 shows the synthesis route used to prepare the conjugates. The cyclic cRGD peptide ligands with or without the PEG linker and glutamic acid residue (cRGD, cRGD-Linker, cRGD-(Linker)2 and cRGD-(Linker)2-Glu) were prepared using the Fmoc solid-phase peptide synthesis protocol with 2-chlorotrityl chloride resin as the solid support (see the synthetic details in the Supporting Information). The synthesis of SiPc-PQ 2 with amine handles started from the commercially available SiPcCl2 1 with 42% yield by reaction with 1-piperazineethanol in pyridine. Then SiPc-COOH 3 with carboxylic group handles was obtained with 76% yield by coupling SiPc-PQ 2 with oxalic anhydride. SiPc-COOH 3 was then coupled with cRGD or PEG-modified cRGD peptides via the typical condensation reaction between the carboxyl and the free amino groups at the N-termini of the cRGD ligands at room temperature to produce conjugates 4, 5, 6 and 7. These conjugates could be obtained in pure form by purification by high-performance liquid chromatography (HPLC) after repeated precipitations with diethyl ether and dissolution in DMSO. The green color of the Pc moiety assisted with the precipitation process. The conjugates were characterized by spectroscopy and analysis of their mass spectra, and their purity was analyzed by HPLC (Fig. S2 in the Supporting Information).
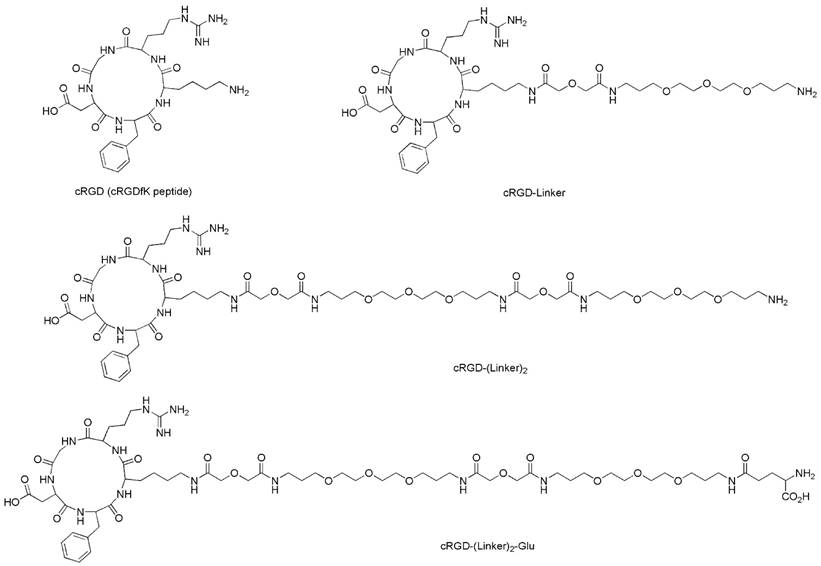
The structures of the cRGD ligands (cRGD, cRGD-Linker, cRGD-(Linker)2 and cRGD-(Linker)2-Glu).
Electronic absorption and photophysical properties
Absorption spectrum study
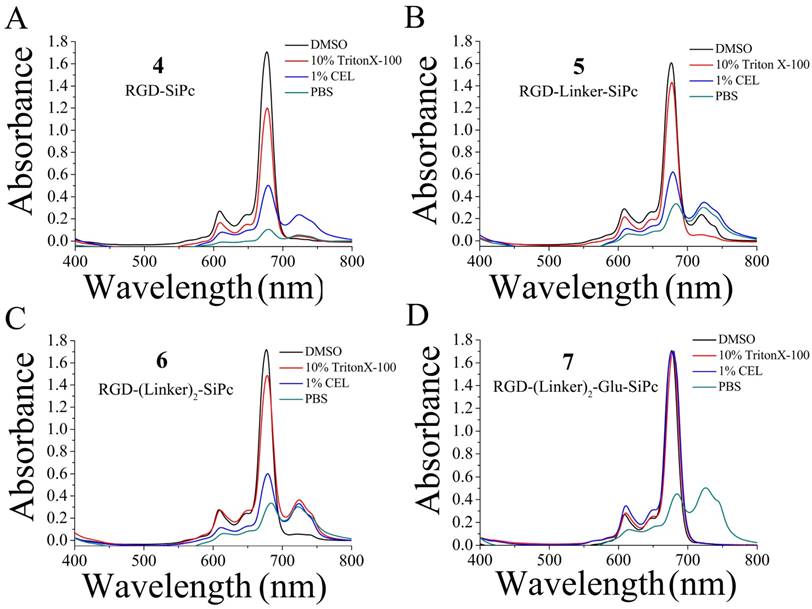
The UV-Vis absorption spectra of conjugates 4, 5, 6 and 7 showed an intense, sharp Q band at λ = 680 nm in DMSO (Fig. 2 and Table 1), a typically non-aggregated form, which strictly followed the Beer-Lambert law (Fig. S3). DMSO is known to prevent the aggregation of Pcs; however, it is not an appropriate solvent for the evaluation of biological applications. In contrast to the non-conjugated Pcs, all of the conjugates showed good solubility in aqueous solution, and especially conjugate 7, which had an extra glutamic acid residue. Therefore, the conjugates' photochemical properties were further tested in aqueous solutions, including 10% Triton X-100 aqueous solution, 1% Cremophor EL (CEL) aqueous solution (VCEL/Vwater = 1:99) and phosphate-buffered saline (PBS) solution. As shown in Fig. 2, all of these compounds aggregated in PBS solution, as shown by the broadened Q band at λ = 600-700 nm. However, the spectra for the conjugates in the 10% Triton X-100 aqueous solution were similar to those for the conjugates in DMSO, especially for RGD-(Linker)2-Glu-SiPc, whose absorption spectrum nearly completely recovered from aggregation. Using a 1% CEL aqueous solution as the solvent, which is a relatively weak lipid environment, several of these conjugates remained in aggregated forms, characterized by broad Q bands, whereas RGD-(Linker)2-Glu-SiPc showed nearly 100% recovery of its spectra, as indicated by the same pattern as for the conjugate in DMSO. These results indicate that conjugation with the hydrophilic peptide and modification with the PEG linker and extra carboxylic acid group can significantly influence the water solubility of SiPcs and are very helpful for solving the aggregation problem of SiPcs. Although the conjugates still existed in aggregated forms in PBS, the addition of 10% Triton and even 1% CEL significantly disaggregated the peptide-conjugated SiPcs, especially for RGD-(Linker)2-Glu-SiPc, which has a longer PEG linker and an extra carboxylic acid group. Adding a glutamic acid residue could obviously increase the solubility of the conjugates, causing the conjugates to be more easily de-aggregated. A lipid aqueous solution could better mimic the physiologic conditions of the cell membrane environment than a pure water solution; this is especially the case for 1% CEL in aqueous solution, which has a very low lipid concentration. The disaggregation of the photosensitizers under physiologic conditions is very important for their biological application, as only the disaggregated form of photosensitizers has photodynamic activity.
Synthesis route for the axially substituted SiPc-PQ 2; SiPc-COOH 3; and the peptide conjugates RGD-SiPc 4, RGD-Linker-SiPc 5, RGD-(Linker)2-SiPc 6, and RGD-(Linker)2-Glu-SiPc 7.
UV-Vis absorption spectra of the conjugates RGD-SiPc 4 (A), RGD-Linker-SiPc 5 (B), RGD-(Linker)2-SiPc 6 (C) and RGD-(Linker)2-Glu-SiPc 7 (D) in different solvents (i.e., DMSO, 10% Triton X-100 aqueous solution, 1% CEL aqueous solution and PBS solution). The concentration of the compounds for absorption determination was 10 μM.
Fluorescence quantum yields, lifetimes and singlet oxygen quantum yield
The fluorescence data and singlet oxygen quantum yields of the conjugates were determined in DMSO as described previously [18, 19]. As shown in Table 2, the cRGD moiety did not exert a significant influence on the electronic absorption and photophysical properties of the Pc core. The conjugates had similar maximum emission and excitation wavelengths with non-conjugated SiPc-PQ 2 and SiPc-COOH 3. Their fluorescence quantum yields are also similar, but they showed a slightly reduced fluorescence decay time compared with SiPc-PQ 2 and SiPc-COOH 3. All of the conjugates also had relatively high singlet oxygen quantum yields, but interestingly, conjugation with the peptide and linkers seemed to slightly increase the singlet oxygen quantum yields of the conjugates. RGD-(Linker)2-Glu-SiPc 7 had the highest yield (0.39). Singlet oxygen (1O2) is believed to be the major cytotoxic agent involved in PDT, and an increase in the singlet oxygen quantum yield should be beneficial for PDT.
In vitro photodynamic activity
The in vitro photodynamic activity of the conjugates was evaluated against tumor cell lines by MTT assay. Human glioblastoma U87-MG cells, human prostate carcinoma 22RV1 and PC3 cells, and human epidermoid carcinoma A431 cells were used for the evaluation. U87-MG, 22RV1 and PC3 cells highly express the ανβ3 integrin receptor (ανβ3+), and A431 cells show low levels of expression of the ανβ3 integrin receptor (ανβ3-). All of the cells were incubated with the conjugates, which were dissolved in aqueous culture media in the presence of 1.0% CEL, for 4 h before light illumination at a dosage of 40 mW/cm2 for 15 min (36 J/cm2) at 670 nm. Fig. 3 and Fig. 4 show the corresponding dose-dependent survival curves without or with laser illumination, and Table 3 shows the corresponding EC50 values of the conjugates toward the cells. None of the four conjugates showed significant cytotoxicity in the dark toward the above cell lines at concentrations up to 0.5 μM (to avoid solubility problems, no higher concentrations were tested), and light irradiation alone did not affect cell viability (Fig. 3). However, upon illumination with light, cell viability was strongly reduced in the presence of very low concentrations of the conjugates. All of the conjugates had EC50 values in the nanomolar range, and the conjugate RGD-(Linker)2-Glu-SiPc 7 had the most potent photodynamic activity, with EC50 values of 17.1 nM, 16.7 nM, 16.2 nM and 50.4 nM toward U87-MG, 22RV1, PC3 and A431 cells, respectively (Fig. 4 and Table 3). The carboxylic group of the glutamic acid residue seems to have played a significant role in the improvement of the PDT activity of the conjugates.
Absorption spectral data for SiPc-PQ 2, SiPc-COOH 3, RGD-SiPc 4, RGD-Linker-SiPc 5, RGD-(Linker)2-SiPc 6 and RGD-(Linker)2-Glu-SiPc 7 in DMSO, 10% Triton X-100 aqueous solution, 1% CEL aqueous solution and PBS solution.
The difference in the photocytotoxicities of the conjugates toward receptor-positive and receptor-negative cells was not significant. Similar results were observed for the recently reported cRGD-conjugated pyropheophorbide a and zinc Pc, which exhibited similar photocytotoxicities toward the ανβ3+ U87-MG cells and ανβ3- A431 or MCF-7 cells after drug incubation [34, 37]. The uptake of these conjugates may not be solely due to the number of receptors on the cell surface; another route, such as through the direct anchoring of these conjugates to the cell surface membrane due to the strong hydrophobicity of the SiPc moiety, may exist. In the future, strategies to reduce the strong hydrophobicity of the SiPc moiety may be required to increase the cell selectivity of the conjugates.
In vivo PDT
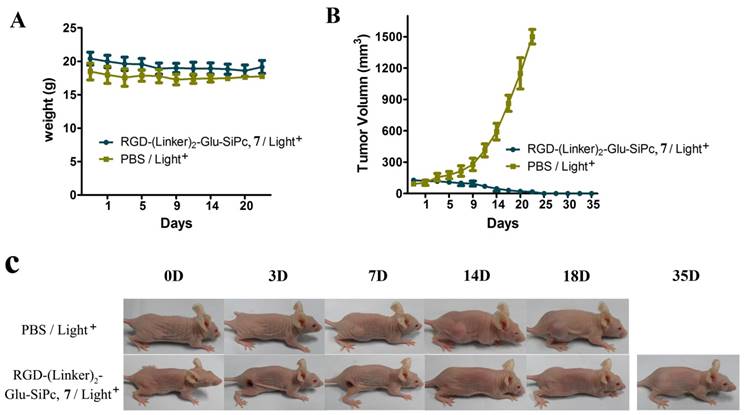
RGD-(Linker)2-Glu-SiPc 7 is a better photosensitizer than the other three conjugates for tumor PDT. This conjugate had the highest PDT activity in the cell-based activity assay, had better water solubility, and was more easily disaggregated in aqueous solution. Therefore, this conjugate was selected for the animal treatment experiment. We specifically used U87-MG murine xenograft model to study the photodynamic effect of this conjugate. In this model, U87-MG cells were injected subcutaneously into the right flanks of BALB/c nude mice. When the tumor volume reached 100 mm3, the conjugate RGD-(Linker)2-Glu-SiPc 7 or saline, as the control, was injected intravenously via the caudal vein, followed by light illumination (200 mW/cm2 for 16 min). As shown in Fig. 5, in the control group, which received the saline injection followed by light illumination, the treatment had no effect on reducing the tumor growth rate, and the tumor sizes were found to increase dramatically, up to 1500 mm2, in less than 25 days. In contrast, the tumor sizes in the conjugate treatment groups, which received both the conjugate injection and light illumination, were greatly attenuated. All tumors were irradiated at approximately day 14, and no tumors grew again during observation for up to 35 days. During the whole treatment, no detectable body weight loss was observed, indicating that neither the irradiation nor the conjugate RGD-(Linker)2-Glu-SiPc 7 caused serious side effects in the mice. The extremely high efficacy of the PDT based on the conjugate RGD-(Linker)2-Glu-SiPc 7 in the U87-MG tumors demonstrated that this conjugate has high clinical potential for cancer therapy.
Conclusion
Herein, we designed and synthesized peptide-conjugated SiPcs by conjugation of an axially substituted SiPc with the cRGD peptide ligand, with the aim of developing new potent photosensitizers for tumor PDT. Conjugation with the cRGD peptide greatly increased the water solubility of the SiPc. After introducing two PEG linkers and one strong hydrophilic carboxylic acid group to further increase the hydrophilicity, the prepared conjugate RGD-(Linker)2-Glu-SiPc 7 showed excellent properties and photodynamic activity, with EC50 values of 10-20 nM toward various cancer cells. This conjugate eradicated the U87-MG tumors in the xenograft murine tumor model after only one dose of photodynamic treatment, and no tumors grew again during observation for up to 35 days. These results demonstrate that PDT based on the conjugate RGD-(Linker)2-Glu-SiPc 7 has high potential for use in tumor treatments.
Photophysical and photochemical parameters for SiPc-PQ 2, SiPc-COOH 3, RGD-SiPc 4, RGD-Linker-SiPc 5, RGD-(Linker)2-SiPc 6 and RGD-(Linker)2-Glu-SiPc 7 in DMSO. ΦF: fluorescence quantum yield; τF: fluorescence lifetime; ΦΔ: singlet oxygen quantum yield. For the reference standard, ZnPc was used; ΦΔ = 0.67.
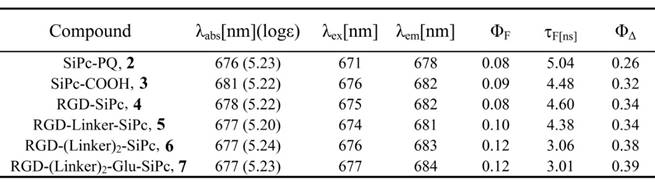
EC50 values of SiPc-COOH 3, RGD-SiPc 4, RGD-Linker-SiPc 5, RGD-(Linker)2-SiPc 6 and RGD-(Linker)2-Glu-SiPc 7 toward U87-MG, 22RV1, PC3 and A431 cells. Data acquisition was performed using GraphPad Prism software.
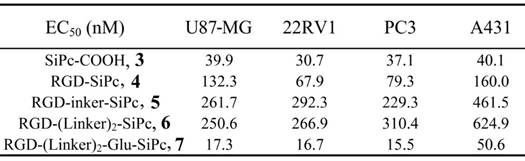
Cytotoxicity of the conjugates in the dark and cytotoxicity of the light alone. The viability of U87-MG cells (A) incubated with SiPc-COOH 3, RGD-SiPc 4, RGD-Linker-SiPc 5, RGD-(Linker)2-SiPc 6 and RGD-(Linker)2-Glu-SiPc 7 without light and the viability of U87-MG, 22RV1, PC3 and A431 cells with light illumination alone (36 J/cm2) (B) were determined by MTT assay.
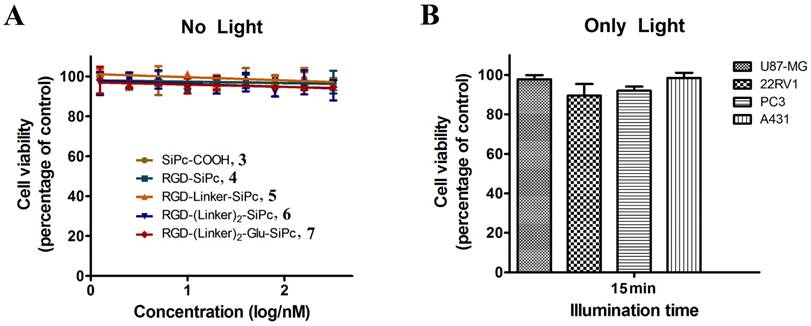
In vitro concentration-dependent photocytotoxicity of SiPc-COOH 3 (circles, dark yellow), RGD-SiPc 4 (squares, olive), RGD-Linker-SiPc 5 (upward triangles, orange), RGD-(Linker)2-SiPc 6 (downward triangles, blue) and RGD-(Linker)2-Glu-SiPc 7 (diamonds, red) on receptor-positive U87-MG (A), 22RV1 (B), and PC3 (C) cells and receptor-negative A431 cells (D) (n = 3, mean ± SEM), as determined by MTT assay. The cells were treated with various concentrations of the conjugates for 4 h before illumination with light (40 mW/cm2, 15 min), and cell viability was determined by MTT assay.
In vivo phototherapeutic activity of the conjugate RGD-(Linker)2-Glu-SiPc 7. The body weight (A) and tumor volume (B) changes of the BALB/c nude mice were monitored every other day. Photographs (C) were taken to observe the more visible tumor changes (one representative mouse from each group is shown). All of the mice were irradiated once with 670-nm light for 16 min (288 J/cm2) 4 h after injection with PBS or RGD-(Linker)2-Glu-SiPc 7 (50 nmol per mouse).
Experimental section
Chemicals and materials
Silicon Pc dichloride (SiPcCl2, 1), 1,13-diamino-4,7,10-trioxatridecane, 1-ethyl-3-(3-dimethyllaminopropyl) carbodiimide hydrochloride (EDC·HCl) and piperazine ethanol were purchased from Sigma-Aldrich (St. Louis, MO, USA). N-hydroxysuccinimide (NHS), diglycolic anhydride and diisopropylethylamine (DIPEA) were purchased from Heowns (Tianjin, China). Fetal bovine serum (FBS) and antibiotics (penicillin/streptomycin) were obtained from HyClone (Logan, UT, USA). Other chemical reagents were obtained from J&K Chemical Ltd. (Beijing, China).
Cells and animals
U87-MG, 22RV1, PC3 and A431 cells were obtained from the Cell Bank of the Chinese Academy of Sciences and cultured in DMEM supplemented with 10% (v/v) FBS and 1% (v/v) penicillin/streptomycin at 37 °C in a 5% CO2 environment. Cells were typically passaged every day at a 1:2 ratio following harvest with 0.25% trypsin-EDTA (1X) and phenol red. Female BALB/c nude mice (6 weeks, 18-22 g) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). All of the mice were treated according to the guidelines of the Committee on Animals of Nankai University.
Instruments
1H and 13C NMR spectra were recorded in DMSO-d6 solutions on a Bruker AV400 400 MHz spectrometer. Mass spectra were recorded on Varian 7.0T FTMS. UV-Vis spectra were recorded with a USA Cary 5000 spectrophotometer (Varian Co., Palo Alto, USA) using a 1-cm path-length cuvette at room temperature. Fluorescence measurements were performed on a PTI QM/TM/NIR system (Photon Technology International, Birmingham, NJ, USA) equipped with a quartz cell (1 cm × 1 cm). HPLC (Shimadzu, Kyoto, Japan) was used for purification and analysis.
Synthesis experiments
Synthesis of SiPc-PQ 2
1-Piperazineethanol (20.8 mg, 0.016 mmol) and SiPcCl2 1 (10.0 mg, 0.016 mmol) were added to a mixed solution of toluene and pyridine (1 mL, v/v = 5:1) and refluxed for approximately 10 h. The solvent was then removed under reduced pressure. The residue was diluted with CH2Cl2 and washed with saline three times, and the combined organic layer was dried over Na2SO4. The product was concentrated and purified by silica gel chromatography eluted with MeOH, CH2Cl2 and a small amount of TEA to yield SiPc-PQ 2 (5.0 mg, 42%). HRMS (ESI): m/z calculated for C44H42N12O2Si [M + H]+ 799.3396, found 799.3392.
Synthesis of SiPc-COOH 3
SiPc-PQ 2 (10.0 mg, 0.013 mmol) and oxydiacetic anhydride (9.0 mg, 0.078 mmol) were added to 200 μL anhydrous DMF and stirred at room temperature for 2 h. The mixture was precipitated and washed with anhydrous diethyl ether to yield SiPc-COOH 3 (10.0 mg, 76%). HRMS (ESI): m/z calculated for C52H51N12O10Si [M + H]+ 1031.3615, found 1031.3613.
Synthesis of RGD-SiPc 4
SiPc-COOH 3 (10.0 mg, 0.010 mmol), EDC (1.8 mg, 0.009 mmol) and NHS (1.1 mg, 0.010 mmol) were added to 1.0 mL anhydrous DMF and stirred at room temperature for 4 h. DIPEA (2.5 mg, 0.019 mmol) and cRGD (1.7 mg, 0.003 mmol) were then added to the mixture and stirred at room temperature overnight. The final mixture was precipitated and washed with ethyl ether and dichloromethane three times, followed by HPLC purification to yield RGD-Linker-SiPc 4 (3.8 mg, 78%). HRMS (ESI): m/z calculated for C79H90N21O16Si [M + H]+ 1616.6638, found 1616.6705.
Synthesis of RGD-Linker-SiPc 5
SiPc-COOH 3 (10.0 mg, 0.010 mmol), EDC (1.8 mg, 0.009 mmol), and NHS (1.1 mg, 0.010 mmol) were added to 200 μL anhydrous DMF and stirred at room temperature for 4 h. DIPEA (2.5 mg, 0.019 mmol) and cRGD-Linker (2.6 mg, 0.003 mmol) were then added to the mixture and stirred at room temperature overnight. The final mixture was precipitated and washed with ethyl ether and dichloromethane three times, followed by HPLC purification to yield RGD-Linker-SiPc 5 (3.3 mg, 57%). HRMS (ESI): m/z calculated for C93H116N23O22Si [M + H]+ 1934.8429, found 1934.8512.
Synthesis of RGD-(Linker)2-SiPc 6
SiPc-COOH 3 (10.0 mg, 0.010 mmol), EDC (1.8 mg, 0.009 mmol), and NHS (1.1 mg, 0.010 mmol) were added to 200 μL anhydrous DMF and stirred at room temperature for 4 h. DIPEA (2.5 mg, 0.019 mmol) and cRGD-(Linker)2 (3.6 mg, 0.003 mmol) were then added to the mixture and stirred at room temperature overnight. The final mixture was precipitated and washed with ethyl ether and dichloromethane three times, followed by HPLC purification to yield RGD-(Linker)2-SiPc 6 (2.6 mg, 40%).
Synthesis of RGD-(Linker)2-Glu-SiPc 7
SiPc-COOH 3 (10.0 mg, 0.010 mmol), EDC (1.8 mg, 0.009 mmol), and NHS (1.1 mg, 0.010 mmol) were added to 200 μL anhydrous DMF and stirred at room temperature for 4 h. DIPEA (2.5 mg, 0.019 mmol) and cRGD-(Linker)2-Glu (4.0 mg, 0.003 mmol) were then added to the mixture and stirred at room temperature overnight. The final mixture was precipitated and washed with ethyl ether and dichloromethane three times, followed by HPLC purification to yield RGD-(Linker)2-Glu-SiPc 7 (2.5 mg, 32%).
Photophysical and photochemical properties
UV-Vis spectra
The UV-Vis spectra were measured using a USA Cary 5000 with a 1-cm path-length cuvette. The concentration of Pcs was 10 μM in the solvents, including DMSO, 10% Triton X-100 aqueous solution, 1% CEL aqueous solution and PBS solution. Baselines and spectra were recorded at room temperature in the wavelength range from 500-900 nm, with a 1-nm resolution and a 600 nm/min scan rate.
Fluorescence spectra
The fluorescence emission spectra were recorded in the wavelength range of 600-900 nm upon excitation at 680 nm, and the fluorescence excitation spectra were recorded in the wavelength range of 500-900 nm upon emission at 695 nm. The concentration of Pcs was 2.0 μM in DMSO. The slit widths were 1 and 2 nm for excitation and emission, respectively.
The natural radiative lifetimes, absolute fluorescence quantum yields and singlet oxygen quantum yields of these compounds were all determined according to our published procedures [18, 19]. Briefly, the fluorescence decay curves were measured on a PTI QM-4m system (Photon Technology International, Birmingham, NJ, USA) with Felix32 software; the absolute fluorescence quantum yields at room temperature were determined on a PTI QM/TM/NIR system (Photon Technology International, Birmingham, NJ, USA) with an integration sphere attachment (Labsphere, North Sutton, NH, USA); the singlet oxygen quantum yields were determined via a steady-state method using DPBF (1,3-diphenylisobenzofuran, 20 μM) as the scavenger in DMSO (10 μM of the conjugates) with ZnPc (ΦΔ = 0.67 in DMSO) as the standard.
In vitro photodynamic activity
In vitro PDT was performed on U87-MG, 22RV1, PC3 and A431 cells. The cells were seeded in 96-well plates (1 × 106 cells per well) and cultured for 24 h prior to PDT. The cells were then incubated with various concentrations of conjugate 4, 5, 6 or 7 in complete DMEM for 4 h, followed by light illumination (670 nm, 40 mW/cm2, 15 min). Four hours later, the cell monolayers were incubated with 500 μg/mL MTT solution at 37 °C for 4 h. Next, the formazan crystals that formed were dissolved in DMSO, and the absorbance of the dissolved formazan crystals at 490 nm was measured using a 96-well plate reader (BioTek microplate reader). Meanwhile, we carried out experiments to assess the toxicity of the conjugates toward the cell lines in the dark. The cell viability was determined as described above but without light illumination after incubation of the cells with various concentrations of the conjugates. Each experiment was repeated three times with three replicates at each concentration of the conjugate and for each cell line. The cell phototoxicity curves were plotted as a function of the photosensitizer dose, and the EC50 values were calculated.
In vivo anticancer efficacy
The in vivo PDT efficacy of the conjugate RGD-(Linker)2-Glu-SiPc 7 was investigated using the U87-MG subcutaneous xenograft mouse model. Tumors were established in 8-week-old female BALB/c nude mice by subcutaneous inoculation of U87-MG cell suspension (3 × 106 cells per mouse) into the right thigh of each mouse. When the tumors reached 100 mm3 in volume, the mice were randomly divided into two groups (5 mice per group) with equivalent average starting tumor sizes. The treatment group was intravenously administered RGD-(Linker)2-Glu-SiPc 7 (50 nmol per mouse), followed by light illumination (200 mW/cm2, 670 nm, 16 min) 4 h after the injection. The control group received an equivalent volume of PBS, followed by the same light illumination. Tumor volume and body weight evolution were monitored for all mice for 35 days after the PDT. Tumor volumes were measured with a digital caliper every other day and were calculated as follows: (length × width2) / 2. Photographs of the mice were also taken every two days to observe the more visible tumor changes. Mice were considered dead when the tumor volume exceeded 1500 mm3.
Supplementary Material
Supplementary figures.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (NO. 31270926), the Ph.D. Candidate Research Innovation Fund of Nankai University (No. 96172128), the Major Program of the National Natural Science Foundation of China (No. 31527801) and the Natural Science Foundation of Tianjin (17JCYBJC24600).
Note
Electronic Supplementary Information (ESI) available: [Synthetic procedure for cRGDfK peptide and linker-modified peptides and HPLC chromatograms and ESI-HRMS of the conjugates]. See DOI:
Competing Interests
The authors have declared that no competing interest exists.
References
1. Agostinis P, Berg K, Cengel KA. et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250-281
2. Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535
3. Celli JP, Spring BQ, Rizvi I. et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010;110:2795-2838
4. Mitsunaga M, Ogawa M, Kosaka N. et al. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685-1691
5. Ethirajan M, Chen Y, Joshi P. et al. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem Soc Rev. 2011;40:340-362
6. Jiang Z, Shao J, Yang T. et al. Pharmaceutical development, composition and quantitative analysis of phthalocyanine as the photosensitizer for cancer photodynamic therapy. J Pharmaceut Biomed. 2014;87:98-104
7. Josefsen LB, Boyle RW. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics. 2012;2:916-966
8. O'Connor AE, Gallagher WM, Byrne AT. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol. 2009;85:1053-1074
9. Mckeown NB. Phthalocyanine materials: synthesis, structure and function. Cambridge University Press: UK. 1998 ISBN 0-521-49623-3
10. Makhseed S, Machacek M, Alfadly W. et al. Water-soluble non-aggregating zinc phthalocyanine and in vitro studies for photodynamic therapy. Chem Commun. 2013;49:11149-11151
11. DeRosa MC, Crutchley RJ. Photosensitized singlet oxygen and its applications. Chem Rev. 2002;233:351-371
12. Lunardi CN, Tedesco AC. Synergic photosensitizers: a new trend in photodynamic therapy. Curr Org Chem. 2005;9:813-821
13. Bugaj AM. Targeted photodynamic therapy-a promising strategy of tumor treatment. Photoch Photobio Sci. 2011;10:1097-1109
14. Luan L, Fang W, Liu W. et al. Phthalocyanine-cRGD conjugate: synthesis, photophysical properties and in vitro biological activity for targeting photodynamic therapy. Org Biomol Chem. 2016;14:2985-2992
15. Mahato R, Tai W, Cheng K. Prodrugs for improving tumor targetability and efficiency. Adv Drug Deliver Rev. 2011;63:659-670
16. Ranyuk E, Cauchon N, Klarskov K. et al. Phthalocyanine-peptide conjugates: receptor-targeting bifunctional agents for imaging and photodynamic therapy. J Med Chem. 2013;56:1520-1534
17. Ongarora BG, Fontenot KR, Hu X. et al. Phthalocyanine-peptide conjugates for epidermal growth factor receptor targeting. J Med Chem. 2012;55:3725-3738
18. Li YX, Wang J, Zhang XX. et al. Highly water-soluble and tumor-targeted photosensitizers for photodynamic therapy. Org Biomol Chem. 2015;13:7681-7694
19. Li F, Liu Q, Liang ZZ. et al. Synthesis and biological evaluation of peptideconjugated phthalocyanine photosensitizers with highly hydrophilic modifications. Org Biomol Chem. 2016;14:3409-3422
20. Xu P, Chen J, Chen Z. et al. Receptor-targeting phthalocyanine photosensitizer for improving antitumor photocytotoxicity. PloS ONE. 2012;7:e37051
21. Sibrian-Vazquez M, Ortiz J, Nesterova IV. et al. Synthesis and properties of cell-targeted Zn(II)-phthalocyanine-peptide conjugates. Bioconjugate Chem. 2007;18:410-420
22. Kucinska M, Skupin-Mrugalska P, Szczolko W. et al. Phthalocyanine derivatives possessing 2-(morpholin-4-yl)ethoxy groups as potential agents for photodynamic therapy. J Med Chem. 2015;58:2240-2255
23. Machacek M, Cidlina A, Novakova V. et al. Far-red-absorbing cationic phthalocyanine photosensitizers: synthesis and evaluation of the photodynamic anticancer activity and the mode of cell death induction. J Med Chem. 2015;58:1736-1749
24. Lo PC, Chan CMH, Liu JY. et al. Highly photocytotoxic glucosylated silicon(IV) phthalocyanines. Effects of peripheral chloro substitution on the photophysical and photodynamic properties. J Med Chem. 2007;50:2100-2107
25. Lau JTF, Lo PC, Tsang YM. et al. Unsymmetrical β-cyclodextrin-conjugated silicon(IV) phthalocyanines as highly potent photosensitisers for photodynamic therapy. Chem Commun. 2011;47:9657-9659
26. Hofman JW, Zeeland FV, Turker S. et al. Peripheral and axial substitution of phthalocyanines with solketal groups: synthesis and in vitro evaluation for photodynamic therapy. J Med Chem. 2007;50:1485-1494
27. Jiang XJ, Lo PC, Tsang YM. et al. Phthalocyanine-polyamine conjugates as pH-controlled photosensitizers for photodynamic therapy. Chem Eur J. 2010;16:4777-4783
28. Baron ED, Malbasa CL, Santo-Domingo D. et al. Silicon phthalocyanine (Pc 4) photodynamic therapy is a safe modality for cutaneous neoplasms: results of a phase 1 clinical trial. Laser Surg Med. 2010;42:728-735
29. Miller JD, Baron ED, Scull H. et al. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: the case experience with preclinical mechanistic and early clinical-translational studies. Toxicol Appl Pharm. 2007;224:290-299
30. Nkepang G, Bio M, Rajaputra P. et al. Folate receptor-mediated enhanced and specific delivery of far-red light-activatable prodrugs of combretastatin A-4 to FR-positive tumor. Bioconjugate Chem. 2014;25:2175-2188
31. Thapa P, Li M, Bio M. et al. Far-red light-activatable prodrug of paclitaxel for the combined effects of photodynamic therapy and site-specific paclitaxel chemotherapy. J Med Chem. 2016;59:3204-3214
32. Wang F, Li Y, Shen Y. et al. The functions and applications of RGD in tumor therapy and tissue engineering. Int J Mol Sci. 2013;14:13447-13462
33. Chen K, Chen X. Integrin targeted delivery of chemotherapeutics. Theranostics. 2011;1:189-200
34. Ke MR, Ng DKP, Lo PC. Synthesis and in vitro photodynamic activities of an integrin-targeting cRGD-conjugated Zinc(II) phthalocyanine. Chem Asian J. 2014;9:554-561
35. Guo Y, Yuan H, Rice WL. et al. The PEG-fluorochrome shielding approach for targeted probe design. J Am Chem Soc. 2012;134:19338-19341
36. Liu F, Deng D, Chen X. et al. Folate-polyethylene glycol conjugated near-infrared fluorescence probe with high targeting affinity and sensitivity for in vivo early tumor diagnosis. Mol Imaging Biol. 2010;12:595-607
37. Avinash S, Manivannan E, Suresh KP. et al. Conjugation of cRGD peptide to chlorophyll a based photosensitizer (HPPH) alters its pharmacokinetics with enhanced tumor-imaging and photosensitizing (PDT) efficacy. Mol Pharm. 2011;8:1186-1197
Author contact
![]() Corresponding authors: Zhangyong Hong, E-mail: hongzyedu.cn; Tel.: +86 022 23498707. Wenjie Wu, E-mail: wwjieedu.cn; Tel.: +86 022 23502875. Kai Wang, E-mail: wangkai0286com. Tel.: +86 022 23003610.
Corresponding authors: Zhangyong Hong, E-mail: hongzyedu.cn; Tel.: +86 022 23498707. Wenjie Wu, E-mail: wwjieedu.cn; Tel.: +86 022 23502875. Kai Wang, E-mail: wangkai0286com. Tel.: +86 022 23003610.

 Global reach, higher impact
Global reach, higher impact