3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(14):2518-2524. doi:10.7150/jca.25824 This issue Cite
Research Paper
Association of endothelial nitric oxide synthase (eNOS) polymorphisms with EGFR-mutated lung adenocarcinoma in Taiwan
1. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
2. Department of Pulmonary Medicine, Buddhist Tzu Chi General Hospital, Taipei Branch, New Taipei City, Taiwan
3. Cancer Research Center, Changhua Christian Hospital, Changhua, Taiwan
4. Graduate Institute of Biomedical Sciences, China Medical University, Taichung, Taiwan
5. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
6. School of Medical Laboratory and Biotechnology, Chung Shan Medical University, Taichung, Taiwan
7. Department of Chest Medicine, Cheng-Ching General Hospital, Taichung, Taiwan
8. School of Medicine, Chung Shan Medical University, Taichung, Taiwan
9. Division of Chest, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan
#These authors contributed equally to the work.
Received 2018-3-1; Accepted 2018-5-20; Published 2018-6-15
Abstract
EGFR mutation of Non-small cell lung cancers (NSCLC) was predominantly seen in Asian population and it was considered as a predictor of responsiveness. Eendothelial nitric oxide synthase (eNOS) plays a vital role in chronic inflammation and carcinogenesis. In this study, we aimed to explore the association between the genetic polymorphisms of eNOS (-786T/C and 894 G/T) and EGFR mutation in patients with lung adenocarcinoma. A total of 277 patients with diagnosed lung adenocarcinoma were recruited between years 2012 and 2015. All study subjects underwent the analysis of eNOS genetic variants (-786 T/C and 894 G/T) using real-time polymerase chain reaction (PCR) genotyping. Our results showed that, among the 277 patients, variant types (GT + TT) of eNOS 894 G/T polymorphism were significantly positively correlated with EGFR mutation type, specifically exon 19 in-frame deletion. With the subgroup of EGFR L858R mutation, variant genotypes (GT + TT) of eNOS 894 G/T were significantly associated with lymph node invasion. Moreover, in silico analysis indicated that eNOS 894 G/T altered the eNOS expression. In conclusion, our study showed that eNOS 894 G/T variants were significantly associated with EGFR mutation types of lung adenocarcinoma, specifically exon 19 in-frame deletion. This may be utilized as a prediction of tumor invasiveness and therapy responsiveness.
Keywords: Adenocarcinoma, Lung cancer, Endothelial nitric oxide synthase (eNOS) gene, Polymorphism, Epidermal growth factor receptor (EGFR)
Introduction
Lung cancer is the leading cause of cancer mortality in the United States, but also throughout the world. In the United States, lung cancer is estimated to be diagnosed in approximately 234,000 people annually and causes approximately 154,000 deaths [1]. In Taiwan, lung cancer was the top three incidence (13,312 people) of cancer diagnosed in year 2015 and the top one cancer mortality (9,232 deaths) based on the latest report published in Dec 2017. With advancements in early detection and treatment, likelihood of long-term survival is increasing. But still, high mortality was expected since diagnosed. Survival rates of females were much higher than males (i.e. 5-year survival rate: male - 14.3%, female - 28.4%). These data of survival rates were the lowest among the top ten incidences of cancers in Taiwan.
About 80 percent of all lung cancers are classified as non-small cell lung cancer (NSCLC), and the rest are mostly small cell lung cancer (SCLC). This distinction is necessary for appropriate staging, treatment, and prognosis. Adenocarcinoma, classified as a NSCLC, is the most common type of lung cancer recently and accounting for about 50% of all lung cancers. Patients with advanced lung adenocarcinoma and other NSCLC should have their tumors assessed for the presence of a driver mutation [2]. At present, there are more and more documented genotypes with approved targeted therapies, such as epidermal growth factor receptor (EGFR) mutation, ROS1 translocation, anaplastic lymphoma kinase (ALK) translocation, BRAF mutation, HER2 mutation, MET abnormalities, RET translocation, etc. There are also some genotypes with ongoing clinical trials.
Mutations in EGFR tyrosine kinase are noted in about 15% of NSCLC adenocarcinoma in the Unites States [3] and more often in women and non-smokers. In Asian population, the incidence of EGFR mutation is extremely higher, up to 62% [4]. Advanced NSCLCs that contains characteristic mutations in EGFR are highly sensitive to EGFR-TKIs (EGFR-tyrosine kinase inhibitors), and hence, considered as a predictor of responsiveness.
Nitric oxide (NO), a small free radical, is involved in various physiologic and pathophysiologic processes, including immunity, neurotransmission and carcinogenesis [5, 6]. NO is synthesized from L-arginine, NADPH and oxygen by NO synthase (NOS) [7]. Endothelial NOS (eNOS), one of the major isoform of NOS, is constitutively expressed and is therefore also referred to as constitutive NOS (cNOS). Several studies have shown that NO can both promote and inhibit tumor metastasis and progression at different concentration [7]. Moreover, eNOS can regulate the proinflammatory molecules expression [8], such as cyclooxygenase-2 or nuclear factor-κB (NF-κB) [9].
Several researches have reported that single nucleotide polymorphisms (SNPs) of eNOS may regulate gene transcription, thus, affecting the causing the protein levels and activity of eNOS [10]. The eNOS gene is located on chromosome 7q36.1. Among the known polymorphisms, there were three extensively studied genetic polymorphisms that could modify its transcription and endogenous NO production: a single nucleotide polymorphism (SNP) -786T > C (rs2070744) in the promoter region, a 27-bp variable nucleotide tandem repeat (VNTR) in intron 4, and another SNP 894G > T (rs1799983) in exon 7 [11, 12]. Numerous studies have investigated how the eNOS polymorphisms influencing the risk of carcinogenesis [13-18], including breast cancer, prostate cancer and colorectal cancer, but no conclusive results were obtained [14]. So far, there are very scarce studies about the relationship between lung cancer and eNOS gene polymorphisms. In this study, we aimed to explore the association between the genetic polymorphisms of eNOS (-786T/C and 894 G/T) and EGFR mutation in patients with lung adenocarcinoma.
Material and Methods
Study Subjects
We recruited 277 patients diagnosed of lung adenocarcinoma at Cheng-Ching General Hospital in Taichung, Taiwan from years 2012 to 2015. This study protocol was approved by the Institutional Review Board of Cheng-Ching General Hospital (No. HP120009; 22 September 2012). Informed written consent was obtained from each individual before initiation of the study. For all lung adenocarcinoma patients, the tumor-frozen specimen and paraffin-embedded tissues were collected for EGFR gene sequencing and whole-blood specimens collected from all participants were placed in sterile tubes containing ethylenediaminetetraacetic acid (EDTA) for eNOS genotyping. The clinical information of the patients was staged at the time of diagnosis following the tumor/node/metastasis staging system of the AJCC.
Study Variables
The main endpoint of this study was the prevalence of EGFR mutation among Taiwanese patients with lung adenocarcinoma. Independent variable of this study was eNOS polymorphisms (-786 T/C, 894 G/T, and combination genotypes -786 T/C / 894 G/T). The relative variables obtained from each participant's medical record included demographics (age, gender, cigarette smoking status) and clinicopathogical characteristics of disease (AJCC classification and differentiation).
DNA Extraction and EGFR gene sequencing
The DNA was extracted from tumor-frozen specimen and paraffin-embedded tissues using QIAamp DNA Tissue kits (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Exons 18-21 of the EGFR gene were amplified using polymerase chain reaction (PCR) and then DNA sequencing reaction and subjected to electrophoresis using the ABI PRISM 3130XL System (Applied Biosystems, Foster City, CA, USA) as previously described [19].
Genomic DNA Extraction and eNOS Genotyping
The genomic DNA was extracted from the whole blood leukocyte samples using QIAamp DNA blood mini kits (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. The eNOS polymorphisms -786 T/C (C_15903863_10) and 894 G/T (C_3219460_20) have previously been found to modify its transcription and endogenous NO production and were determined by real-time polymerase chain reaction (PCR) genotyping using the ABI StepOne™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA).
Statistical Analysis
Categorical variables, including demographics, cigarette smoking status, tumor characteristics and eNOS genotypes polymorphisms were summarized as number and percentage stratified by EGFR mutation status; continuous variables were also expressed as mean and standard deviation. Genotype-Tissue Expression (GTEx) database were used to identify correlations between SNPs and the transformed fibroblasts cell gene expression levels. The distributions of demographics, clinical characteristics and genotype frequencies among lung adenocarcinoma patients, as well as clinicopathological characteristics in different genotypes, were analyzed with a χ2-test. The odds ratio with corresponding 95% confidence interval (CI) and p-values of the associations between the genotype frequencies and risk of EGFR mutation, as well as their relationships with the clinicopathologic characteristics, were estimated using multiple logistic regression models after controlling for other covariates. P values less than 0.05 were statistically considered as significant. All statistical analyses were conducted using SAS statistical software (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of Study Subjects
This study included 125 males and 152 females for analysis, with a mean age of 65 years. The baseline demographics and clinical characteristic of recruited participants were summarized in Table 1. There were 109 (39.4%) and 168 (60.6%) patients in the EGFR wild-type and mutation type groups, respectively. These two groups differed significantly in the aspects of gender, cigarette smoking status, and tumor differentiation (p-value ≤0.001). The EGFR mutation group, as compared with subjects in the EGFR wild-type group, were predominantly female (64.3% vs. 40.4%, respectively), non-smoker (77.4% vs. 45.0%), and more well-differentiated (12.5% vs. 7.3%) and moderately-differentiated (81.5% vs. 72.5%) tumors.
Baseline demographics and clinical characteristics of 277 patients with lung adenocarcinoma by EGFR mutation status
| Variable | Wild type (N=109) | EGFR mutation (N=168) | p-value |
|---|---|---|---|
| Age, n (%) | |||
| <30 | 1 (0.9%) | 1 (0.6%) | p=0.656 |
| 30-39 | 3 (2.8%) | 2 (1.2%) | |
| 40-49 | 10 (9.2%) | 16 (9.5%) | |
| 50-59 | 21 (19.2%) | 44 (26.2%) | |
| 60-69 | 26 (23.9%) | 31 (18.5%) | |
| ≥70 | 48 (44.0%) | 74 (44.0%) | |
| Mean ± SD | 65.45 ± 13.34 | 65.69 ± 13.58 | p=0.885 |
| Gender, n (%) | |||
| Male | 65 (59.6%) | 60 (35.7%) | p<0.001 |
| Female | 44 (40.4%) | 108 (64.3%) | |
| Cigarette smoking, n (%) | |||
| Non-smoker | 49 (45.0%) | 130 (77.4%) | p<0.001 |
| Smoker | 60 (55.0%) | 38 (22.6%) | |
| PPK ± SD | 46.34 ± 28.41 | 19.94 ± 23.83 | p<0.001 |
| Tumor AJCC staging, n (%) | |||
| IA | 10 (9.2%) | 17 (10.1%) | p=0.557 |
| IB | 9 (8.3%) | 23 (13.7%) | |
| IIA | 5 (4.6%) | 7 (4.2%) | |
| IIB | 1 (0.9%) | 0 (0%) | |
| IIIA | 10 (9.2%) | 11 (6.5%) | |
| IIIB | 17 (15.6%) | 19 (11.3%) | |
| IV | 57 (52.2%) | 91 (54.2%) | |
| Tumor differentiation, n (%) | |||
| Good | 8 (7.3%) | 21 (12.5%) | p=0.001 |
| Moderate | 79 (72.5%) | 137 (81.5%) | |
| Poor | 22 (20.2%) | 10 (6.0%) |
Distribution of eNOS Genotypes of Study Subjects and Its Association with EGFR Mutation
The distribution frequency of two eNOS polymorphisms (-786 T/C, rs2070744 and 894 G/T, rs1799983) and haplotypes of patients with lung adenocarcinoma was shown in Table 2. The alleles with the highest distribution frequency for -786 T/C and 894 G/T among recruited patients were homozygous T/T and homozygous G/G for both EGFR wild-type and mutation type groups, respectively.
The associations between each genotype of eNOS polymorphisms and the risk of EGFR mutation of lung adenocarcinoma were summarized in Table 2. For -786 T/C polymorphisms, there was no statistically significant association demonstrated between variant types (TC, CC, and TC+CC) and EGFR mutation type. For 894 G/T polymorphisms, GT + TT genotypes were significantly highly associated with EGFR mutation type (Adjusted odds ratio (AOR) = 2.617, 95% confidential interval (CI) = 1.067-6.416). For combination genotypes of -786 T/C and 894 G/T, no statistical significant relationship was seen between different haplotypes (T/G, C/T, and others) and EGFR mutation type.
Association between eNOS Polymorphisms and EGFR Hotspot Mutations among Lung Adenocarcinoma Patients
Table 3 demonstrated the association between the polymorphisms of eNOS and the EGFR hotspot mutation. Two hotspot mutations were analyzed in our study, namely L858R and exon 19 in-frame deletions. Again, no significant association was detected between -786 T/C polymorphisms and either hotspot mutations of EGFR. On the other hand, significant associations were observed between exon 19 in-frame deletion and GT genotype (OR = 2.619, 95% CI = 1.242-5.524) and GT + TT genotypes (OR = 2.857, 95% CI = 1.368-5.967) of 894 G/T polymorphisms. No statistically significant relationship was noted between 894 G/T genotypes and L858R mutation of EGFR.
Subgroup Analysis of EGFR L858R Mutation Based on Polymorphic Genotypes of eNOS 894 G/T
The AJCC Tumor, Node, Metastasis (TNM) staging system [20] is an internationally accepted system to describe the extent of tumor. It combines features of the tumor into disease stage groups that correlate with survival and are linked to recommendations for treatment, as well as an indicator of prognosis. To further investigate any relationship between clinicopathological characteristics of EGFR L858R mutation and eNOS 894 G/T polymorphism, we performed a subgroup analysis of L858R mutation, as shown in Table 4. In general, as compared with wild type of GG, GT + TT genotypes were related with a more aggressive disease in regards to AJCC staging, “TNM” classification, and tumor differentiation. Unfortunately, they didn't reach statistically significant difference except the lymph node status (“N” classification, p=0.008). This finding indicated that GT + TT genotypes of eNOS 894 G/T were highly associated with lymph node invasion of lung adenocarcinoma.
Distribution frequency of eNOS genotypes of patients with lung adenocarcinoma and multiple logistic regression analysis of its association with EGFR mutation status
| Genotypes SNP | Wild type (N=109) n (%) | Mutation type (N=168) n (%) | OR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|
| eNOS -786T/C (rs2070744) | ||||
| TT | 84 (77.1%) | 135 (80.4%) | 1.00 | 1.00 |
| TC | 21 (19.3%) | 31 (18.5%) | 0.919 (0.495-1.703) | 1.327 (0.592-2.973) |
| CC | 4 (3.6%) | 2 (1.1%) | 0.311 (0.056-1.736) | 0.684 (0.067-6.928) |
| TC+CC | 25 (22.9%) | 33 (19.6%) | 0.821 (0.457-1.477) | 1.243 (0.577-2.680) |
| eNOS 894 G/T (rs1799983) | ||||
| GG | 95 (87.2%) | 130 (77.4%) | 1.00 | 1.00 |
| GT | 14 (12.8%) | 36 (21.4%) | 1.879 (0.960-3.678) | 2.391 (0.967-5.914) |
| TT | 0 (0%) | 2 (1.2%) | --- | --- |
| GT+TT | 14 (12.8%) | 38 (22.6%) | 1.984 (1.018-3.866) | 2.617 (1.067-6.416) |
| eNOS -786T/C /894 G/T | ||||
| TT/GG | 71 (65.1%) | 101 (60.1%) | 1.00 | 1.00 |
| Others | 37 (33.9%) | 63 (37.5%) | 1.197 (0.721-1.987) | 1.176 (0.685-2.020) |
| CC/TT | 1 (0.9%) | 4 (2.4%) | 2.812 (0.308-25.690) | 1.745 (0.187-16.301) |
| Others + CC/TT | 38 (34.9%) | 67 (39.9%) | 1.239 (0.751-2.045) | 1.195 (0.701-2.039) |
The AORs with 95% CIs were estimated by multiple logistic regression models after controlling for age and smoking.
Note: Bold text indicated a significant association with p-value <0.05.
Abbreviations: SNP, single nucleotide polymorphism; AOR, adjusted odds ratio; CI, confidence interval.
The associations between the polymorphisms of eNOS and the EGFR hotspot mutations in patients with lung adenocarcinoma
| Genotypes | Wild type | L858R | Exon 19 in-frame deletion | ||
|---|---|---|---|---|---|
| (N=109) n (%) | (N=78) n (%) | OR (95% CI) | (N=81) n (%) | OR (95% CI) | |
| eNOS -786T/C (rs2070744) | |||||
| TT | 84 (77.1%) | 58 (74.4%) | 1.00 | 68 (84.0%) | 1.00 |
| TC | 21 (19.3%) | 18 (23.1%) | 1.241 (0.609-2.532) | 13 (16.0%) | 0.765 (0.357-1.638) |
| CC | 4 (3.6%) | 2 (2.5%) | 0.724 (0.128-4.085) | 0 (0%) | --- |
| TC+CC | 25 (22.9%) | 20 (25.6%) | 1.159 (0.589-2.279) | 13 (16.0%) | 0.642 (0.306-1.350) |
| eNOS 894 G/T (rs1799983) | |||||
| GG | 95 (87.2%) | 66 (84.6%) | 1.00 | 57 (70.4%) | 1.00 |
| GT | 14 (12.8%) | 12 (15.4%) | 1.234 (0.537-2.837) | 22 (27.2%) | 2.619 (1.242-5.524) |
| TT | 0 (0%) | 0 (0%) | --- | 2 (2.4%) | --- |
| GT+TT | 14 (12.8%) | 12 (15.4%) | 1.234 (0.537-2.837) | 24 (29.6%) | 2.857 (1.368-5.967) |
Note: There were 9 patients not included in the analysis, due to unknown or inconclusive real-time mutation results.
Note: Bold text indicated a significant association with p-value <0.05.
Abbreviations: OR, odds ratio; CI, confidence interval.
Clinicopathologic characteristics of lung adenocarcinoma patients with EGFR L858R mutation, stratified by polymorphic genotypes of eNOS 894 G/T (rs1799983)
| Variables | GG (N=66) | GT + TT (N=12) | p-value |
|---|---|---|---|
| Tumor AJCC stages, n (%) | |||
| I+II | 22 (33.3%) | 1 (8.3%) | p=0.081 |
| III+IV | 44 (66.7%) | 11 (91.7%) | |
| Tumor “T” classification, n (%) | |||
| T1+T2 | 50 (75.8%) | 7 (58.3%) | p=0.211 |
| T3+T4 | 16 (24.2%) | 5 (41.7%) | |
| Lymph node status, n (%) | |||
| Negative | 26 (39.4%) | 0 (0%) | p=0.008* |
| Positive | 40 (60.6%) | 12 (100%) | |
| Distant Metastasis, n (%) | |||
| Negative | 37 (56.1%) | 6 (50.0%) | p=0.698 |
| Positive | 29 (43.9%) | 6 (50.0%) | |
| Tumor differentiation, n (%) | |||
| Good | 9 (13.6%) | 2 (16.7%) | p=0.912 |
| Moderate | 53 (80.3%) | 9 (75.0%) | |
| Poor | 4 (6.1%) | 1 (8.3%) |
eNOS displays a significant expression quantitative trait locus (eQTL) association with rs1799983 genotypes in the transformed fibroblasts cell of the Genotype-Tissue Expression (GTEx) database.
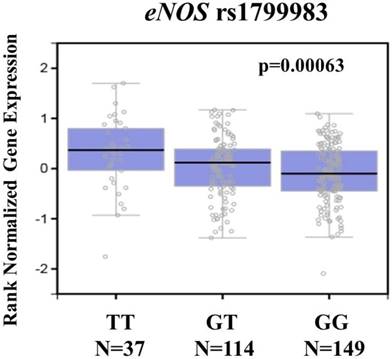
To further support our findings, we assessed eNOS expression in the transformed fibroblasts cell using the Genotype-Tissue Expression database (GTEx) database to conduct the putative functional relevance of eNOS SNP rs1799983. As shown in figure 1, the GTEx database revealed a statistically significant upregulation of eNOS mRNA expression in the transformed fibroblasts cell of rs1799983-variant genotypes (GT or TT) compared with that of the WT homozygous GG genotype (p=0.00063) (Figure 1). The data suggested that changes in eNOS expression due to genetic polymorphisms may affect the development of cervical cancer.
Discussion
The purpose of this study was to examine the associations between eNOS gene polymorphism and EGFR mutation of lung adenocarcinoma, which refers to a predictor of responsiveness to targeted therapy. Among the 277 patients, variant types (GT + TT) of eNOS 894 G/T polymorphism (rs1799983) were significantly positively correlated with EGFR mutation type, specifically exon 19 in-frame deletion. With the subgroup of EGFR L858R mutation, variant genotypes (GT + TT) of eNOS 894 G/T were significantly associated with lymph node invasion.
EGFR mutation of NSCLCs was predominantly observed among Asian population, as well as females and non-smokers [3]. Hsu CH et al. [21] also performed a study in 2016, utilizing National Taiwan Lung Cancer Registry, and noticed EGFR mutation prevalence higher than 50%. Our study revealed a consistently high prevalence of EGFR mutation. Within this subgroup, females and non-smokers were predominant, as well as better differentiation of lung tumors.
In recent two decades, polymorphisms of eNOS gene have gained attention for susceptibility of tumorigenesis or carcinogenesis, as well as cancer invasion and metastasis. Several studies focused on the associations of its three major polymorphisms, namely G894T, T-786C and VNTR 4a/b, and different cancers, such as bladder [13-15, 22], colorectum [14, 15, 23], breast [11, 13, 14, 24-26], prostate [14, 17, 27-33], stomach [14, 34], lung [35-41], liver [10, 14, 42], gallbladder [43], pancreas [44], and others [14, 45-47]. There were also different researches investigating correlation of eNOS polymorphisms and responsiveness of different therapies toward malignancies, such as chemotherapy, targeted therapy and radiotherapy [5, 10, 38, 48-53]. There were three significant meta-analyses performed in 2014-2015 regarding the associations between three eNOS polymorphisms and cancer risks. Wu X et al. [15] concluded: 1) eNOS -786 variants were associated with increased cancer risk; 2) eNOS G894T was associated with risk for females and for breast cancer; and 3) eNOS intron 4a/b polymorphisms increased susceptibility was revealed for prostate cancer. Conversely, Haque S et al. [14] collected 29 research articles and revealed that eNOS 4a/b and G894T polymorphisms were not associated with cancer risk. Still, no consistent conclusion was obtained from these meta-analyses.
In the field of lung cancer, unlike other malignancies, there were very few studies examining the role of eNOS gene polymorphism in carcinogenesis and invasiveness. And yet, the answer was conflicting so far. One study showed that VNTR polymorphism was the culprit [35], while another believed high expression of NOS was a favorable prognostic sign [40]. Moreover, Donnini et al. also reports that NO and PGE2 exist cross-talk effects in EGFR-driven epithelial tumor cells and may as the better therapeutic strategies for cancer treatment [54]. In our study, we performed analysis of lung adenocarcinoma patients based on their EGFR mutation types and eNOS gene polymorphisms (-786 T/C and 894 G/T). There was significantly positive correlation between 894 G/T variant types (GT + TT) and EGFR mutation type, but no significant relationship was found between EGFR mutation type and -786 T/C (T/G, C/T, and others). To further exploring this association, we noticed that 894 G/T genotypes (GT and GT + TT) was highly correlated with EGFR mutation type of exon 19 in-frame deletion, instead of L858R. To determine if there was any correlation between EGFR L858R mutation and eNOS 894 G/T genotypes, we performed a subgroup analysis of L858R mutation based on clinicopathological characteristics of lung cancer. We noticed that GT + TT genotypes were associated with more invasive disease as compared with the wild type of GG, represented by higher AJCC staging (stage III + IV) percentage, higher AJCC “T” classification (T3 + T4) percentage, positive lymph node invasion, more distant metastasis, and poorer tumor differentiation. But these findings did not reach statistically significant difference, except lymph node invasion. This is mostly due to limited study sample. The limitation of our study is lacking of healthy control group. The possible association of eNOS gene polymorphisms with the risk of developing lung cancer is worth for further investigation, which will be included in our future work. To our best knowledge, our study is the first one investigating association between eNOS polymorphisms and EGFR mutation of lung adenocarcinoma. Further exploration of this correlation with a large-scale study population was mandatory for better understanding of this gene prediction.
To apply the knowledge of our findings in the clinical practice, we may utilize genetic analysis of eNOS polymorphism to predict future cancer risk, especially among NSCLC. Also, with the significant association between eNOS 894 G/T variant types and EGFR mutation of lung cancer, which was considered as a predictor of therapy responsiveness, we may provide with EGFR-TKI therapy in the early phase of disease once diagnosed and predict a better response.
In conclusion, our study showed that eNOS 894 G/T variant were significantly associated with EGFR mutation types of lung adenocarcinoma, specifically exon 19 in-frame deletion. This may be utilized as a prediction of tumor invasiveness and therapy responsiveness.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30
2. Travis WD, Brambilla E, Nicholson AG. et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243-1260
3. Kawaguchi T, Koh Y, Ando M. et al. Prospective Analysis of Oncogenic Driver Mutations and Environmental Factors: Japan Molecular Epidemiology for Lung Cancer Study. J Clin Oncol. 2016;34:2247-2257
4. Shi Y, Au JS, Thongprasert S. et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154-162
5. Fujita S, Masago K, Hatachi Y. et al. Genetic polymorphisms in the endothelial nitric oxide synthase gene correlate with overall survival in advanced non-small-cell lung cancer patients treated with platinum-based doublet chemotherapy. BMC Med Genet. 2010;11:167
6. Su SC, Hsieh MJ, Yang WE, Chung WH, Reiter RJ, Yang SF. Cancer metastasis: Mechanisms of inhibition by melatonin. J Pineal Res. 2017:62
7. Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521-534
8. Bao XC, Mao AR, Fang YQ. et al. Simvastatin decreases hyperbaric oxygen-induced acute lung injury by upregulating eNOS. Am J Physiol Lung Cell Mol Physiol. 2018;314:L287-L297
9. Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007;67:1407-1410
10. Casadei Gardini A, Marisi G. et al. eNOS polymorphisms and clinical outcome in advanced HCC patients receiving sorafenib: final results of the ePHAS study. Oncotarget. 2016;7:27988-27999
11. Chen CH, Wu SH, Tseng YM, Hou MF, Tsai LY, Tsai SM. Distinct role of endothelial nitric oxide synthase gene polymorphisms from menopausal status in the patients with sporadic breast cancer in Taiwan. Nitric Oxide. 2018;72:1-6
12. Ben Nasr H, Bchir S, Ben Anes A. et al. The -786 T/C polymorphism of NOS3 gene is a susceptibility marker of COPD among Tunisians that correlates with nitric oxide levels and airflow obstruction. Cytokine. 2017;93:66-73
13. Gao X, Wang J, Wang W, Wang M, Zhang J. eNOS Genetic Polymorphisms and Cancer Risk: A Meta-Analysis and a Case-Control Study of Breast Cancer. Medicine (Baltimore). 2015;94:e972
14. Haque S, Mandal RK, Akhter N. et al. G894T and 4a/b polymorphisms of NOS3 gene are not associated with cancer risk: a meta-analysis. Asian Pac J Cancer Prev. 2015;16:2929-2937
15. Wu X, Wang ZF, Xu Y, Ren R, Heng BL, Su ZX. Association between three eNOS polymorphisms and cancer risk: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:5317-5324
16. Zhang L, Chen LM, Wang MN. et al. The G894t, T-786c and 4b/a polymorphisms in Enos gene and cancer risk: a meta-analysis. J Evid Based Med. 2014;7:263-269
17. Zhao C, Yan W, Zu X. et al. Association between endothelial nitric oxide synthase 894G>T polymorphism and prostate cancer risk: a meta-analysis of literature studies. Tumour Biol. 2014;35:11727-11733
18. Di Salvatore M, Pietrantonio F, Orlandi A. et al. IL-8 and eNOS polymorphisms predict bevacizumab-based first line treatment outcomes in RAS mutant metastatic colorectal cancer patients. Oncotarget. 2017;8:16887-16898
19. Yang SY, Yang TY, Chen KC. et al. EGFR L858R mutation and polymorphisms of genes related to estrogen biosynthesis and metabolism in never-smoking female lung adenocarcinoma patients. Clin Cancer Res. 2011;17:2149-2158
20. Goldstraw P, Chansky K, Crowley J. et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39-51
21. Hsu CH, Tseng CH, Chiang CJ. et al. Characteristics of young lung cancer: Analysis of Taiwan's nationwide lung cancer registry focusing on epidermal growth factor receptor mutation and smoking status. Oncotarget. 2016;7:46628-46635
22. Polat F, Diler SB, Azazi I, Oden A. T-786C, G894T, and intron 4 VNTR (4a/b) polymorphisms of the endothelial nitric oxide synthase gene in bladder cancer cases. Asian Pac J Cancer Prev. 2015;16:2199-2202
23. Jang MJ, Jeon YJ, Kim JW. et al. Association of eNOS polymorphisms (-786T>C, 4a4b, 894G>T) with colorectal cancer susceptibility in the Korean population. Gene. 2013;512:275-281
24. Goel S, Gupta N, Walcott BP. et al. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J Natl Cancer Inst. 2013;105:1188-1201
25. Ramirez-Patino R, Figuera LE, Puebla-Perez AM. et al. Intron 4 VNTR (4a/b) polymorphism of the endothelial nitric oxide synthase gene is associated with breast cancer in Mexican women. J Korean Med Sci. 2013;28:1587-1594
26. Gauthier N, Lohm S, Touzery C. et al. Tumour-derived and host-derived nitric oxide differentially regulate breast carcinoma metastasis to the lungs. Carcinogenesis. 2004;25:1559-1565
27. Aiello A, Bacci L, Re A. et al. MALAT1 and HOTAIR Long Non-Coding RNAs Play Opposite Role in Estrogen-Mediated Transcriptional Regulation in Prostate Cancer Cells. Sci Rep. 2016;6:38414
28. Ceylan GG, Ceylan C, Gulmemmedov B. et al. Polymorphisms of eNOS, catalase, and myeloperoxidase genes in prostate cancer in Turkish men: preliminary results. Genet Mol Res. 2016:15
29. Diler SB, Oden A. The T -786C, G894T, and Intron 4 VNTR (4a/b) Polymorphisms of the Endothelial Nitric Oxide Synthase Gene in Prostate Cancer Cases. Genetika. 2016;52:249-254
30. Polat F, Turaclar N, Yilmaz M, Bingol G, Cingilli Vural H. eNOS gene polymorphisms in paraffin-embedded tissues of prostate cancer patients. Turk J Med Sci. 2016;46:673-679
31. Fu Q, Liu X, Liu Y, Yang J, Lv G, Dong S. MicroRNA-335 and -543 suppress bone metastasis in prostate cancer via targeting endothelial nitric oxide synthase. Int J Mol Med. 2015;36:1417-1425
32. Safarinejad MR, Safarinejad S, Shafiei N. Effects of the T-786C, G894T, and Intron 4 VNTR (4a/b) polymorphisms of the endothelial nitric oxide synthase gene on the risk of prostate cancer. Urol Oncol. 2013;31:1132-1140
33. Ziaei SA, Samzadeh M, Jamaldini SH, Afshari M, Haghdoost AA, Hasanzad M. Endothelial nitric oxide synthase Glu298Asp polymorphism as a risk factor for prostate cancer. Int J Biol Markers. 2013;28:43-48
34. Krishnaveni D, Amar Chand B, Shravan Kumar P. et al. Association of endothelial nitric oxide synthase gene T-786C promoter polymorphism with gastric cancer. World J Gastrointest Oncol. 2015;7:87-94
35. Peddireddy V, Badabagni SP, Gundimeda SD, Mundluru HP. Association of eNOS and ACE gene polymorphisms and plasma nitric oxide with risk of non-small cell lung cancer in South India. Clin Respir J. 2018;12:207-217
36. Shiozawa T, Iyama S, Toshima S. et al. Dimethylarginine dimethylaminohydrolase 2 promotes tumor angiogenesis in lung adenocarcinoma. Virchows Arch. 2016;468:179-190
37. Ye S, Yang W, Wang Y. et al. Cationic liposome-mediated nitric oxide synthase gene therapy enhances the antitumor effects of cisplatin in lung cancer. Int J Mol Med. 2013;31:33-42
38. Nagai H, Yasuda H, Hatachi Y. et al. Nitric oxide (NO) enhances pemetrexed cytotoxicity via NOcGMP signaling in lung adenocarcinoma cells in vitro and in vivo. Int J Oncol. 2012;41:24-30
39. Masri FA, Comhair SA, Koeck T. et al. Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med. 2005;172:597-605
40. Puhakka A, Kinnula V, Napankangas U. et al. High expression of nitric oxide synthases is a favorable prognostic sign in non-small cell lung carcinoma. Apmis. 2003;111:1137-1146
41. Fujimoto H, Sasaki J, Matsumoto M. et al. Significant correlation of nitric oxide synthase activity and p53 gene mutation in stage I lung adenocarcinoma. Jpn J Cancer Res. 1998;89:696-702
42. Huang CY, Batzorig U, Cheng WL. et al. Glucose-regulated protein 94 mediates cancer progression via AKT and eNOS in hepatocellular carcinoma. Tumour Biol. 2016;37:4295-4304
43. Suksawat M, Techasen A, Namwat N. et al. Upregulation of endothelial nitric oxide synthase (eNOS) and its upstream regulators in Opisthorchis viverrini associated cholangiocarcinoma and its clinical significance. Parasitology International. 2017;66:486-493
44. Wang J, Yang S, He P. et al. Endothelial Nitric Oxide Synthase Traffic Inducer (NOSTRIN) is a Negative Regulator of Disease Aggressiveness in Pancreatic Cancer. Clin Cancer Res. 2016;22:5992-6001
45. Yanar K, Cakatay U, Aydin S. et al. Relation between Endothelial Nitric Oxide Synthase Genotypes and Oxidative Stress Markers in Larynx Cancer. Oxid Med Cell Longev. 2016;2016:4985063
46. Shang ZJ, Li ZB, Li JR. In vitro effects of nitric oxide synthase inhibitor L-NAME on oral squamous cell carcinoma: a preliminary study. Int J Oral Maxillofac Surg. 2006;35:539-543
47. Broholm H, Rubin I, Kruse A. et al. Nitric oxide synthase expression and enzymatic activity in human brain tumors. Clin Neuropathol. 2003;22:273-281
48. Di Salvatore M, Lo Giudice L, Rossi E. et al. Association of IL-8 and eNOS polymorphisms with clinical outcomes in bevacizumab-treated breast cancer patients: an exploratory analysis. Clin Transl Oncol. 2016;18:40-46
49. Zhang J, Li BS, Zhou CC. et al. Single nucleotide polymorphisms in NOS2A and NOS3 genes are not associated with treatment response of non-small cell lung cancer patients following the definitive radiochemotherapy. Neoplasma. 2012;59:631-640
50. Zeybek ND, Inan S, Ekerbicer N, Vatansever HS, Karakaya J, Muftuoglu SF. The effects of Gemcitabine and Vinorelbine on inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) distribution of MCF-7 breast cancer cells. Acta Histochemica. 2011;113:62-67
51. Hildebrandt MA, Komaki R, Liao Z. et al. Genetic variants in inflammation-related genes are associated with radiation-induced toxicity following treatment for non-small cell lung cancer. PLoS One. 2010;5:e12402
52. Lee HC, An S, Lee H. et al. Activation of epidermal growth factor receptor and its downstream signaling pathway by nitric oxide in response to ionizing radiation. Mol Cancer Res. 2008;6:996-1002
53. Sonveaux P, Brouet A, Havaux X. et al. Irradiation-induced angiogenesis through the up-regulation of the nitric oxide pathway: implications for tumor radiotherapy. Cancer Res. 2003;63:1012-1019
54. Donnini S, Bazzani L, Ziche M, Terzuoli E. Nitric Oxide and PGE-2 Cross-Talk in EGFR-Driven Epithelial Tumor Cells. Crit Rev Oncog. 2016;21:325-331
Author contact
![]() Corresponding authors: Thomas Chang-Yao Tsao MD, PhD. or Shun-Fa Yang, PhD. Institute of Medicine, Chung Shan Medical University, 110, Section 1, Chien-Kuo N. Road, Taichung, Taiwan, ROC. Fax: 886-4-24723229. E-mail: his885889com (Tsao TC); ysfedu.tw (Yang SF)
Corresponding authors: Thomas Chang-Yao Tsao MD, PhD. or Shun-Fa Yang, PhD. Institute of Medicine, Chung Shan Medical University, 110, Section 1, Chien-Kuo N. Road, Taichung, Taiwan, ROC. Fax: 886-4-24723229. E-mail: his885889com (Tsao TC); ysfedu.tw (Yang SF)

 Global reach, higher impact
Global reach, higher impact