3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(15):2713-2722. doi:10.7150/jca.26207 This issue Cite
Research Paper
Long noncoding RNA SNHG1 promotes cell proliferation through PI3K/AKT signaling pathway in pancreatic ductal adenocarcinoma
1. Department of General Surgery, Tongji hospital, Tongji University School of Medicine, Shanghai 200065, China.
2. Department of Pancreas and Hepatobiliary Surgery, Pancreas Cancer Institute, Fudan University Shanghai Cancer Center, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China.
3. Department of General Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200433, China.
Received 2018-3-21; Accepted 2018-6-2; Published 2018-6-23
Abstract
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most common causes of cancer-related death. Recently, long noncoding RNAs (lncRNAs) have emerged as significant regulators in numerous cancers, including PDAC. LncRNA small nucleolar RNA host gene 1 (SNHG1) has been reported in the development of several tumors, but the biological roles of it in PDAC remain to be illuminated. This study aims to investigate the function of lncRNA SNHG1, revealing its molecular mechanism and clinical significance in PDAC. Herein, we found that SNHG1 was highly expressed in PDAC tissues in comparison with adjacent noncancerous tissues, being closely related to tumor size and TNM stage. Functionally, silencing of SNHG1 could significantly inhibit cell proliferation, promote cell apoptosis, as well as alter cell cycle progression, whereas the contrary results could be presented in the overexpression of SNHG1. In addition, in vivo xenograft experiment also further confirmed the above results. Finally, an activator (740Y-P) and inhibitor (LY294002) of the PI3K/AKT signaling pathway were used in the western blot assays and the following rescue experiments, demonstrating that SNHG1 facilitates cell proliferation and tumorigenicity partly via the PI3K/AKT signaling pathway in PDAC. Hence, SNHG1 may be a prospective therapeutic target.
Keywords: long noncoding RNA SNHG1, cell proliferation, cell apoptosis, PI3K/AKT signaling pathway, pancreatic ductal adenocarcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal human malignancies with an extremely high mortality rate and a 5-year survival rate of less than 5% [1, 2]. On account of the early diagnosis of PDAC is difficult, the majority of patients are diagnosed at an advanced stage with distant metastasis and not eligible for surgical resection [3, 4]. Therefore, it is extremely essential to find out the molecular mechanisms underlying pancreatic cancer progression.
Long noncoding RNAs (lncRNAs) refer to a kind of RNA molecule that are over 200 nucleotides in length with no protein coding capability [5]. LncRNAs are involved in various biological processes of cancer and the dysregulation of lncRNA may be a major contributor to development of cancer [6-8]. For instance, lncRNA HOTTIP is overexpressed in esophageal cancer, promoting tumorigenesis and metastasis by regulating HOXA13 at both the transcriptional and posttranscriptional levels [9].
SNHG1 is a novel lncRNA localized at the chromosomal locus 11q12.3, and accumulating evidence has indicated that the dysregulation of SNHG1 is implicated in the progression of human cancers, such as glioma [10], osteosarcoma [11], colorectal cancer [12], hepatocellular carcinoma [13] and esophageal cancer [14]. However, to the best of our knowledge, this is the first study to report the biological role and clinical significance of SNHG1 in PDAC.
In the present study, we observed that lncRNA SNHG1 was aberrantly expressed in PDAC tissues and cell lines, and investigated the relationship between SNHG1 and clinicopathological features of PDAC. Furthermore, the influences of SNHG1 silencing and overexpression on proliferation and growth of PDAC cells were evaluated by cellular function assays, respectively. Relative function mechanism assays revealed that SNHG1 exerts its function in PDAC partly through PI3K/AKT signaling pathway. Our data shed new light on the candidate therapeutic target in PDAC.
Materials and methods
Clinical specimens
A total of 46 PDAC tissues and the paired adjacent noncancerous tissues were obtained from Fudan University Shanghai Cancer Center. All the collected tissues were instantly snap-frozen in liquid nitrogen after surgical resection and stored at -80°C until required. The PDAC diagnosis was confirmed by two pathologists. None of the patients received local or systemic therapy before surgery. The protocol was approved by the Ethics Committee of Fudan University Shanghai Cancer Center, and informed consent was obtained from all subjects.
Cell culture
The human pancreatic cancer cell lines (Panc-1, BxPC-3, SW1990) and immortalized human pancreatic ductal epithelial cells (HPDE6) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cell lines were cultured in DMEM Medium (Invitrogen) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Invitrogen), and maintained in a humidified incubator at 37°C with 5% CO2.
RNA transfection
The sh-SNHG1, si-SNHG1, plasmid pcDNA3.1-SNHG1 and relative negative control RNA were synthesized and purchased from Genepharm (Shanghai, China). Pancreatic cancer cells were transfected with Lipofectamine 2000 (Invitrogen, USA) following the manufacturer's instructions. The cells were incubated for 48h before used in assays. The siRNA sequences for SNHG1 were 5'-CAGCAGTTGAGGGTTTGCTGTGTAT-3'.
Total RNA isolation and qRT-PCR assays
Total RNA was extracted from tissues or cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. RNA quantity and quality were determined by NanoDrop2000c (Thermo Scientific, Waltham, MA, USA). Reverse transcription for mRNAs were performed using the PrimeScriptTM RT Master Mix (TaKaRa, Shiga, Japan). The cDNA templates were amplified by real-time RT-PCR using the SYBR Green PCR Kit (TaKaRa). The qRT-PCR reactions were run in triplicate with the ABI7500 System (Applied Biosystems, USA) and accompanying analytical software. GAPDH was used as internal control. The relative expression levels of lncRNA and mRNA were calculated by the 2-ΔΔCt method. The primer sequences were as follows: SNHG1, forward: 5′-AGGCTGAAGTTACAGGTC-3′ and reverse: 5′-TTGGCTCCCAGTGTCTTA-3′; GAPDH, forward: 5′-TGCCATCAATGACCCCTTC-3′ and 5′-CATCGCCCCACTTGATTTTG-3′.
Cell proliferation assays
Cell proliferation assays were performed with Cell Counting Kit-8 (Dojindo, Japan) according to the manufacturer's protocol. The proliferative ability of cells was detected every 24h. Absorbance at 450nm was determined spectrophotometrically using a microplate reader. Colony formation assays were also used to assess ability of cell proliferation. The transfected cells were placed in six-well plates with a density of 800 cells/well and incubated for 12 days. Subsequently, cells were fixed in 4% paraformaldehyde for 15min and stained with 0.1% crystal violet for 15min. The plates were photographed and visible colonies for each well were quantified using Image J software.
Flow cytometry
The transfected cells were harvested and fixed with 70% cold ethanol for 1h on ice. Then, appropriate amount of RNase was added and incubated at 37°C for 30min. For the apoptosis analysis, cells were stained with Annexin V-APC and 7-AAD in the dark and then were analyzed by the BD FACSVerse flow cytometer (BD Biosciences, USA). In terms of the cell cycle analysis, cells were stained with Propidium Iodide (PI), and then were analyzed by flow cytometer. The percentages of cells in the G0/G1, S, and G2/M phases were counted.
Western blotting
Total protein lysates (RIPA lysis buffer) of each group cells were separated with 12% SDS-PAGE, and transferred to a PVDF membrane (Millipore). The membranes were blocked with 5% fat-free milk for 4 h at room temperature, then incubated with specific primary antibodies overnight at 4°C. Followed by washed with PBST, the PVDF membrane was incubated with the appropriate secondary antibody for 2h. The proteins were visualized using a detection system of enhanced chemiluminescence (ECL) and exposed to X-ray film. GAPDH served as the internal reference.
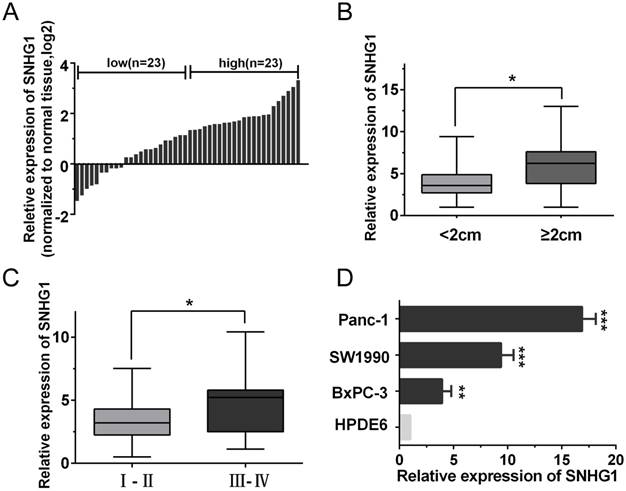
The expression of SNHG1 is upregulated in PDAC tissues and cell lines. (A) qRT-PCR analysis of SNHG1 expression levels in 46 PDAC tissues compared with adjacent normal tissues. The results were expressed as log2(2-ΔΔCt). (B, C) Relative expression of SNHG1 in PDAC patients was stratified by tumor size or clinical stage. (D) Expression of SNHG1 in PDAC cell lines (Panc-1, SW1990 and BxPC-3) and immortalized human pancreatic ductal epithelial cells (HPDE6). Mean±SD, *P<0.05, **P<0.01 and ***P<0.001.
In vivo xenograft experiment
Male BALB/c nude mice (4-5 weeks old) were obtained from Shanghai Experimental Animal Center of Chinese Academy of Sciences and maintained in pathogen-free conditions. Mice were randomly classified into two groups and each group consisted of four mice. The logarithmic growth phase of cells (1×107/ml) which were Panc-1 transfected with sh-SNHG1, BxPC-3 transfected with pc-SNHG1 or nonspecific vector inoculated subcutaneously in the left groin of the nude mice with 100μl of suspended cells. The tumors were measured once a week by vernier calipers and its volume was calculated as length×width2×1/2. The mice were sacrificed at day 42 and tumors were harvested and weighed. The protocol was approved by the Ethics Committee of Tongji University (Shanghai, China). This study was implemented in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Statistical analysis
Data are represented as the Mean±(SD) from at least three independent experiments. Statistical analysis and graph presentation was carried out using SPSS software (version 17.0) and GraphPad Prism software (version 5.0). The significance of differences between groups was assessed by Student's t-test or one-way ANOVA. Chi-square test was performed as statistical analysis of categorical data. P-values of statistical significance are represented as *P<0.05, **P<0.01 and ***P<0.001.
Results
Expression of SNHG1 is upregulated in PDAC tissues and cell lines
To explore the role of SNHG1 in PDAC progression, the qRT-PCR analysis was performed to evaluate the expression of SNHG1 in 46 pairs of human PDAC and adjacent noncancerous tissues. As shown in Fig. 1A, the expression of SNHG1 was significantly upregulated in cancerous tissues compared with normal counterparts (P<0.01). To further investigate the relationship between SNHG1 expression and clinical pathological features, the 46 PDAC patients were divided into high (above the median, n=23) and low (below the median, n=23) SNHG1 expression groups according to the median value (Fig. 1A). After statistical analysis, we found that high expression of SNHG1 was positively associated with larger tumor size (P=0.017) and advanced TNM stage (P=0.026) (Fig. 1B-C). However, there was no significantly associated with age, gender, differentiation and lymph node metastasis (Table 1). Moreover, compared to immortalized human pancreatic ductal epithelial cells (HPDE6), the expression levels of SNHG1 in the PDAC cell lines (Panc-1, BxPC-3 and SW1990) were also significantly upregulated (Fig. 1D).
Correlation between SNHG1 expression and clinicopathological characteristics of PDAC patients (N=46)
| Parameters | Total | SNHG1 expression | P-value | |
|---|---|---|---|---|
| Low (n=23) | High (n=23) | |||
| Age(years) | 0.139 | |||
| <60 | 25 | 15 | 10 | |
| ≥60 | 21 | 8 | 13 | |
| Gender | 0.555 | |||
| Male | 24 | 13 | 11 | |
| Female | 22 | 10 | 12 | |
| Tumor differentiation | 0.200 | |||
| Well | 14 | 9 | 5 | |
| Moderate-poor | 32 | 14 | 18 | |
| Tumor size (cm) | 0.017* | |||
| <2 | 20 | 14 | 6 | |
| ≥2 | 26 | 9 | 17 | |
| Lymph node metastasis | 0.116 | |||
| Positive | 31 | 18 | 13 | |
| Negative | 15 | 5 | 10 | |
| TNM stage | 0.026* | |||
| Ⅰ-Ⅱ | 37 | 22 | 15 | |
| Ⅲ-Ⅳ | 9 | 1 | 8 | |
*P<0.05
SNHG1 promotes PDAC cell proliferation, inhibits cell apoptosis and induces cell cycle progression
To investigate the biological function of SNHG1 in PDAC cells, we downregulated SNHG1 in Panc-1 cells by transfected with si-RNA and overexpressed SNHG1 in BxPC-3 cells by transfected with pcDNA, respectively. Then, the successful knockdown of SNHG1 in Panc-1 cells and the successful overexpression of SNHG1 in BxPC-3 cells were confirmed by qRT-PCR (Fig. 2A-B). CCK-8 assays and colony formation assays displayed that depletion of SNHG1 reduced cell viability and proliferative capacity compared with si-Control group in Panc-1 cells. Reciprocally, overexpression of SNHG1 increased cell viability and proliferative capacity compared with pc-Control group in BxPC-3 cells (Fig. 2C-D).
Furthermore, cell apoptosis and cell cycle distribution were assessed by using flow cytometry analysis. The results indicated that deficiency of SNHG1 induced apoptosis in Panc-1 cells and enrichment of SNHG1 reduced apoptosis in BxPC-3 cells (Fig. 2E). Similarly, downregulation of SNHG1 increased G0/G1 phase arrest and decreased the population of S phase, whereas upregulation of SNHG1 decreased G0/G1 phase arrest (Fig. 2F).
Silencing SNHG1 inhibits PDAC cells growth in vivo
In order to further determine whether SNHG1 expression could affect PDAC cells growth in vivo, Panc-1 cells stably transfected with sh-SNHG1, BxPC-3 cells transfected with pc-SNHG1 and respective negative controls were subcutaneously injected into four groups of immune-compromised mice and tumor growth was monitored. At 6 weeks post-injection, the tumor growth of the sh-SNHG1 group was obviously smaller than the sh-Control group (Fig. 3A). Correspondingly, tumor weights and volumes were dramatically decreased compared with controls (Fig. 3B-C). qPCR analysis confirmed that the average level of SNHG1 expression in sh-SNHG1 group was lower than that in control group (Fig. 3D). The opposite results were obtained in the SNHG1 overexpression group (Fig. 3E-H). Collectively, these data were in concordance with the results of the in vitro experiments, and revealed that the level of SNHG1 expression is significantly associated with proliferation capacity of PDAC cells, and may play a crucial role in PDAC progression.
SNHG1 regulates the activity of PI3K/AKT-mediated signaling pathway in PDAC
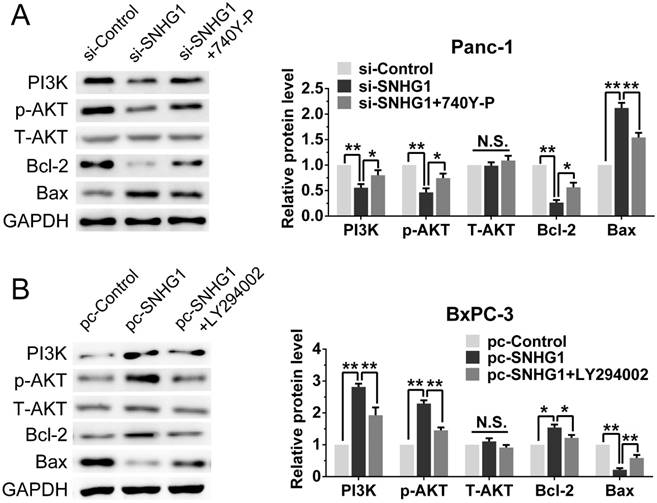
As the PI3K/AKT pathway was activated abnormally in various types of tumor and was closely related to tumor proliferation [15]. Hence, we posited that SNHG1 may modulate PDAC cell proliferation via PI3K/AKT signaling pathway. In order to examine our hypothesis, western blot assays were performed to analyze the alteration of relative protein of the PI3K/AKT pathway in transfected cells. The deficiency of SNHG1 remarkably decreased the expression of PI3K and phosphorylated AKT (p-AKT), whereas overexpression of SNHG1 increased the level of PI3K and p-AKT. However, the expression of total AKT (T-AKT) had no significant changes (Fig. 4A-B). Next, we detected the downstream genes of PI3K/AKT signaling pathway—anti-apoptosis related factor (Bcl-2) and apoptosis stimulating protein (Bax). Compared with control group, SNHG1 knockdown reduced the levels of Bcl-2, and enhanced expression of Bax in Panc-1, which was contrary to the results of SNHG1 overexpression in BxPC-3 cells (Fig. 4A-B).
Stimulation or suppression of the PI3K/AKT signaling pathway affects the oncogenic function of SNHG1
For the purpose of further corroborating the above conclusion, we performed a series of rescue experiments. First, we verified that Panc-1 cells transfected with si-SNHG1 were treated with 740Y-P (an activator of PI3K/AKT pathway) could restore the expression levels of PI3K, p-AKT and Bcl-2, while decrease the expression of Bax (Fig. 5A). As expected, BxPC-3 cells transfected with pcDNA-SNHG1 were treated with LY294002 (an inhibitor of PI3K/AKT pathway) could reverse the elevated levels of PI3K, p-AKT and Bcl-2, and the reduced expression of Bax (Fig. 5B). These results preliminarily confirmed that the regulatory function of SNHG1 at least partly through PI3K/AKT-mediated signaling pathway in PDAC.
SNHG1 promotes PDAC cell proliferation, inhibits cell apoptosis and induces cell cycle progression. (A) SNHG1 knockdown in Panc-1 cells transfected with related siRNA. (B) SNHG1 overexpression in BxPC-3 cells transfected with relative pcDNA. (C, D) CCK-8 assays and colony formation assays were performed to assess the proliferation capacity and viability of PDAC cells in the case of knockdown or overexpression of SNHG1. (E, F) Relative cell apoptosis progression and cell cycle distribution were evaluated by flow cytometry assays in Panc-1 cells transfected with si-SNHG1 and BxPC-3 cells transfected with pc-SNHG1. Mean±SD, *P<0.05, **P<0.01 and ***P<0.001.
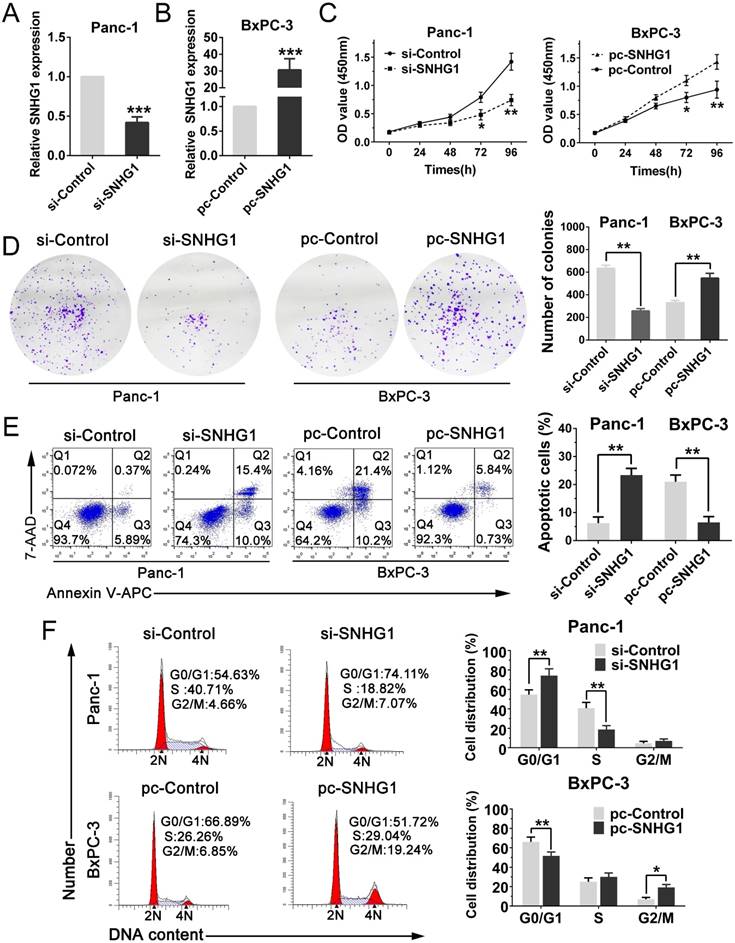
Silencing SNHG1 inhibits PDAC cells growth in vivo. (A, E) Tumors were photographed after harvested from the nude mice which were inoculated Panc-1 cells transfected with sh-SNHG1 or BxPC-3 cells transfected with pc-SNHG1. (B, F) Tumor volumes were calculated once a week until the sixth week. (C, G) Tumor weights of nude mice were measured in each group. (D, H) Real-time PCR was used to confirm the expression levels of SNHG1 in xenograft tumors. Mean±SD, *P<0.05, **P<0.01.
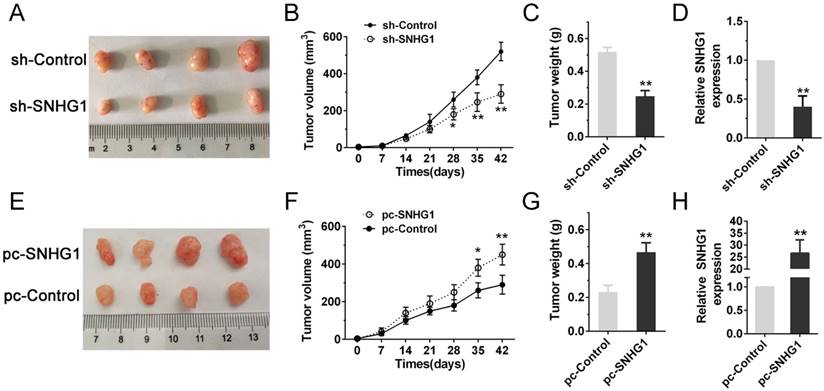
SNHG1 regulates the activity of PI3K/AKT signaling pathway in PDAC. (A, B) Western blot analysis detected the protein levels of PI3K, p-AKT, T-AKT and apoptosis-related protein Bcl-2/Bax in the context of depletion or enrichment of SNHG1. Mean±SD, *P<0.05, **P<0.01, ***P<0.001 and N.S. not significant.
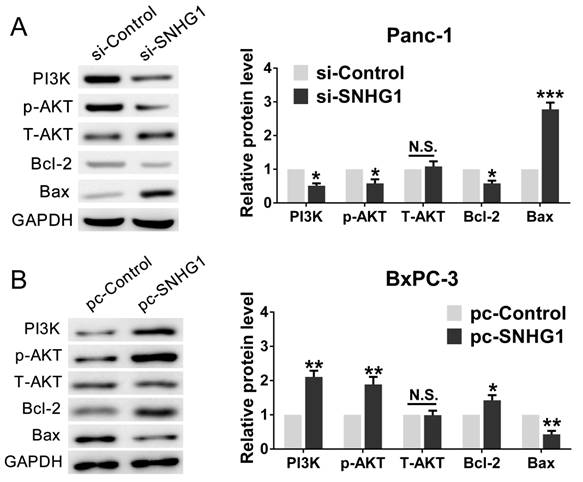
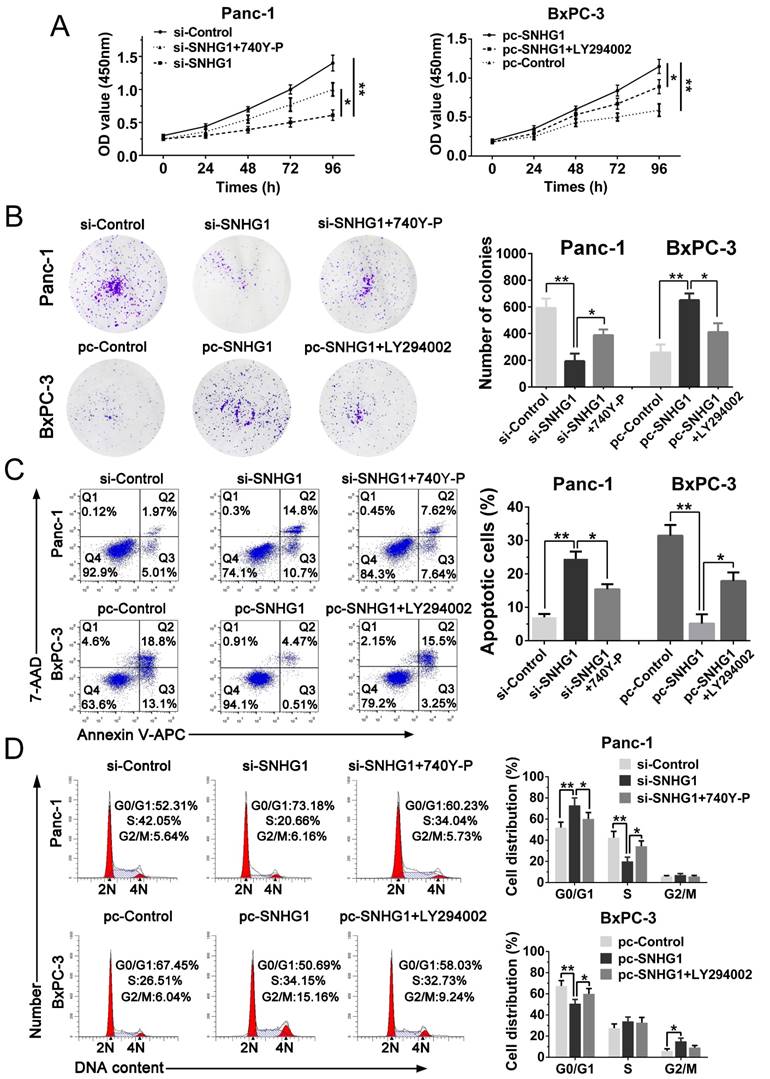
In addition, cell function assays were tested again. The CCK-8 and colony formation assays demonstrated that 740Y-P rescued cell proliferation ability compared with si-SNHG1 group, whereas LY294002 reversed the raised ability of proliferation (Fig. 6A-B). Analogously, flow cytometry analysis turned out that 740Y-P reduced G0/G1 stages arrest and inhibited cell apoptosis compared with si-SNHG1 group, while LY294002 partially abrogated the effects induced by SNHG1 upregulation (Fig. 6C-D). Altogether, SNHG1 exerts biological effect on PDAC cells partly by means of PI3K/AKT signaling pathway.
Stimulation or suppression of the PI3K/AKT signaling pathway affects the oncogenic function of SNHG1. (A, B) An agonist (740Y-P) and inhibitor (LY294002) of PI3K/AKT signaling pathway were used in the downregulation and upregulation group, respectively. The protein levels of PI3K, p-AKT, T-AKT, Bcl-2 and Bax were detected by western blot assays. Mean±SD, *P<0.05, **P<0.01 and N.S. not significant.
Discussion
Earlier studies indicated that the initiation and development of pancreatic cancer are associated with multifarious lncRNAs [16-18]. For example, the silencing of lncRNA NEAT1 observably inhibited cell proliferation via facilitating cell apoptosis in PDAC cells [19]. HOTAIR expression was correlated with more aggressive tumors and exhibited pro-oncogenic activity in PDAC [20]. Nevertheless, the function and the specific mechanism of lncRNAs in PDAC remained largely unknown.
Recently, it has been revealed that the oncogenic effects of SNHG1 involved in human cancers by activating different signaling pathways [21-23]. Cui et al. showed that upregulated SNHG1 facilitated the progression of non-small cell lung cancer by Wnt/β-catenin signaling pathway [24]. Li et al. found that SNHG1 as a ceRNA adjusted miR-199a-3p/CDK7 axis, and finally affected cell proliferation in prostate cancer [25]. Zhang et al. indicated that knockdown of SNHG1 inhibited the Notch signaling pathway, and subsequently suppressed cell proliferation and invasion in esophageal squamous cell cancer [26]. However, the relationship between SNHG1 and PDAC remained to be elucidated.
In this study, we used qRT-PCR to analyze SNHG1 expression pattern in our cohort containing 46 pair human PDAC tissues, and discovered that expression of SNHG1 in PDAC tissues was notably higher than corresponding adjacent tissues. Moreover, we also discovered that SNHG1 was upregulated in PDAC cell lines in comparison with HPDE6. Furthermore, high expression of SNHG1 in PDAC patients was related to advanced TNM stage and larger tumor size. To some extent, these results revealed that SNHG1 may play an important role in the development of PDAC, although the sample sizes need to be further expanded so as to augment persuasion.
Next, the functions of PDAC proliferation, apoptosis and cell cycle were tested by transfected with si-SNHG1 in Panc-1 and transfected with pcDNA-SNHG1 in BxPC-3. We revealed that downregulation of SNHG1 could notably impair cell proliferation, facilitate cell apoptosis and induce G0/G1 phases arrest, and the contrary results were presented in upregulated group. In vivo xenograft experiment also further confirmed that knockdown of SNHG1 repressed the tumor growth, which was in line with the results in vitro. These results were similar to the role of SNHG1 in gastric cancer and prostate cancer.
Stimulation or suppression of the PI3K/AKT signaling pathway affects the oncogenic function of SNHG1. (A, B) CCK-8 and colony formation assays were performed to evaluate the effect of 740Y-P on Panc-1 cells transfected with si-SNHG1 and LY294002 on BxPC-3 cells transfected with pcDNA-SNHG1 in terms of the cell proliferation ability. (C, D) Cell apoptosis and cell cycle distribution were detected by flow cytometry assays to assess the impact of 740Y-P and LY294002 on the role of SNHG1 knockdown and overexpression, respectively. Mean±SD, *P<0.05, **P<0.01.
The PI3K/AKT pathway is a cellular growth, proliferation, metabolism and survival signaling axis that is perhaps the most commonly activated signaling pathway in human cancer [15, 27]. Wang et al. showed that lncRNA AB073614 modulated proliferation and metastasis of colorectal cancer through the PI3K/AKT pathway [28]. Liu et al. indicated that the enhanced lncRNA CRNDE promoted non-small cell lung carcinoma growth also by activating PI3K/AKT signaling [29]. These reports inspired us to ascertain whether SNHG1 exerted a similar role in PDAC via the PI3K/AKT signaling pathway. Fortunately, we found that the expression of PI3K, p-AKT and antiapoptotic protein Bcl-2 was significantly attenuated, while apoptosis “effectors” Bax was enhanced in SNHG1 depletion cells. Correspondingly, the opposite results were observed in SNHG1 overexpression cells. In addition, we used activator (740Y-P) and inhibitor (LY294002) of PI3K/AKT signaling pathway to further validate that SNHG1 promoted proliferation by virtue of PI3K/p-AKT in PDAC. Regardless of western blot assays or the relevant cellular function experiments, 740Y-P and LY294002 partly reversed the previous effects of SNHG1 knockdown in Panc-1 cells and SNHG1 overexpression in BxPC-3 cells, respectively. Consequently, these findings suggested that SNHG1 promotes PDAC cell proliferation, inhibits cell apoptosis and induces cell cycle progression partially through activating the PI3K/AKT signaling pathway, and other molecular mechanisms may be involved and need us to further explore. Sun et al. found that SNHG1 was involved in the AKT signaling pathway in colorectal and lung tumor [30], which was in concordance with our findings in pancreatic cancer.
In conclusion, this study indicates that SNHG1 is upregulated in PDAC tissues and could promote PDAC cell proliferation and tumorigenesis by activating the PI3K/AKT signaling pathway. Therefore, SNHG1 acts as an oncogenic lncRNA and may serve as a potential therapeutic target in PDAC.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China [No. 81572388].
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30
2. Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford). 2008;10:58-62
3. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049-57
4. Moniri MR, Dai LJ, Warnock GL. The challenge of pancreatic cancer therapy and novel treatment strategy using engineered mesenchymal stem cells. Cancer Gene Therapy. 2014;21:12-23
5. Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nature Reviews Genetics. 2014;15:7-21
6. Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38
7. Hauptman N, Glavac D. Long non-coding RNA in cancer. Int J Mol Sci. 2013;14:4655-69
8. Li H, Yu B, Li J, Su L, Yan M, Zhu Z. et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318-29
9. Lin C, Wang Y, Wang Y, Zhang S, Yu L, Guo C. et al. Transcriptional and posttranscriptional regulation of HOXA13 by lncRNA HOTTIP facilitates tumorigenesis and metastasis in esophageal squamous carcinoma cells. Oncogene. 2017;36:5392-406
10. Wang Q, Li Q, Zhou P, Deng D, Xue L, Shao N. et al. Upregulation of the long non-coding RNA SNHG1 predicts poor prognosis, promotes cell proliferation and invasion, and reduces apoptosis in glioma. Biomed Pharmacother. 2017;91:906-11
11. Wang J, Cao L, Wu J, Wang Q. Long non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326 and promotes tumorigenesis in osteosarcoma. Int J Oncol. 2018;52:77-88
12. Sun X, Wang Z, Yuan W. Down-regulated long non-coding RNA SNHG1 inhibits tumor genesis of colorectal carcinoma. Cancer Biomark. 2017;20:67-73
13. Zhang M, Wang W, Li T, Yu X, Zhu Y, Ding F. et al. Long noncoding RNA SNHG1 predicts a poor prognosis and promotes hepatocellular carcinoma tumorigenesis. Biomed Pharmacother. 2016;80:73-9
14. Yan Y, Fan Q, Wang L, Zhou Y, Li J, Zhou K. LncRNA Snhg1, a non-degradable sponge for miR-338, promotes expression of proto-oncogene CST3 in primary esophageal cancer cells. Oncotarget. 2017;8:35750-60
15. Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627-44
16. Li Z, Zhao X, Zhou Y, Liu Y, Zhou Q, Ye H. et al. The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer. J Transl Med. 2015;13:84
17. Li J, Liu D, Hua R, Zhang J, Liu W, Huo Y. et al. Long non-coding RNAs expressed in pancreatic ductal adenocarcinoma and lncRNA BC008363 an independent prognostic factor in PDAC. Pancreatology. 2014;14:385-90
18. Gao S, Wang P, Hua Y, Xi H, Meng Z, Liu T. et al. ROR functions as a ceRNA to regulate Nanog expression by sponging miR-145 and predicts poor prognosis in pancreatic cancer. Oncotarget. 2016;7:1608-18
19. Huang B, Liu C, Wu Q, Zhang J, Min Q, Sheng T. et al. Long non-coding RNA NEAT1 facilitates pancreatic cancer progression through negative modulation of miR-506-3p. Biochem Biophys Res Commun. 2017;482:828-34
20. Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R. et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616-25
21. Sahu D, Hsu CL, Lin CC, Yang TW, Hsu WM, Ho SY. et al. Co-expression analysis identifies long noncoding RNA SNHG1 as a novel predictor for event-free survival in neuroblastoma. Oncotarget. 2016;7:58022-37
22. Zhang H, Zhou D, Ying M, Chen M, Chen P, Chen Z. et al. Expression of Long Non-Coding RNA (lncRNA) Small Nucleolar RNA Host Gene 1 (SNHG1) Exacerbates Hepatocellular Carcinoma Through Suppressing miR-195. Med Sci Monit. 2016;22:4820-9
23. Zhang HY, Yang W, Zheng FS, Wang YB, Lu JB. Long non-coding RNA SNHG1 regulates zinc finger E-box binding homeobox 1 expression by interacting with TAp63 and promotes cell metastasis and invasion in Lung squamous cell carcinoma. Biomed Pharmacother. 2017;90:650-8
24. Cui Y, Zhang F, Zhu C, Geng L, Tian T, Liu H. Upregulated lncRNA SNHG1 contributes to progression of non-small cell lung cancer through inhibition of miR-101-3p and activation of Wnt/beta-catenin signaling pathway. Oncotarget. 2017;8:17785-94
25. Li J, Zhang Z, Xiong L, Guo C, Jiang T, Zeng L. et al. SNHG1 lncRNA negatively regulates miR-199a-3p to enhance CDK7 expression and promote cell proliferation in prostate cancer. Biochem Biophys Res Commun. 2017;487:146-52
26. Zhang Y, Jin X, Wang Z, Zhang X, Liu S, Liu G. Downregulation of SNHG1 suppresses cell proliferation and invasion by regulating Notch signaling pathway in esophageal squamous cell cancer. Cancer Biomark. 2017;21:89-96
27. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655-7
28. Wang Y, Kuang H, Xue J, Liao L, Yin F, Zhou X. LncRNA AB073614 regulates proliferation and metastasis of colorectal cancer cells via the PI3K/AKT signaling pathway. Biomed Pharmacother. 2017;93:1230-7
29. Liu XX, Xiong HP, Huang JS, Qi K, Xu JJ. Highly expressed long non-coding RNA CRNDE promotes cell proliferation through PI3K/AKT signalling in non-small cell lung carcinoma. Clin Exp Pharmacol Physiol. 2017;44:895-902
30. Sun Y, Wei G, Luo H, Wu W, Skogerbo G, Luo J. et al. The long noncoding RNA SNHG1 promotes tumor growth through regulating transcription of both local and distal genes. Oncogene. 2017;36:6774-83
Author contact
![]() Corresponding author: Kaixing Ai, Department of General Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, No.507 Zhengmin Road, Shanghai 200433, China. E-mail: akxing8258edu.cn
Corresponding author: Kaixing Ai, Department of General Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, No.507 Zhengmin Road, Shanghai 200433, China. E-mail: akxing8258edu.cn

 Global reach, higher impact
Global reach, higher impact