3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(1):43-50. doi:10.7150/jca.26723 This issue Cite
Research Paper
Circulating miR-1290 and miR-320d as Novel Diagnostic Biomarkers of Human Colorectal Cancer
1. Central Laboratory, Nanjing First Hospital, Nanjing Medical University, Nanjing 210000, China.
2. Medical School of Southeast University, 210009, China.
*These authors contribute to the study equally.
Received 2018-4-17; Accepted 2018-10-2; Published 2019-1-1
Abstract
Background: The lack of screening methods with high diagnostic utility leads to colorectal cancer (CRC) patients usually diagnosed in advanced stages which results in high mortality. This study aimed to identify novel circulating miRNAs as biomarkers for the early detection of CRC.
Materials and Methods: Total 205 participants were enrolled in this study. First, two dysregulated candidate miRNAs were selected after integrated analysis of four GEO datasets. Then, the expression of these two miRNAs in plasma samples were tested through qRT-PCR. Training phase and validation phase were designed to verify the diagnostic value of these two miRNAs using receiver operating characteristic curve (ROC) analysis.
Results: After integrated analysis of GEO datasets, we discovered miR-1290 and miR-320d were dysregulated in colorectal adenoma and adenocarcinoma tissues, and circulating miR-1290 and miR-320d in CRC patients were tumor-derived. Thereafter, circulating miR-1290 and miR-320d were selected to further investigate their potential for early diagnosis of CRC. Plasma miR-1290 expression could differentiate adenoma and CRC patients from healthy controls with area under the curve (AUC) of 0.78 and 0.88. Similarly, plasma miR-320d expression could discriminate adenoma and CRC patients from healthy controls with AUC of 0.74 and 0.81.
Conclusions: Circulating miR-1290 and miR-320d are novel promising biomarkers for early diagnosis of CRC.
Keywords: circulation, microRNA, colorectal cancer, diagnostic biomarker
Introduction
Colorectal cancer, one of the most common cancers worldwide, caused over 1.2 million new cases and 608,700 mortality estimated annually[1]. In the United States, the five-year survival rate is 93.2% for CRC patients with stage I, but only 8.1% for patients with stage IV[2]. It is well known that the development of CRC follows the sequential progression from adenoma to carcinoma. Thus, there are opportunities to diagnose CRC at early stage.
Despite the benefit of fecal occult blood tests (FOBT), X-Ray barium enema, and colonoscopy examinations in early detection of CRC, the wide use for screening the general population was limited due to the low efficacy, high cost, or invasion[3, 4]. Besides, the currently available circulating tumor biomarkers are neither very sensitive nor specific such as carcinoembryonic antibody (CEA), carbohydrate antibody 19‐9 (CA19‐9) and carbohydrate antibody 72‐4 (CA72‐4)[5]. Therefore, novel non-invasive biomarkers with high sensitivity and specificity for early detection of CRC are urgently needed.
Recently, circulating miRNAs as diagnostic biomarkers of cancers attracted more and more attentions. MiRNAs, short single-stranded non-coding RNAs (about 22 nucleotides), can functionally carry out biological effects through direct binding to the 3' untranslated regions (UTR) of their target mRNAs by inducing mRNAs degradation and/or translational repression[6].Tumor-derived circulating miRNAs exist in peripheral blood steadily because they could not only be packaged into some kinds of particles, such as apoptotic bodies and exosomes, but also combine with protein complexes, such as argonaute (AGO) and high-density lipoproteins, which confer them the ability to resist to endogenous ribonuclease activity, extreme pH and temperature [7-9].
In this study, we conducted an integrated analysis of four GEO datasets and selected two candidate miRNAs (miR-1290 and miR-320d) which were significantly dysregulated in both CRC tissues and peripheral blood of CRC patients. In addition, we discovered that circulating miR-1290 and miR-320d could discriminate colorectal adenomas and cancers from healthy controls. Therefore, we identified novel circulating miRNAs as promising biomarkers for early diagnosis of CRC.
Materials and Methods
Study population
A total of 205 participants were enrolled in this study. CRC and adenoma patients as well as healthy controls were recruited from Nanjing First Hospital affiliated to Nanjing Medical University between May 2016 and October 2017. All CRC and adenoma patients were confirmed through histopathological analysis of surgical resected tissues and the tumor stage was determined according to the tumor-node-metastasis (TNM) system. Thereafter, tissues were stored in liquid nitrogen until RNA extraction. None of the patients in this study received chemotherapy or radiation therapy before peripheral blood collected. Healthy plasma samples were collected from volunteers participating in the physical examination. Written informed consent was obtained from all participants, and this study have been approved by the Research and Ethical Committee of Nanjing First Hospital.
Study design
As shown in Figure 1, in the discovery phase, we carried out a comprehensive analysis of miRNA expression profiles in GSE108153 and GSE81581. The source of miRNAs selected was explored in GSE55139. The expression of candidate miRNAs in colorectal adenomas and adenocarcinomas tissues were investigated in GSE41655. In the training phase, the expression and diagnostic value of candidate miRNAs were confirmed by qRT-PCR in 45 plasma samples (15 healthy controls and 15 colorectal adenoma patients and 15 CRC patients). In the validation phase, the differential expression of miR-1290 and miR-320d were verified in 20 CRC tissues, 20 adenoma tissues, and 20 normal intestinal mucosa tissues. The tumor-derived trait of circulating miR-1290 and miR-320d were validated using 15 paired pre- and post-operation plasma samples as well as the positive correlation of expression between matched tissue and plasma samples. Moreover, the diagnostic parameters of circulating miR-1290 and miR-320d were independently calculated in additional 160 subjects (80 CRC patients, 50 adenomas patients and 30 healthy controls). No significant difference in the factors of age, gender and drinking status exists among these groups. The clinical characteristics of these subjects were presented in Table 1.
Clinical characteristics of subjects enrolled in this study.
| Variables | Training phase | Validation phase | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plasma samples | Tissues samples | Plasma samples | ||||||||
| CRC | adenoma | HC | CRC | adenoma | HC | Pre/post | CRC | adenoma | HC | |
| Number | 15 | 15 | 15 | 20 | 20 | 20 | 15 | 80 | 50 | 30 |
| Age (mean±SD) | 62.4 ± 10.9 | 61.2 ± 12.1 | 61.4 ± 11.2 | 61.5 ± 10.6 | 60.2 ± 11.7 | 58.4 ± 10.8 | 62.6 ± 9.3 | 63.7 ± 9.2 | 62.2 ± 9.6 | 59.4 ± 10.3 |
| Gender | ||||||||||
| Male | 10 | 12 | 10 | 14 | 15 | 13 | 11 | 53 | 34 | 19 |
| Female | 5 | 3 | 5 | 6 | 5 | 7 | 4 | 27 | 16 | 11 |
| Drinking status | ||||||||||
| never | 4 | 2 | 3 | 6 | 4 | 6 | 3 | 29 | 19 | 12 |
| ever | 11 | 13 | 12 | 14 | 16 | 14 | 12 | 51 | 31 | 18 |
| Tumor stage | ||||||||||
| I | 5 | 5 | 4 | 24 | ||||||
| II | 6 | 8 | 6 | 31 | ||||||
| III | 2 | 4 | 3 | 16 | ||||||
| IV | 2 | 3 | 2 | 9 | ||||||
The flow diagram of this study designed.
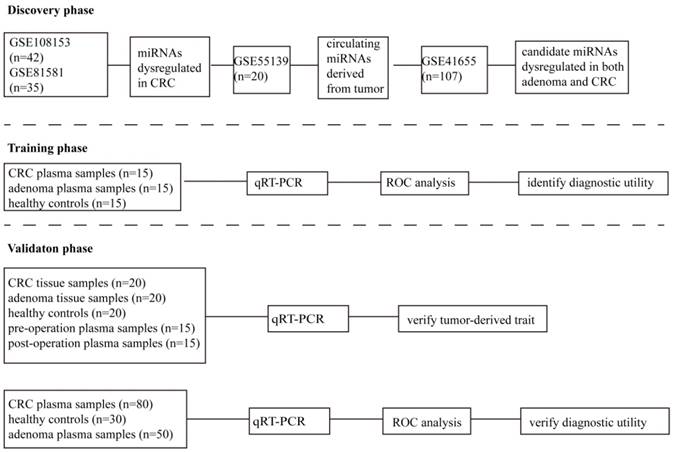
Samples processing
Plasma samples were collected from venous blood of participants stored in EDTA bottles after being centrifuged at 4000 rpm for 10min, and subsequently stored at -80 ℃ until further analysis. Total RNA was isolated using Trizol LS reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer's protocol. 1ul cel-miR-39 (GenePharma, Shanghai, China) at a concentration of 1 umol/L was added to each sample acting as the external reference.
qRT-PCR for miRNAs quantitation
Reverse transcription and qRT-PCR for miR-1290, miR-320d, endogenous control U6 snRNA, and external reference miR-39 were performed using Hairpin-itTM microRNA RT-PCR Quantitation Kit (GenePharma, Shanghai, China) according to the manufacturer's instructions. The reactions were initiated with denaturation at 95℃ for 3min, followed by 40 cycles of 95℃ for 15s and 62℃ for 34s. The relative expression levels of miR-1290 and miR-320d were calculated with 2 -△CtCt method. △Ct=CtmiRNA-CtmiR-39, △CtCt=△Ctpatient- Ctcontrol.
Statistical analysis
All data were presented as mean ± SD. Clinicopathological characteristics among groups were compared by chi-square test. The different expression of miRNAs among groups was determined using ANOVA or unpaired t test. ROC curve and AUC were established for discriminating colorectal adenoma patients and CRC patients from healthy individuals. A cutoff value of the miR-1290 and miR-320d expression were determined by Youden index from ROC curves. A p-value < 0.05 was considered statistically significant. The statistical analysis was performed using SPSS 19.0 (IBM, Chicago, IL, USA) and GraphPad 5.0 (GraphPad Software, USA).
Results
Integrated analysis of four GEO datasets identified two candidate circulating miRNAs
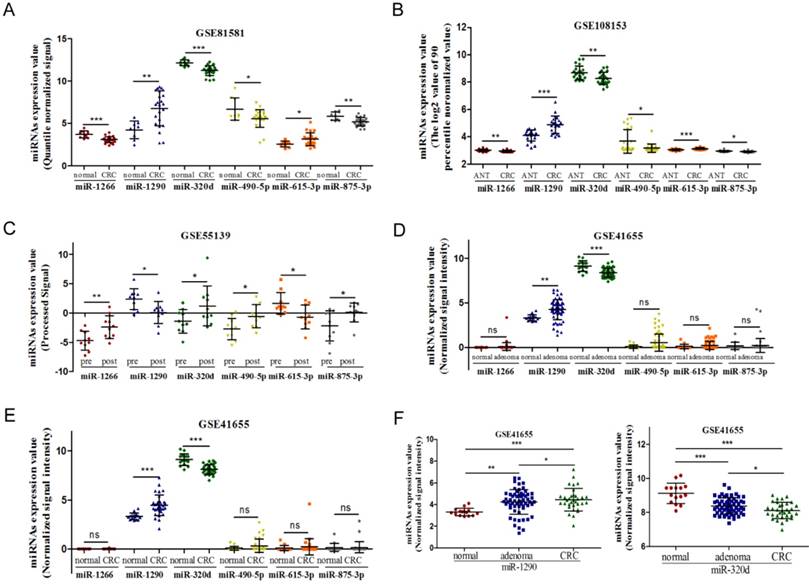
First, two GEO datasets (GSE108153 and GSE81581) which conducted a comprehensive comparative analysis of miRNA expression profiles between CRC tissue and normal intestinal mucosa were explored, and we found 16 consistently dysregulated miRNAs. Then, results of GSE55139 revealed that six miRNAs (miR-1290, miR-320d, miR-875-3p, miR-490-5p, miR-1266, and miR-615-3p) were tumor-derived (Figure 2A-C). Another GEO dataset (GSE41655) showed that only miR-1290 and miR-320d were significantly dysregulated in both colorectal adenoma and CRC (Figure 2D-F). Given the tumor-derived trait and the dysregulated expression in colorectal adenoma and CRC, selected candidate miR-1290 and miR-320d act as potential circulating biomarkers for the early detection of CRC.
Dysregulated miR-1290 and miR-320d were selected to further explore their potential diagnostic value after integrated analysis of GEO datasets. A-B. Partial miRNAs which were significantly dysregulated in CRC tissues. C. Circulating miRNAs that differentially expressed after operation. D-E. Dysregulated miRNAs in both adenoma and CRC tissues. F. miR-1290 and miR-320d were significantly dysregulated in both adenoma and CRC tissues. *P<0.05, **P<0.01, ***P<0.001.
Circulating miR-1290 and miR-320d serve as potential diagnostic biomarkers of CRC
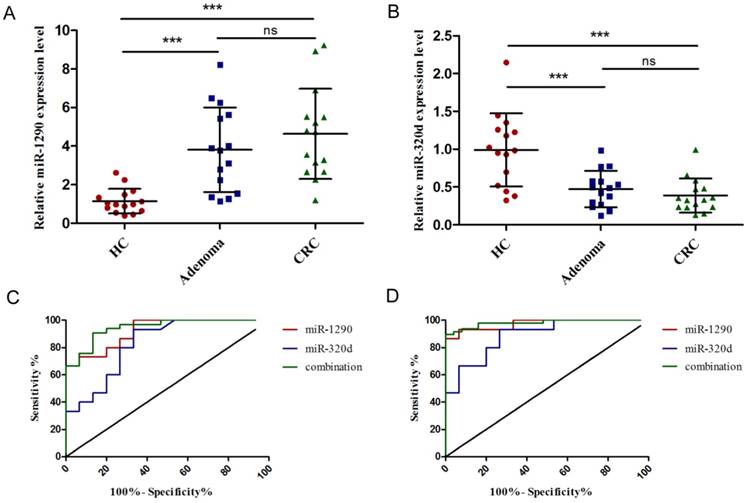
In the training phase, the expression of circulating miR-1290 and miR-320d was detected in a small set of 15 CRC patients, 15 colorectal adenoma patients and 15 healthy individuals. The relative expression levels of miR-1290 and miR-320d were statistically significantly dysregulated in both colorectal adenoma and CRC plasma samples compared to that in healthy controls (Figure 3A-B). To assess the potential diagnostic role of miRNAs, ROC curve analysis was performed. The AUC was 0.92 (95%CI: 0.83 to 1.00) for miR-1290 with 79.66% sensitivity and 86.67% specificity, 0.83 (95%CI: 0.68 to 0.97) for miR-320d with 81.36% sensitivity and 73.33% specificity, and 0.94 (95 % CI: 0.88-1.00) for 2-miRNAs combination with 80.72 sensitivity and 87.57% specificity to differentiate colorectal adenoma patients from healthy controls respectively (Figure 3C). Furthermore, AUC was 0.96 (95%CI: 0.92 to 1.00) for miR-1290 with 78.79% sensitivity and 93.33% specificity, 0.89 (95%CI: 0.77 to 1.00) for miR-320d with 93.94% sensitivity and 73.33% specificity, and 0.98 (95 % CI: 0.96-1.00) for 2-miRNAs combination with 90.91% sensitivity and 93.33% specificity to discriminate CRC patients from healthy controls respectively (Figure 3D).
Validation of tumor-derived circulating miR-1290 and miR-320d as CRC diagnostic biomarkers
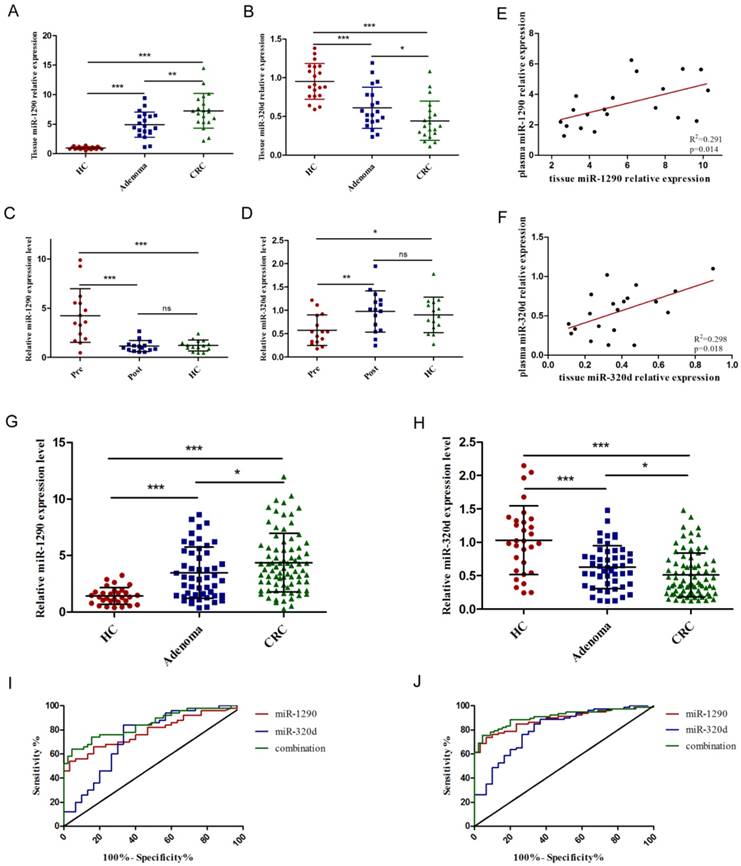
The expression of miR-1290 and miR-320d in colorectal adenoma and CRC tissues was significantly dysregulated compared to normal mucosa, respectively (Figure 4A-B). After analysis of 15 paired pre- and post-operation plasma samples of CRC patients, we found the expression of miR-1290 and miR-320d returned to normal levels after surgical resection of malignancies (Figure 4C-D). Interestingly, we observed a significant positive correlation of miR-1290 and miR-320d expression levels between 20 matched primary CRC tissues and plasma samples (Figure 4E-F).
To further validate the diagnostic role of miR-1290 and miR-320d, a larger independent cohort composed of 80 CRC patients, 50 adenoma patients, and 30 healthy controls was conducted. As shown in Figure 4G-H, The relative expression of plasma miR-1290 and miR-320d were significantly dysregulated in the adenoma and CRC patients compared to that in healthy controls. ROC curve revealed that the AUC was 0.78 (95%CI: 0.69 to 0.88) for miR-1290 with 75.53% sensitivity and 87.41% specificity, 0.74 (95%CI: 0.62 to 0.86) for miR-320d with 79.64% sensitivity and 71.55% specificity, and 0.82 (95 % CI: 0.75-0.89) for 2-miRNAs combination with 77.46% sensitivity and 88.69% specificity to differentiate colorectal adenoma patients from healthy controls respectively (Figure 4I). AUC was 0.88 (95%CI: 0.82 to 0.95) for miR-1290 with 76.65% sensitivity and 90.23% specificity, 0.81 (95%CI: 0.72 to 0.90) for miR-320d with 88.83% sensitivity and 71.66% specificity, and 0.91 (95 % CI: 0.85-0.96) for 2-miRNAs combination with 81.21% sensitivity and 90.74% specificity to discriminate CRC patients from healthy controls respectively (Figure 4J). Besides, plasma miR-1290 expression levels were significantly higher in CRC patients with larger tumor size (p=0.045), advanced TNM stage (p=0.008), lymph node metastasis (p=0.012), and distant metastasis (p=0.043). In contrast, plasma miR-320d expression levels were significantly lower in CRC patients with larger tumor size (p=0.031), advanced TNM stage (p=0.003), lymph node metastasis (p=0.038) (Table 2).
Correlations between plasma miR-1290 and miR-320d expression levels and clinicopathological features of CRC patients.
| Variables | Number | Plasma miR-1290 expression level | Plasma miR-320d expression level | ||
|---|---|---|---|---|---|
| Mean± SD | P value | Mean± SD | P value | ||
| <63 | 38 | 4.37±2.92 | 0.42±0.29 | ||
| ≥63 | 42 | 4.34±2.35 | 0.956 | 0.48±0.27 | 0.532 |
| Gender | |||||
| Male | 53 | 4.62±2.67 | 0.41±0.27 | ||
| Female | 27 | 4.08±2.59 | 0.526 | 0.49±0.26 | 0.35 |
| Well/moderately | 68 | 4.26±1.98 | 0.52±0.23 | ||
| poorly | 12 | 4.91±3.0 | 0.422 | 0.39±0.19 | 0.146 |
| Tumor size (cm) | |||||
| <4 | 39 | 3.79±1.88 | 0.57±0.31 | ||
| ≥4 | 41 | 5.38±2.87 | 0.045 | 0.39±0.18 | 0.031 |
| I+II | 55 | 3.55±1.97 | 0.61±0.27 | ||
| III+IV | 25 | 5.62±2.64 | 0.008 | 0.33±0.21 | 0.003 |
| Negative | 53 | 3.74±2.07 | 0.65±0.25 | ||
| Positive | 27 | 5.57±2.31 | 0.012 | 0.47±0.30 | 0.038 |
| Negative | 69 | 4.04±2.66 | 0.54±0.32 | ||
| Positive | 11 | 5.77±2.62 | 0.043 | 0.36±0.22 | 0.062 |
Exploration to identify circulating miR-1290 and miR-320d as novel biomarkers for early diagnosis of CRC. A-B. Plasma miR-1290 and miR-320d were significantly dysregulated in both adenoma and CRC patients. C-D. The diagnostic utility of plasma miR-1290 and miR-320d to distinguish adenoma and CRC patients from healthy controls. ***P<0.001.
Further validation of tumor-derived circulating miR-1290 and miR-320d as novel biomarkers for early diagnosis of CRC. A-B. Tissue miR-1290 and miR-320d were significantly dysregulated in both adenoma and CRC patients. C-D. The expression of miR-1290 and miR-320d returned to normal levels after surgical resection of malignancies. E-F. The positive correlation of miR-1290 and miR-320d expression levels between matched primary CRC tissues and plasma samples. G-H. Plasma miR-1290 and miR-320d were significantly dysregulated in both adenoma and CRC patients. I-J. The diagnostic utility of plasma miR-1290 and miR-320d to distinguish adenoma and CRC patients from healthy controls. *P<0.05, **P<0.01, ***P<0.001.
Discussion
In this study, we carried out an integrated analysis of four GEO datasets and discovered miR-1290 and miR-320d were significantly dysregulated in colorectal adenoma and adenocarcinoma. Furthermore, we validated plasma miR-1290 and miR-320d were tumor-derived and served as novel biomarkers for early diagnosis of CRC due to their ability to differentiate patients with colorectal adenoma and adenocarcinoma from healthy controls.
Previous studies have reported that miR-1290 and miR-320d were significantly dysregulated in various cancers, including CRC[10-14]. Mao et al. declared that miR-1290 functions as a tumor oncogene to promote cancer progression by targeting nuclear factor I/X (NFIX) in esophageal squamous cell carcinoma (ESCC)[15]; Ma et al. reported that miR-1290 expression increases in CRC tissues and could contribute to cancer cell proliferation by targeting inositol Polyphosphate 4-Phosphatase B (INPP4B) [16]; Tadano et al. demonstrated miR-320 family, including miR-320a, miR-320b, miR-320c, miR-320d, and miR-320e, were frequently downregulated in colorectal adenoma and submucosal invasive carcinoma tissues and regulated tumor proliferation by targeting CDK6 [17]; In addition, Qin et al. revealed that the expression of miR-320d is significantly decreased in glioma tissues and cell lines and overexpression of miR-320d could suppress cell growth, migration and invasion, and induce cell apoptosis as well as cell cycle at G0/G1 arrest[18]. Similarly, our results exhibited that circulating miR-1290 was significantly associated with tumor size, TNM stage, lymph node metastasis, and distant metastasis, and circulating miR-320d was significantly correlated with tumor size, advanced TNM stage, lymph node metastasis, and distant metastasis. Discoveries described above indicate that both miR-1290 and miR-320d significantly modulate the development and progression of CRC.
The value of circulating miR-1290 as tumor biomarker has been comprehensively investigated. Huang et al. reported that plasma exosomal miR-1290 and miR-375 are promising prognostic biomarkers for castration-resistant prostate cancer (CRPC)[19].
Li et al. demonstrated serum miR-1290 is elevated in pancreatic cancer and could accurately distinguish low-stage pancreatic cancer patients from healthy controls[20]. Additionally, Imaoka et al. identified circulating miRNA-1290 as a novel diagnostic and prognostic biomarker in human colorectal cancer[21]. In contrast, the diagnostic role of circulating miR-320d has not been explored in cancers. Our study is the first to demonstrate that plasma miR-320d acts as a potential biomarker for the early detection of colorectal malignancy. Besides, when compared to that of single circulating miR-1290, the diagnostic utility of combination of plasma miR-1290 and miR-320d significantly increased which was supported by the higher AUC value derived from comparison between CRC patients and healthy controls. More importantly, plasma miR-1290 and miR-320d expression levels could discriminate patients with colorectal adenoma from healthy controls in a non-invasive manner with a AUC values of 0.82 which is crucial to the prognosis of CRC patients because almost 90% of mortality could be prevented if CRC patients diagnosed at early stage[22].
Although circulating miR-1290 and miR-320d appear to be promising biomarkers for early diagnosis of CRC, there are some limitations in our study. First, the sample size included was relatively small, and we believe that prospective studies with larger sample numbers are required to clarify the usefulness of circulating miR-1290 and miR-320d as novel diagnostic biomarkers of CRC. Second, the cut-off values of plasma miR-1290 and miR-320d were different in training phase and validation phase which were not shown. Several reasons may explain this phenomenon including the heterogeneity of CRC, the difference of sample size, and the hemolysis of blood cells.
In summary, we demonstrated that plasma miR-1290 and miR-320d are novel promising biomarkers for the early detection of colorectal malignancy with non-invasion.
Acknowledgements
This project was supported by grants from the National Nature Science Foundation of China (No. 81472027) to SKW; Key Project of Science and Technology Development of Nanjing Medicine (ZDX16001); Innovation team of Jiangsu provincial health-strengthening engineering by science and education (CXTDB2017008) to SKW. Jiangsu Youth Medical Talents Training Project to B.H (QNRC2016066) and Y.P (QNRC2016074); Nanjing Medical Science and Technique Development Foundation to BSH (No. JQX13003).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
2. Jeong SY, Chessin DB, Schrag D. et al. Re: Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2005;97:1705-6 author reply 6-7
3. Launoy G, Smith TC, Duffy SW. et al. Colorectal cancer mass-screening: estimation of faecal occult blood test sensitivity, taking into account cancer mean sojourn time. Int J Cancer. 1997;73:220-4
4. Hassan C, Pickhardt PJ, Laghi A. et al. Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm: model simulation with cost-effectiveness analysis. Arch Intern Med. 2008;168:696-705
5. Carpelan-Holmstrom M, Louhimo J, Stenman UH. et al. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 2002;22:2311-6
6. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351-79
7. Mitchell PS, Parkin RK, Kroh EM. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513-8
8. Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087-92
9. Arroyo JD, Chevillet JR, Kroh EM. et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003-8
10. Li M, He XY, Zhang ZM. et al. MicroRNA-1290 promotes esophageal squamous cell carcinoma cell proliferation and metastasis. World J Gastroenterol. 2015;21:3245-55
11. Kim KB, Kim K, Bae S. et al. MicroRNA-1290 promotes asiatic acidinduced apoptosis by decreasing BCL2 protein level in A549 nonsmall cell lung carcinoma cells. Oncol Rep. 2014;32:1029-36
12. Lin M, Shi C, Lin X. et al. sMicroRNA-1290 inhibits cells proliferation and migration by targeting FOXA1 in gastric cancer cells. Gene. 2016;582:137-42
13. Scaravilli M, Porkka KP, Brofeldt A. et al. MiR-1247-5p is overexpressed in castration resistant prostate cancer and targets MYCBP2. Prostate. 2015;75:798-805
14. Wu PY, Zhang XD, Zhu J. et al. Low expression of microRNA-146b-5p and microRNA-320d predicts poor outcome of large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone. Hum Pathol. 2014;45:1664-73
15. Mao Y, Liu J, Zhang D. et al. MiR-1290 promotes cancer progression by targeting nuclear factor I/X(NFIX) in esophageal squamous cell carcinoma (ESCC). Biomed Pharmacother. 2015;76:82-93
16. Ma Q, Wang Y, Zhang H. et al. MiR-1290 Contributes to Colorectal Cancer Cell Proliferation by Targeting INPP4B. Oncol Res. 2017
17. Tadano T, Kakuta Y, Hamada S. et al. MicroRNA-320 family is downregulated in colorectal adenoma and affects tumor proliferation by targeting CDK6. World J Gastrointest Oncol. 2016;8:532-42
18. Qin CZ, Lv QL, Yang YT. et al. Downregulation of MicroRNA-320d predicts poor overall survival and promotes the growth and invasive abilities in glioma. Chem Biol Drug Des. 2017;89:806-14
19. Huang X, Yuan T, Liang M. et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67:33-41
20. Li A, Yu J, Kim H. et al. MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin Cancer Res. 2013;19:3600-10
21. Imaoka H, Toiyama Y, Fujikawa H. et al. Circulating microRNA-1290 as a novel diagnostic and prognostic biomarker in human colorectal cancer. Ann Oncol. 2016;27:1879-86
22. Smith RA, von Eschenbach AC, Wender R. et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001-testing for early lung cancer detection. CA Cancer J Clin. 2001;51:38-75 quiz 7-80
Author contact
![]() Corresponding author: Dr. Shukui Wang. No. 32 Gongqingtuan Road, Nanjing, China. Tel +86 25 52271163; Fax +86 25 52269924; Email sk_wangedu.cn.
Corresponding author: Dr. Shukui Wang. No. 32 Gongqingtuan Road, Nanjing, China. Tel +86 25 52271163; Fax +86 25 52269924; Email sk_wangedu.cn.

 Global reach, higher impact
Global reach, higher impact