3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(2):287-292. doi:10.7150/jca.28331 This issue Cite
Research Paper
Amlexanox and UPF1 Modulate Wnt Signaling and Apoptosis in HCT-116 Colorectal Cancer Cells
Department of Medical Education, Geisinger Commonwealth School of Medicine, 525 Pine Street, Scranton, PA 18509, USA
Received 2018-7-5; Accepted 2018-11-26; Published 2019-1-1
Abstract
Deregulated Wnt signaling initiates most cases of colorectal cancer (CRC). Butyrate, a product of dietary fiber, hyperactivates Wnt signaling, resulting in induction of CRC cell apoptosis, which may in part explain the protective action of fiber. Nonsense mediated decay (NMD) of mRNAs containing premature stop codons (PTCs) affects tumorigenesis and upregulates Wnt signaling in human embryonic stem cells. However, it is unknown how NMD affects Wnt activity in CRC cells that exhibit constitutively activated Wnt signaling. We hypothesize that expression of genes that contain PTCs modulates Wnt signaling and response to butyrate in CRC cells. Amlexanox is a clinically utilized anti-allergic and anti-inflammatory drug that inhibits NMD and promotes PTC read-through. Therefore, Amlexanox is a relevant agent for assessing the role of NMD and PTC read-through in the response of CRC cells to butyrate. To test our hypothesis, we treated HCT-116 CRC cells with Amlexanox and determined effects on Wnt signaling levels, apoptosis, and response to butyrate. Amlexanox enhanced basal Wnt signaling levels; however, it repressed butyrate-induced Wnt signaling hyperactivation and suppressed apoptosis. To evaluate the contribution of NMD and altered expression of PTC-containing genes to these effects, we upregulated NMD by overexpression of up-frameshift protein 1 (UPF1), and observed effects opposite to these of Amlexanox (i.e., Wnt signaling hyperactivation by butyrate was enhanced and levels of apoptosis were increased). Our results support the possibility that altered expression of PTC-containing genes affects butyrate sensitivity of CRC cells.
Keywords: nonsense mediated decay, premature termination codon, colorectal cancer, Amlexanox, Wnt signaling, butyrate
Introduction
Nonsense mediated decay (NMD) degrades mRNAs that contain nonsense mutations resulting in premature stop codons (PTCs) [1-4], and influences gene expression [1-6]. The relationship between NMD and carcinogenesis is complex and likely dependent upon tumor type and stage of neoplastic progression. With respect to neoplastic initiation, NMD decreases expression of mutated tumor suppressor genes, promoting early carcinogenesis [7]. In certain contexts, NMD promotes progression, particularly in microsatellite instability positive (MSI+) colorectal cancers (CRCs) that accumulate PTC-containing mRNAs [6]. The NMD-promoting factor up-frameshift protein 1 (UPF1) is upregulated in MSI+ CRCs compared to normal tissue, and gene expression analysis supports NMD driving MSI-mediated tumorigenesis [6]. However, in other contexts, neoplastic progression is promoted via suppression of NMD by the tumor microenvironment [8]. Understanding the interactions between NMD and neoplasia can optimize preventive and therapeutic approaches against cancer.
Deregulated Wnt signaling is a key feature of most CRCs. The preventive activity of dietary fiber against CRC is associated with its fermentation product, the histone deacetylase inhibitor (HDACi) butyrate [9-15], which induces CRC cell apoptosis in part by hyperactivating Wnt signaling [11-18]. Wnt activity can be exogenously stimulated in human embryonic stem cells, and in such cells, NMD upregulates Wnt signaling and the expression of genes targeted by the pathway [19]. However, it is unknown how NMD affects Wnt activity in CRC cells that exhibit constitutively activated Wnt signaling; for example, NMD may influence the ability of butyrate to hyperactive Wnt signaling and modulate CRC cell apoptosis.
Certain reagents promote PTC read-through, and are selective for PTCs, not affecting wild-type termination codons [20-25]. However, optimized production of functional protein from PTC-containing transcripts should combine PTC read-through with NMD inhibition, which would increase the levels of PTC-containing mRNAs subject to read-through translation. Amlexanox, a clinically relevant anti-allergic and anti-inflammatory drug, both promotes PTC read-through and suppresses NMD [21]. We evaluated how Amlexanox influences Wnt signaling and apoptosis in the MSI+ HCT-116 CRC cell line, in the presence and absence of a physiologically relevant concentration of butyrate. To validate our results, we determined whether stimulation of NMD, and decreased expression of PTC-containing genes, achieved through overexpression of UPF1, exhibited effects on Wnt signaling and apoptosis opposite to those of Amlexanox.
Materials and Methods
Reagents, cell lines, vectors, transfection, and luciferase assays
Amlexanox, butyrate, and NMDI-14 were from Sigma; Ataluren was from Selleckchem. HCT-116 cells were from Cancer Research UK. NMD vectors were from Dr. Andreas Kulozik, TOP/FOP luciferase reporters were from Dr. Hans Clevers, and pRLTK was from Promega. Control and Rent1 (UPF1) CRISPR activation vectors were from Santa Cruz Biotechnology. Transfection and luciferase assays were conducted as previously described [11-18]. Stable transfections were selected with 200 μg/ml hygromycin, 20 μg/ml blasticidin, and 5 μg/ml puromycin; selective agents were from Santa Cruz Biotechnology. Description of the TOPFlash/FOPFlash system and UPF1 CRISPR is in Supplementary Information.
Western blot and apoptosis assays
Western blot and caspase 3/7 assays were performed as previously described [11-18]. Antibodies to CBP, p300, and Rent1 (UPF1) were from Santa Cruz. Actin antibody was from Sigma. Band images were adjusted for brightness and contrast to enhance visualization.
Statistics
Student's T-test was utilized, with statistical significance set at P < 0.05. In Figures, * represents statistical significance; for Fig. S2E, + represents P = 0.053.
Results
NMD can be measured by a beta-globin-Renilla luciferase NMD reporter. Amlexanox is capable of both NMD inhibition and PTC read-through, and this drug is already used in the clinic for its anti-allergic and anti-inflammatory properties [21]. We therefore determined the optimal concentration of this agent for HCT-116 cells.
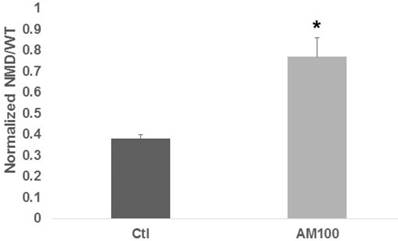
Titration of Amlexanox determined that 100 μM and 150 μM were most effective (data not shown); 100 μM was subsequently used for all experiments. In HCT-116 cells, 100 μM Amlexanox resulted in a two-fold increase in expression (P < 0.002) from the NMD (PTC) reporter vector compared to control (Fig. 1).
Amlexanox stimulates expression from a NMD reporter vector. HCT-116 CRC cells were cotransfected with a wild-type or NMD (PTC) reporter vector and a control vector for normalization, and were treated with 100 µM Amlexanox (AM100) or mock treated (Ctl) for 48 hr. Cells were assayed for luciferase expression, measuring normalized readings of the NMD (PTC) reporter values divided by those of the wild-type reporter. Data from three independent experiments. Bars, SDs.
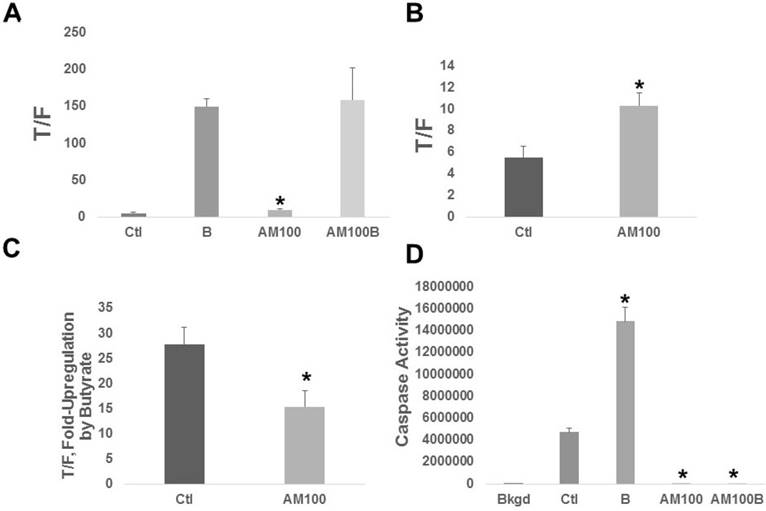
Both basal Wnt activity and the fold upregulation of Wnt activity by butyrate were affected by Amlexanox (Fig. 2A). Amlexanox treatment enhanced basal Wnt activity (Fig. 2B) by 1.9-fold (P < 0.008) and the fold-upregulation of Wnt by butyrate in the presence of Amlexanox (Fig. 2C) was 55% of that observed in mock-treated cells (P < 0.02). Wnt signaling hyperactivation causally correlates to CRC cell apoptosis [10-17]. We observed the expected butyrate-induced upregulation of apoptosis (3.2-fold, P < 0.001), as measured by caspase 3/7 activity, in HCT-116 cells not exposed to Amlexanox (Fig. 2D); however, treatment with Amlexanox completely abrogated caspase 3/7 activity. Differences in caspase activity between control and Amlexanox treated cells were statistically significant (P < 0.001), both in the presence and absence of butyrate.
CBP and p300 are Wnt signaling coactivators that influence response to butyrate in CRC cells [13-15,18]. HCT-116 cells exhibit hemizygous expression of p300; the expressed allele produces a PTC-containing mRNA that partially escapes NMD because the PTC is in the last exon, producing a truncated but functional protein [26-29]. A fraction of HCT-116 cells also exhibit a heterozygous truncating mutation of CBP [27]. Considering the role of CBP and p300 in the response to butyrate and the presence of PTCs in these genes, we evaluated whether Amlexanox affects the levels of these factors in HCT-116 cells. However, no differences in the expression of CBP and p300 were observed (Fig. S1).
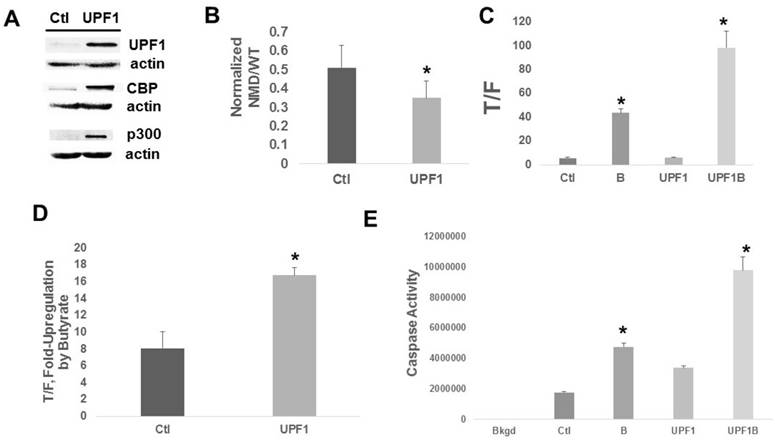
Increased NMD efficiency in suppressing expression of PTC-containing genes is expected to exhibit opposite effects compared to Amlexanox. We determined the effects of enhanced NMD in HCT-116 cells, achieved through increased expression of UPF1. Cells stably transfected with the UPF1 CRISPR activation system overexpress UPF1 compared to control cells (Fig. 3A). As expected, upregulation of UPF1 resulted in a significant (P < 0.01) decline in expression from the NMD/PTC reporter compared to control, consistent with enhanced NMD (Fig. 3B). Basal Wnt activity was unaffected by UPF1 overexpression (Fig. 3C). However, Wnt activity levels after exposure to butyrate were markedly higher (Fig. 3C) in UPF1-overexpressing cells compared to control cells (P < 0.005) and the fold-increase in Wnt activity after exposure to butyrate was two-fold higher (Fig. 3D) in UPF1-overexpressing cells compared to control cells (P < 0.003). Whereas UPF1 overexpression did not significantly affect the fold-change in caspase 3/7 activity after butyrate treatment, absolute levels of apoptotic activity were upregulated in the presence (P < 0.002) and absence (P < 0.001) of butyrate (Fig. 3E). We evaluated whether overexpression of UPF1 affected the levels of CBP or p300; the UPF1-overexpressing cells exhibited increased expression of both CBP and p300 (Fig. 3A).
Amlexanox affects butyrate-modulated Wnt signaling and apoptosis of CRC cells. (A) Wnt signaling. HCT-116 cells transfected with the TOPFLASH or FOPFLASH Wnt signaling reporters and pRLTK normalization vector. Cells were treated with 100 µM Amlexanox (AM100) or mock-treated (Ctl) for 24 hr, and then mock-treated or treated with 100 µM Amlexanox (AM100) and/or 5 mM butyrate [labeled as B] and (AM100B) for a further 24 hr. (B) The basal Wnt activity from (A) is shown to better visualize differences between Ctl and AM100. (C) The fold up-regulation of Wnt activity after exposure to butyrate based upon the data in (A). (D) Apoptosis assays were performed as previously described [11-15]; cells were treated as in (A). Data from three independent experiments. Bars, SDs.
Upregulation of UPF1 in HCT-116 cells. (A) Representative Western blot showing expression of UPF1, CBP, and p300. (B) NMD assayed by the reporter system as in Fig. 1. Data are from nine independent experiments. (C) Wnt signaling assayed as in Fig. 2. (D) The fold up-regulation of Wnt activity after exposure to butyrate from the data of (C). (E) Apoptosis assays were performed as previously described [11-15]. For (C-E), data from three independent experiments are shown. Bars, SDs
To further evaluate NMD inhibition and PTC read-through in the anti-apoptotic effects of Amlexanox, we investigated a specific inhibitor of NMD, NMDI-14 [31]; a specific read-through activator, Ataluren [21-24]; and the combination of these agents. Unexpectedly, treatment with NMDI-14 (alone or with Ataluren) potentiated NMD in HCT-116 cells as demonstrated by decreased expression from the reporter (Fig. S2A). The apoptotic data (Fig. S2B) suggest that the suppression of apoptosis observed with Amlexanox is likely due to its PTC read-through activity, since this anti-apoptotic effect was replicated by Ataluren. Considering this finding, we evaluated the effect of Ataluren on basal and butyrate-induced Wnt activity (Fig. S2C). Ataluren treatment resulted in slight suppression (P < 0.005) of basal Wnt activity (Fig. S2C,D), a greater than two-fold decrease (P < 0.005) in the levels of butyrate-induced Wnt activity (Fig. S2C), and a modest inhibition of the fold-induction of Wnt activity by butyrate (P = 0.053) (Fig. S2C,E).
Discussion
NMD influences tumorigenesis [5,6,8]; therefore, we evaluated the effects of Amlexanox, an inhibitor of NMD that also activates PTC read-through, in HCT-116 CRC cells. As expected, Amlexanox enhanced relative expression from the NMD reporter (Fig. 1). Amlexanox enhanced basal Wnt activity and decreased the fold-upregulation of Wnt activity by butyrate (Fig. 2). Both basal Wnt activity and the fold-upregulation of that activity affect CRC cell physiology; basal Wnt activity is associated with aberrant proliferation during tumorigenesis; whereas, fold-upregulation of Wnt activity by butyrate causally correlates to induction of apoptosis [10-17]. Importantly, caspase 3/7 activity, a terminal step in apoptosis, was completely abrogated by Amlexanox treatment (Fig. 2D).
Amlexanox treatment did not alter expression of CBP and p300, which mediate effects of butyrate on Wnt signaling [11,13,14]. Possibly, Amlexanox-mediated modulation of Wnt activity is due to effects on the expression of other factors and/or on the binding-association of factors (e.g., beta-catenin with CBP and p300). NMD also affects a fraction of mRNAs that encode functional proteins; up to 5-10% of the human transcriptome can be regulated by NMD [30]. Therefore, altered NMD activity could influence the expression of genes involved in modulating Wnt signaling and response to butyrate.
Enhancing NMD (i.e., suppressing expression of PTC-containing genes) through UPF1 overexpression resulted in the opposite effect on butyrate-mediated Wnt signaling hyperactivation compared to Amlexanox (Fig. 2 versus Fig. 3). However, despite effects on Wnt signaling, overexpression of UPF1 did not affect the fold-change of apoptotic activity in butyrate-treated cells. Considering that CRC cell apoptosis has both Wnt-independent and Wnt-dependent components, one possibility is that modulation of NMD affects the Wnt-independent component of apoptosis. An alternative explanation is that Wnt signaling hyperactivation in control-transfected cells is already optimal for maximal Wnt-dependent apoptosis, and therefore higher levels of Wnt activity may not increase apoptosis further. These data suggest that enhanced expression of PTC-containing transcripts modulates CRC cell apoptosis, with possible clinical implications.
Expression of CBP and p300 was upregulated in UPF1-overexpressing cells; this is likely an indirect effect, since the direct effect of enhanced NMD would be to decrease expression of PTC-containing genes. One possibility is that enhanced NMD alters the expression of factors that control CBP and p300 expression. The increased CBP and p300 levels may contribute to the Wnt signaling hyperactivation and apoptosis observed in UPF1-overexpressing cells; however, further studies are required to determine this.
Whereas Amlexanox and UPF1 overexpression had opposite effects on apoptosis and Wnt signaling in butyrate-treated HCT-116 cells, we observed that the increase in basal Wnt activity by Amlexanox was not reciprocated by a suppression of basal Wnt activity in UPF1-overexpressing cells. In addition, Amlexanox did not affect CBP and p300 levels; whereas, UPF1 did so. Therefore, Amlexanox and UPF1 may exert effects on cell physiology independent of NMD and may have Wnt-independent and Wnt-dependent effects. However, the data of Figs. 2 and 3 indicate that altered expression of PTC-containing genes plays a role in the effects of Amlexanox and UPF1 in HCT-116 cells. In addition, the data obtained with Ataluren (Fig. S2B) suggest that it is the read-through component of Amlexanox activity that is most responsible for suppressed apoptosis. Further, the Wnt activity data (Figs. 2, S2) suggest that the effects of these agents on HCT-116 cell apoptosis may be Wnt-independent. Finally, the comparison of the data from Fig. 1 to that of Fig. S2A strongly suggests that optimal expression from PTC-containing genes, as modeled by the NMD reporter, requires both NMD suppression and read-through activation.
Our findings indicate that Amlexanox may interfere with the preventive activity of butyrate. However, the maximal serum concentration of Amlexanox in clinical use is two orders of magnitude lower than that in our study [32]. The long-term effects of continuous low dosage Amlexanox exposure on colonic physiology requires further investigation. Discussion on our findings in relation to the work of El-Bchiri et al. [6] and on the activity of Amlexanox as an HDACi [33] is included in Supplementary Information, as is the discussion of the NMDI-14 and Ataluren experiments.
Abbreviations
NMD: nonsense mediated decay, PTC: premature stop codon, CRC: colorectal cancer, MSI+: microsatellite instability positive, UPF1: frameshift protein 1, HDACi: histone deacetylase inhibitor.
Supplementary Material
Supplementary figures.
Acknowledgements
This work was supported by funding from Geisinger Commonwealth School of Medicine. We acknowledge Drs. Kulozik and Clevers for supplying vectors.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Metze S, Herzog VA, Ruepp MD. et al. Comparison of EJC-enhanced and EJC-independent NMD in human cells reveals two partially redundant degradation pathways. RNA. 2013;19:1432-48
2. Hug N, Longman D, Cáceres JF. Mechanism and regulation of the nonsense-mediated decay pathway. Nucleic Acids Res. 2016;44:1483-95
3. Popp MW, Maquat LE. Leveraging Rules of Nonsense-Mediated mRNA Decay for Genome Engineering and Personalized Medicine. Cell. 2016;165:1319-22
4. Nickless A, Bailis JM, You Z. Control of gene expression through the nonsense-mediated RNA decay pathway. Cell Biosci. 2017;7:26
5. Wengrod J, Martin L, Wang D. et al. Inhibition of nonsense-mediated RNA decay activates autophagy. Mol Cell Bio. 2013;33:2128-35
6. El-Bchiri J, Guilloux A, Dartigues P. et al. Nonsense-mediated mRNA decay impacts MSI-driven carcinogenesis and anti-tumor immunity in colorectal cancers. PLoS One. 2008;3:e2583
7. Lindeboom RGH, Supek F, Lehner B. The rules and impact of nonsense-mediated decay in human cancers. Nat Genet. 2016;48:112-8
8. Wang D, Zavadi Jl, Martin L. et al. Inhibition of nonsense-mediated RNA decay by the tumor microenvironment promotes tumorigenesis. Mol Cell Bio. 2011;31:3670-80
9. Bingham SA, Day NE, Luben R. et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition [EPIC]: an observational study. Lancet. 2003;361:1496-501
10. Bordonaro M, Lazarova D, Sartorelli AC. Hyperinduction of WNT signaling: a new paradigm for the treatment of colorectal cancer? Oncol Res. 2008;17:1-9
11. Bordonaro M, Lazarova DL, Sartorelli AC. The activation of beta-catenin by WNT signaling mediates the effects of histone deacetylase inhibitors. Exp Cell Res. 2007;313:1652-66
12. Chiaro C, Lazarova DL, Bordonaro M. Tcf3 and cell cycle factors contribute to butyrate resistance in colorectal cancer cells. Biochem Biophys Res Commun. 2012;428:121-6
13. Lazarova DL, Chiaro C, Wong T. et al. CBP activity mediates effects of the histone deacetylase inhibitor butyrate on Wnt activity and apoptosis in colon cancer cells. J Cancer. 2013;4:481-90
14. Lazarova DL, Wong T, Chiaro C. et al. p300 influences butyrate-mediated WNT hyperactivation in colorectal cancer cells. J Cancer. 2013;4:491-501
15. Lazarova D, Lee A, Wong T. et al. Modulation of Wnt Activity and cell physiology by butyrate in LT97 microadenoma cells. J Cancer. 2014;5:203-13
16. Lazarova DL, Chiaro C, Bordonaro M. Butyrate induced changes in Wnt-signaling specific gene expression in colorectal cancer cells. BMC Research Notes. 2014;7:226
17. Lazarova DL, Bordonaro M. Extreme fluctuations in Wnt/beta-catenin signaling as an approach for colon cancer prevention and therapy. Advanced Studies in Biology. 2012;4:351-62
18. Lazarova DL, Bordonaro M. p300 knockout promotes butyrate resistance. J Cancer. 2017;8:3405-9
19. Lou CH, Dumdie J, Goetz A. et al. Nonsense-mediated RNA decay influences human embryonic stem cell fate. Stem Cell Reports. 2016;6:844-57
20. Boelz S, Neu-Yilik G, Gehring NH. et al. A chemiluminescence-based reporter system to monitor nonsense-mediated mRNA decay Biochemical and Biophysical Research Communications. 2006; 349:186-91.
21. Gonzalez-Hilarion S, Beghyn T, Jia J. et al. Rescue of nonsense mutations by Amlexanox in human cells. Orphanet J Rare Dis. 2012;7:58
22. Peltz SW, Morsy M, Welch EM. et al. Ataluren as an agent for therapeutic nonsense suppression. Annu Rev Med. 2013;64:407-25
23. Roy B, Friesen WJ, Tomizawa Y. et al. Ataluren stimulates ribosomal selection of near-cognate tRNAs to promote nonsense suppression. Proc Natl Acad Sci. 2016:11312508-13
24. Siddiqui N, Sonenberg N. Proposing a mechanism of action for ataluren. Proc Natl Acad Sci U S A. 2016;113:12353-5
25. Bolze F, Mocek S, Zimmerman A. et al. Aminoglycosides, but not PTC124 (Ataluren), rescue nonsense mutations in the leptin receptor and in luciferase reporter genes. Sci Rep. 2017;7:1020
26. Krubasik D, Iyer NG, English WR. et al. Absence of p300 induces cellular phenotype changes characteristic of epithelial to mesenchyme transition. Br J Cancer. 2006;94:1326-32
27. Ionov Y, Matsui S-I, Cowell JK. A role for p300/CREB binding protein genes in promoting cancer progression in colon cancer cell lines with microsatellite instability. Proc Natl Acad Sci USA. 2004;101:1273-8
28. Martincorena I, Campbell PJ. Somatic mutation in cancer and normal cells. Science. 2015;349:1483-8
29. Iyer NG, Ozdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23:4225-31
30. Peccarelli M, Kebaara B. Regulation of natural mRNAs by the nonsense-mediated mRNA decay pathway. Euk Cell. 2014;13:1126-35
31. Martin L, Grigoryan A, Wang D. et al. Identification and characterization of small molecules that inhibit nonsense mediated RNA decay and suppress nonsense p53 mutations. Cancer Res. 2014;74:3104-13
32. Bell J. Amlexanox for the treatment of recurrent aphthous ulcers. Clin Drug Investig. 2005;25:555-66
33. Hsu C-W, Shou D, Huang R. et al. Identification of HDAC inhibitors using a cell-based HDACI/II assay. J Biomol Screen. 2016;21:643-52
34. Goentoro L, Kirschner MW. Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. Mol Cell. 2009;36:872-84
Author contact
![]() Corresponding author: Geisinger Commonwealth School of Medicine, 525 Pine Street, Scranton, PA 18509. Tel: 570-504-9646; Fax: 570-504-9636; Email: mbordonarogeisinger.edu
Corresponding author: Geisinger Commonwealth School of Medicine, 525 Pine Street, Scranton, PA 18509. Tel: 570-504-9646; Fax: 570-504-9636; Email: mbordonarogeisinger.edu

 Global reach, higher impact
Global reach, higher impact