3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(2):332-340. doi:10.7150/jca.27753 This issue Cite
Research Paper
Optimal Timing for Postsurgical Adjuvant Therapy in Patients with Gastric Cancer: A Propensity Score Matching Study
1. Department of Radiation Oncology, Keelung Chang Gung Memorial Hospital, Keelung, Taiwan
2. Division of Radiation Oncology, Department of Oncology, National Taiwan University Hospital, Taipei, Taiwan
3. Cancer Research Center, National Taiwan University College of Medicine, Taipei, Taiwan
4. Graduate Institute of Clinical Medicine, National Taiwan University College of Medicine, Taipei, Taiwan
5. Clinical Trial Center, Chang Gung Memorial Hospital, Linkou, Taiwan.
6. Department of Radiation Oncology, Proton and radiation therapy center, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan
7. Department of Surgery, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan
8. Division of Hematology and Oncology, Department of Internal Medicine, Keelung Chang Gung Memorial Hospital, Keelung, Taiwan
9. College of Medicine, Chang Gung University, Taoyuan, Taiwan
10. Department of Food Science, National Taiwan Ocean University, Keelung, Taiwan
11. Division of general surgery, Department of surgery, Keelung Chang Gung Memorial Hospital, Keelung, Taiwan
12. Department of Ophthalmology, Keelung Chang Gung Memorial Hospital, Keelung, Taiwan
13. Graduate Institute of Clinical Medical Science, Chang Gung University, Taoyuan, Taiwan
Received 2018-6-8; Accepted 2018-10-3; Published 2019-1-1
Abstract
Background: In clinical trials, adjuvant therapy (AT) has been shown to improve the prognosis in patients with gastric adenocarcinoma who undergo curative gastrectomy and adequate lymph node dissection. However, the optimal timing for initiating AT is still unclear.
Method: We collected data from 538 patients with stage II-III gastric cancer who underwent curative gastrectomy and AT in two tertiary hospitals from 2006 to 2013. Patients were divided into the early group (≤8 weeks, n=393) and the late group (>8 weeks, n=145), based on the interval between gastrectomy and initiation of AT. Propensity score matching was applied according to baseline characteristics.
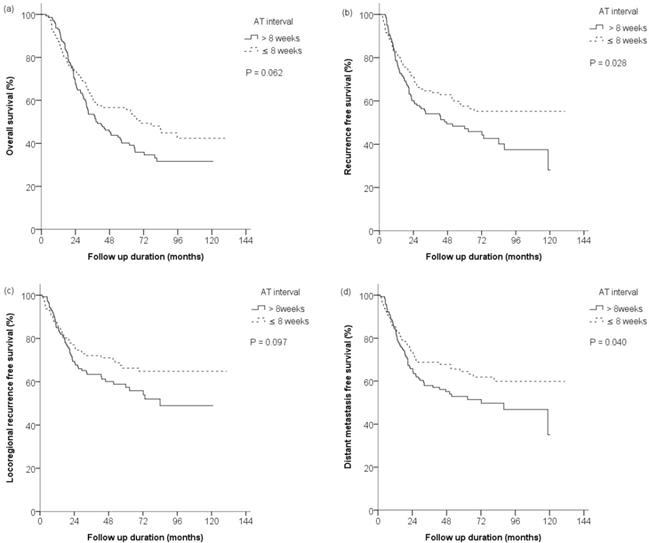
Results: After 1:1 propensity score matching, an even distribution of characteristics in both groups (143:143) was achieved. The 5-year overall survival (OS) rates were 56.6% and 40.2% in the matched early and late groups, respectively (p=0.062), while the corresponding 5-year recurrence-free survival (RFS) rates were 57.6% and 46.4%, respectively (p=0.028). The time to AT initiation was correlated with RFS and had a positive association with OS. The 5-year distant metastasis-free survival was also significantly better (HR 0.682, 95% CI 0.472-0.985, p=0.040), suggesting an early AT results in a better outcome in patients.
Conclusion: We observed that initiation of AT within 8 weeks of curative gastrectomy produces better disease control and may contribute to better overall survival.
Keywords: adjuvant therapy, gastric cancer, survival, gastrectomy, propensity score
Introduction
Gastric cancer is the third most common cause of cancer-specific death worldwide1. The main treatment for gastric cancer is surgical removal of the tumor along with regional lymph node dissection, followed by adjuvant therapy (AT) including chemotherapy or concurrent chemoradiotherapy. In Asian countries, D2 dissection is more commonly performed than D1, and post-surgical adjuvant chemotherapy has been shown to benefit prognosis 2-5. However, the optimal time for AT initiation is still controversial. It has been proposed that AT eradicates residual microscopic disease after curative gastrectomy, thereby reducing recurrence rate and eventually improving survival for patients with stage II gastric cancer or higher. Previous studies comparing AT with gastrectomy alone showed a survival benefit when AT was administered, when therapy was initiated within 3-8 weeks of gastrectomy2, 4, 6. Whether delaying adjuvant therapy, especially by more than 8 weeks, will worsen patient prognosis remains unknown due to controversial results from retrospective studies 7-10. Moreover, clinical trials generally suggest initiate AT within 6-8 weeks post-gastrectomy but without any evidence.8 It is impractical to perform a randomized study to address this question owing to clinical circumstances and ethical concerns. As such, propensity score matching may be a more appropriate approach, as it is applied to balance the baseline characteristic data of patients to minimize treatment selection bias and mimic a randomized trial 11.
Hence, we performed a retrospective study by applying propensity score matching to analyze whether the timing of adjuvant therapy influences the prognosis of gastric cancer patients with post-curative gastrectomy and adequate lymph node dissection.
Material and Methods
This study was reviewed and approved by the institutional review board of Chang Gung Memorial Hospital (Reference No.: 201600551B0C101). All data were stored in the hospital database and used for research.
Study population
We targeted patients with stage II and III primary gastric adenocarcinoma, who underwent curative total or partial gastrectomy with D2 or D1 resection, with 15 or more regional lymph nodes dissected, followed by AT. Staging information was based on the American Joint Committee on Cancer (AJCC) staging manual (seventh edition) 12. Patients with metastatic disease, those who did not have detailed pathology reports, or who received neoadjuvant chemoradiotherapy or neoadjuvant chemotherapy were excluded. Patients who received D1 lymph node dissection of fewer than 15 lymph nodes were also excluded to ensure only patients with D2 or D1 with adequate lymph node dissections were included 13-15. The AT initiation interval was defined as the period between the date of gastrectomy and the date AT commenced. Because initiating systematic treatment with AT regimens generally occurs within 8 weeks after gastrectomy in clinical trials 2, 6 as well as in previously reported studies 8, 9, we divided our cohort into two groups according to the time of AT initiation as the early group (within 8 weeks) and the late group (later than 8 weeks).
Statistical analysis
Propensity score matching was applied to reach a balanced distribution of patient characteristics in the two AT groups, to mimic a randomized patient composition. The propensity score was calculated by using a logistic regression model based on patient clinicopathological parameters, deemed to be related to outcome including sex; age; Eastern Cooperative Oncology Group (ECOG) performance status before gastrectomy; pathologic tumor staging; tumor differentiation; pathologic nodal status; lymph node ratio; number of examined lymph nodes; presence of vascular, lymphatic, or perineural invasion; and surgical method. The matching process was based on the nearest neighbor matching method 16, under a 0.2 caliper to perform a 1:1 matching 17.
Overall survival (OS) was defined by the duration between the date of gastrectomy and death or the date of the last of follow-up, whichever came first, and recurrence-free survival (RFS) was defined as the duration between the date of gastrectomy until the detection of tumor recurrence. The first site(s) of recurrence was collected, and locoregional recurrence-free survival (LRRFS) and distant metastasis-free survival (DMFS) were also determined. Locoregional recurrence was defined as that which occurred at the anastomosis site, duodenal stump, tumor bed, remnant stomach or the regional lymph nodes. Distant metastasis was defined as recurrence in distant nodal basins, peritoneal seeding, liver metastasis, or any metastasis in other extra-abdominal sites. The Kaplan-Meier method was used to estimate OS, RFS, LRRFS, and DMFS, while differences were determined using the log-rank test. Univariable and multivariable Cox regression analysis was used to determine independent clinical factors impacting RFS. A Cox proportional hazards model was used to identify hazard ratios for failure patterns between two groups. All statistical analyses were performed using SPSS® v. 23.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). All p-values were two-sided, and a p-value of <0.05 was considered statistically significant.
Adjuvant therapy
We included all post-curative resection patients who had received AT including chemotherapy or chemotherapy with concurrent radiotherapy. AT is routinely initiated within 3 to 8 weeks after gastrectomy, when the patient's clinical condition is stable. Initiation of AT will be delayed due to prolonged postoperative recovery time, the waiting time for post-gastrectomy examinations, or upon the patient's request. The main chemotherapeutic agent for gastric cancer is 5 Fluorouracil (5-FU). Many types of 5-FU based chemotherapy regimens were used in either hospital, depending on time, running clinical trials, tumor staging, patient tolerance, and/or physician's preference. These combinations can be classified into two major categories based on route of administration: (1) intravenous (IV) fluorouracil-based or fluorouracil plus cisplatin-based regimens, (2) oral 5-FU prodrugs including Tegafur and Uracil (UFUR), TS-1 as part of a clinical trials 2, 18, and capecitabine plus oxaliplatin (XELOX) as part of a previous clinical trial 4. Doses and regimens were adjusted before and during treatment depending on patient tolerance. Chemotherapy is usually ceased temporarily when grade I or II leukopenia occurs.
Radiotherapy was delivered to patients with higher risk of recurrence including those with an advanced stage gastric cancer or in the presence of multiple unfavorable pathologic factors including narrow surgical margins, lymphovascular invasion and extra-capsular spread of lymph nodes to name a few. We applied photons from a 6 or 10 MV linear accelerator using conventional anterioposterior opposing fields, 3-dimensional conformal radiotherapy, intensive modulation radiotherapy, or volumetric modulated arc therapy. The radiotherapy field included the tumor bed, remnant stomach, duodenal stump, anastomosis site and regional lymph nodes. Nodal stations were contoured depending on location and stage of the tumor, including perigastric, celiac, splenic, splenic hilum (optional), porta-hepatic, perioesophageal, and pancreaticoduodenal nodal stations. The median prescribed dose was 45 Gy (range, 23.4- 60 Gy) with 1.8-Gy daily fractions administered over 5-6 weeks.
Post-therapy surveillance
According to the surveillance protocol used in our institution, follow-up clinic appointments were arranged every 3 months during the first 2 years, every 4-6 months during the third and fourth years, and every 6-12 months thereafter; imaging was performed at specific intervals: chest radiography every 3 months, computed tomography every 3 to 6 months, and panendoscopy every 3 to 6 months or when symptoms indicating recurrence were noted.
Complications and Toxicity events
The post-operative condition of patients between gastrectomy and the initiation of AT was evaluated using the Clavien-Dindo classification 19, 20, which is widely used to grade the adverse events (i.e. complications) of surgical procedures. After their recovery and initiation of AT, toxic events during the AT course were collected according to Common Terminology Criteria for Adverse Events (CTCAE) 3.0. 21
Results
Patient characteristics
We enrolled 538 patients diagnosed with gastric adenocarcinoma at two tertiary hospitals affiliated with Chang-Gung Memorial Hospital (the Linkuo and Keelung branches) between January 1, 2006 and December 31, 2013. Of these patients, 393 (73%) started AT within 8 weeks after definitive gastrectomy (the early group), while 145 (27%) commenced AT later than 8 weeks after gastrectomy (the late group). The baseline clinicopathological characteristics between the two groups were statistically uneven, as the proportion of advanced gastric cancer cases seemed to be higher in the late group with higher pathologic nodule stages (N3: 50.3% vs. 40.5%; p=0.040) and a more advanced T stage with near statistically significance (T4: 55.1% vs. 46.1%; p=0.060). Furthermore, a significantly higher number of early-group patients underwent total gastrectomy (66.9% vs. 57.2%; p=0.038).
Among the 538 patients, 143 from each group were matched after 1:1 propensity score matching. The patients' characteristics before and after matching are shown in Table 1. None of the covariates were significantly different between the groups (p>0.05) after matching. In the early group, the median interval between gastrectomy and AT was 6 weeks (25-75%, 4.0 to 7.0 weeks), while the corresponding median interval in the late group was 11 weeks (25-75%, 9.0 to 14.0 weeks). The detailed chemotherapy regimens are presented in Table 2. Around 60% of the patients received oral 5-FU based regimens at both early and late groups, of which, more than half were prescribed UFUR. Among IV 5-FU based chemotherapy, most of the patient received 5-FU plus leucovorin as their adjuvant treatment.
Survival and recurrence
Before matching, the median follow-up time for the entire cohort was 42.4 months. The 5-year OS rates were 52.7% and 40.3% in the early and late groups (p=0.029), with 5-year RFS rates of 51.9% and 47.8%, respectively (p=0.124). The 5-year LRRFS rates were 63.1% in the early (≤8 weeks) and 58.0% in the late group (>8 weeks) (p=0.159), and the DMFS rates were 58.5% and 52.1%, respectively (p=0.132).
Patient characteristics before and after matching
| Before matching | After matching | ||||||
|---|---|---|---|---|---|---|---|
| ≤8 weeks (%) | >8 weeks (%) | P-value | ≤8 weeks (%) | >8 weeks (%) | P-value | ||
| Sex | 0.783 | 0.623 | |||||
| Male | 249 (63.4) | 90 (62.1) | 93 (65.0) | 89 (62.2) | |||
| Female | 144 (36.6) | 55 (37.9) | 50 (35.0) | 54 (37.8) | |||
| ECOG 0-1 | 387 (98.5) | 141 (97.2) | 0.348 | 141 (98.6) | 139 (97.2) | 0.409 | |
| ECOG ≥2 | 6 (1.5) | 4 (2.8) | 2 (1.4) | 4 (2.8) | |||
| Median age (25%-75%) | 61 (50-72) | 64 (53.5-71.5) | 0.154 | 60 (50-71) | 63 (53-71) | 0.100 | |
| pT stage | 0.060 | 0.694 | |||||
| T1-3 | 212 (53.9) | 65 (44.8) | 72 (50.3) | 65 (45.6) | |||
| T4 | 181 (46.1) | 80 (55.2) | 71 (49.7) | 78 (54.4) | |||
| pN Stage | 0.040* | 0.154 | |||||
| N0-2 | 234 (59.5) | 72 (49.7) | 84 (58.7) | 72 (50.3) | |||
| N3 | 159 (40.5) | 73 (50.3) | 59 (41.3) | 71 (49.7) | |||
| Differentiation | 0.832 | 0.802 | |||||
| Well-moderate | 129 (32.8) | 49 (33.8) | 47 (32.8) | 49 (34.2) | |||
| Poor or others | 264 (67.2) | 96 (66.2) | 96 (67.2) | 94 (65.8) | |||
| Gastrectomy | 0.038* | 0.224 | |||||
| Total | 263 (66.9) | 83 (57.2) | 93 (65.0) | 83 (58.0) | |||
| Partial | 130 (33.1) | 62 (42.8) | 50 (35.0) | 60 (42.0) | |||
| LND type | 0.611 | 0.866 | |||||
| D1 and 15+ LND | 64 (16.3) | 21 (14.5) | 20 (14.0) | 21 (14.7) | |||
| D2 | 329 (83.7) | 124 (85.5) | 123 (86.0) | 122 (85.3) | |||
| Median LN number (25%-75%) | 35 (25-45) | 37 (28-53) | 34 (24-44) | 36 (28-53) | |||
| > 35 | 192 (48.9) | 77 (53.1) | 0.382 | 65 (45.5) | 75 (52.4) | 0.237 | |
| ≤ 35 | 201 (51.1) | 68 (46.9) | 78 (54.5) | 68 (47.6) | |||
| Median LN ratio (25%-75%) | 0.12 (0.04-0.32) | 0.17 (0.06-0.36) | 0.323 | 0.13 (0.03-0.29) | 0.17 (0.05-0.35) | 0.498 | |
| Pathologic factors | |||||||
| VI (-) | 283 (72.0) | 116 (80.0) | 0.060 | 101 (70.6) | 114 (79.7) | 0.075 | |
| VI (+) | 110 (28.0) | 29 (20.0) | 42 (29.4) | 29 (20.3) | |||
| LI (-) | 123 (31.3) | 37 (25.5) | 0.193 | 43 (30.1) | 37 (25.9) | 0.429 | |
| LI (+) | 270 (68.7) | 108 (74.5) | 100 (69.9) | 106 (74.1) | |||
| PNI (-) | 166 (42.2) | 64 (44.1) | 0.693 | 62 (43.4) | 62 (43.3) | 1.000 | |
| PNI (+) | 227 (57.8) | 81 (55.9) | 81 (56.6) | 81 (56.7) | |||
| Laurens-intestine | 177 (45.0) | 61 (42.1) | 0.387 | 65 (45.5) | 61 (42.7) | 0.188 | |
| Laurens-diffuse | 152 (38.7) | 65 (44.8) | 50 (35.0) | 63 (44.1) | |||
| Laurens-mixed | 64 (16.3) | 19 (13.1) | 28 (19.5) | 19 (13.3) | |||
| Adjuvant regimen | 0.545 | 0.845 | |||||
| Chemotherapy alone | 359 (91.3) | 130 (89.6) | 129 (90.2) | 128 (89.5) | |||
| CCRT | 34 (8.7) | 15 (10.4) | 14 (9.8) | 15 (10.5) | |||
ECOG, Eastern Cooperative Oncology Group; pT stage, pathologic T stage; pN stage, pathologic N stage; LND, lymph node dissection; LN, lymph node; VI, vascular invasion; LI, lymphatic invasion; PNI, perineural invasion; CCRT, concurrent chemoradiotherapy. *: p value significant.
After matching, the median follow-up time was 68.9 months, the 5-year OS rates were 56.6% and 40.2% in the early and late groups (HR 0.745, 95% CI 0.546 - 1.016, p=0.062), while the corresponding RFS rates were 57.6% and 46.4%, respectively (HR 0.683, 95% CI 0.485 - 0.962, p=0.028). Survival curves are shown in Figure 1a and Figure 1b.
Detailed chemotherapy regimens
| Total (n=286) | ≤8 weeks (n=143) | >8 weeks (n=143) | |
|---|---|---|---|
| Oral 5-FU prodrugs (%) | |||
| UFUR | 126 | 70 (48.9) | 56 (39.1) |
| TS-1 | 32 | 10 (7.0) | 22 (15.4) |
| XELOX | 32 | 10 (7.0) | 22 (15.4) |
| total | 190 | 90 (62.9) | 100 (69.9) |
| IV 5-FU based regimens (%) | |||
| 5-FU + LV | 68 | 37 (25.9) | 31 (21.7) |
| 5-FU + LV + cisplatin based | 28 | 16 (11.2) | 12 (8.4) |
| total | 96 | 53 (37.1) | 43 (30.1) |
5-FU, 5-fluorouracil; UFUR, Tegafur and Uracil; XELOX, capecitabine + oxaliplatin; LV, leucovorin.
The first site(s) of recurrence after AT are presented in Table 2. During the follow-up period, 57 experienced recurrence in the early group, while recurrence was significantly more common in the late group, and occurred in 78 patients (HR 0.700, 95% CI 0.497-0.988, p=0.042). The 5-year LRRFS rates in the early and late groups were 66.3% and 57.4% (HR 0.716, 95% CI 0.482-1.064, p=0.097), while the corresponding 5-year DMFS rates were 64.4% and 52.0%, respectively (HR 0.682, 95% CI 0.472-0.985, p=0.040). Survival curves are shown in Figure 1c and Figure 1d. Both locoregional recurrences and distant metastases were more frequent in the late group, whereas recurrences at distant metastatic sites were significantly lower in the early group.
As shown in Table 4, univariate analyses for RFS showed that early pathologic stage (stage II), chemotherapy interval within 8 weeks, and the absence of pathologic factors including vascular invasion, lymphatic invasion, and perineural invasion were associated with higher RFS. Multivariate analyses showed that pathologic stage II (HR 0.310, 95% CI 0.168-0.571, p<0.001), chemotherapy interval within 8 weeks (HR 0.685, 95% CI 0.478-0.982, p=0.039), and no lymphatic invasion (HR 0.564, 95% CI 0.328-0.968, p=0.038) were independent prognostic factors for RFS. Chemotherapy regimens, whether IVF or oral 5-FU based, did not affect RFS (univariate HR 0.903, 95% CI 0.633-1.287, p= 0.573; multivariate HR 0.901, 95% CI 0.614 - 1.157, p=0.595).
Overall survival (OS), recurrence-free survival (RFS), locoregional recurrence-free survival (LRRFS) and distance metastasis-free survival (DMFS) rates after propensity score matching. (a) The 5-year OS rates were 56.6% and 40.2% in early (≤8 weeks) and late (>8 weeks) groups (p=0.062), with (b) the 5-year RFS rates of 57.6% and 46.4%, respectively (p=0.028), and (c) the 5-year LRRFS rates were 66.3% and 57.4%, respectively (p=0.097). (d) The 5-year DMFS rates were 64.4% and 52.0%, respectively (p=0.040). AT = adjuvant therapy
The site of recurrence in patients who underwent adjuvant chemotherapy post-curative gastrectomy.
| Site | ≤ 8 weeks | >8 weeks | Hazard Ratio for relapse in the early group (95% CI) | P value |
|---|---|---|---|---|
| No of patients (%) | ||||
| Total no. of relapses | 57 (39.9) | 78 (54.5) | 0.683 (0.485 - 0.962) | 0.028 |
| Locoregional | 43 (30.0) | 57 (39.9) | 0.716 (0.482 - 1.064) | 0.097 |
| Distant | 49 (34.3) | 68 (47.6) | 0.682 (0.472 - 0.985) | 0.040 |
Treatment complications
The Clavien-Dindo classification for two groups of patients is presented in Table 5. In both groups, up to 70% of the patients had a normal recovery after gastrectomy, and the complications in more than 90% of patient were classified as less than grade II. However, 5 patients from the late group required intensive care for their surgical complications. The toxicities during AT are presented in Table 6. Toxicities stronger than grade 3 were observed in less than 10% of the patients in either of the two groups, regardless of early or late initiation of AT, although we observed more toxic events in the IV 5-FU group, especially leukopenia, thrombocytopenia and gastrointestinal symptoms.
Discussion
Early initiation of AT has been shown to improve prognosis in certain types of cancer. Randomized trials and meta-analyses of patients with breast cancer or rectal adenocarcinoma have shown that early initiation of AT can produce better outcomes22-24. In particular, a meta-analysis of patients with colon cancer found that AT administered within 8 weeks postoperatively resulted in better survival outcomes than when it was started later 25. For gastric cancer, there also were some retrospective studies showing that early initiation of AT can improve OS, especially when started within 8 weeks 7, 8.
Univariate and Multivariate analysis of RFS
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR(95% CI) | P | HR(95% CI) | P | |
| Age | ||||
| ≥65 | 0.895(0.633-1.266) | 0.531 | 0.848(0.572-1.258) | 0.413 |
| <65 | ||||
| Sex | ||||
| Female | 0.800(0.558-1.147) | 0.223 | 0.815(0.563-1.180) | 0.279 |
| Male | ||||
| pStage | ||||
| II | 0.214(0.130-0.354) | <0.001* | 0.310(0.168-0.571) | <0.001* |
| III | ||||
| Gastrectomy type | ||||
| Total | 0.806(0.570-1.140) | 0.224 | 0.949(0.656-1.372) | 0.781 |
| Partial | ||||
| LND type | ||||
| D2 | 0.932(0.567-1.532) | 0.782 | 0.804(0.470-1.373) | 0.424 |
| D1 with ≥15 nodes | ||||
| Differential type | ||||
| Well to moderate | 0.743(0.513-1.076) | 0.115 | 0.984(0.563-1.719) | 0.954 |
| Poor | ||||
| LN dissection number | ||||
| >35 | 0.885(0.631-1.241) | 0.478 | 0.835(0.590-1.181) | 0.308 |
| ≤35 | ||||
| Chemotherapy interval | ||||
| ≤8 weeks | 0.683(0.485-0.962) | 0.028* | 0.685(0.478-0.982) | 0.039* |
| >8 weeks | ||||
| Chemotherapy form | ||||
| Oral 5-FU form | 0.903(0.633-1.287) | 0.573 | 0.901(0.614-1.322) | 0.595 |
| IV 5-FU form | ||||
| Laurens classification | ||||
| Intestine | 0.782(0.554-1.104) | 0.163 | 0.691(0.413-1.157) | 0.160 |
| Diffuse or mixed | ||||
| Pathologic factors | ||||
| VI(-) | 0.615(0.424-0.892) | 0.010* | 0.908(0.601-1.372) | 0.647 |
| VI(+) | ||||
| LI(-) | 0.301(0.187-0.485) | <0.001* | 0.564(0.328-0.968) | 0.038* |
| LI(+) | ||||
| PNI(-) | 0.396(0.273-0.576) | <0.001* | 0.660(0.427-1.020) | 0.061 |
| PNI(+) | ||||
pStage, pathologic stage; LND, lymph node dissection; LN, lymph node; VI, vascular invasion; LI, lymphatic invasion; PNI, perineural invasion. *: p value significant.
Clavien-Dindo classification of post-gastrectomy patients
| ≤8 weeks, n (%) | >8 weeks, n (%) | |
|---|---|---|
| Grade 0-I | 110 (76.9) | 103 (72.0) |
| Grade II | 25 (17.5) | 26 (18.2) |
| Grade III | 8 (5.6) | 9 (6.3) |
| Grade IV | 0 (0) | 5 (3.5) |
| Grade V | 0 (0) | 0 (0) |
Toxicities during adjuvant therapy.
| ≤8 weeks | >8 weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Oral 5-FU (%) | IV 5-FU (%) | **P value | Total (%) | Oral 5-FU (%) | IV 5-FU (%) | **P value | Total (%) | *P value | |
| Patient number | 90 | 53 | 143 | 100 | 43 | 143 | |||
| Leukopenia | <0.001 | <0.001 | 0.719 | ||||||
| Gr 1-2 | 18 (20.0) | 21 (39.6) | 39 (27.3) | 15 (15.0) | 17 (39.5) | 32 (22.4) | |||
| Gr >3 | 1 (1.1) | 8 (15.1) | 9 (6.3) | 1 (1.0) | 5 (11.6) | 6 (4.2) | |||
| Thrombocytopenia | <0.001 | 0.005 | 0.591 | ||||||
| Gr 1-2 | 16 (17.8) | 29 (54.7) | 45 (31.5) | 28 (28.0) | 22 (51.2) | 50 (35) | |||
| Gr >3 | 1 (1.1) | 2 (3.8) | 3 (2.1) | 3 (3.0) | 2 (4.7) | 5 (3.5) | |||
| Elevated AST level | 0.525 | 0.478 | 0.414 | ||||||
| Gr 1-2 | 17 (18.9) | 12 (22.6) | 29 (20.3) | 22 (22.0) | 6 (14.0) | 28 (19.6) | |||
| Gr >3 | 1 (1.1) | 1 (1.9) | 2 (1.4) | 2 (2.0) | 2 (4.7) | 4 (2.8) | |||
| Elevated ALT level | 0.428 | 0.233 | 0.872 | ||||||
| Gr 1-2 | 10 (11.1) | 7 (13.2) | 19 (13.3) | 14 (14.0) | 10 (23.3) | 24 (16.8) | |||
| Gr >3 | 1 (1.1) | 2 (3.8) | 1 (0.7) | 1 (1.0) | 0 | 1 (0.7) | |||
| Elevated total serum bilirubin level | - | 0.175 | 0.880 | ||||||
| Gr 1-2 | 3 (3.3) | 0 | 3 (2.1) | 10 (10.0) | 1 (2.3) | 11 (7.7) | |||
| Gr >3 | 1 (1.1) | 0 | 1 (0.7) | 2 (2.0) | 1 (2.3) | 3 (2.1) | |||
| Elevated creatinine level | 0.363 | 0.617 | - | ||||||
| Gr 1-2 | 7 (7.7) | 8 (15.1) | 15 (10.5) | 11 (11.0) | 6 (14.0) | 17 (11.9) | |||
| Gr >3 | 2 (2.2) | 0 | 2 (1.4) | 0 | 0 | 0 (0) | |||
| Diarrhea | 0.002 | 0.001 | 0.900 | ||||||
| Gr 1-2 | 17 (18.7) | 20 (37.7) | 37 (25.9) | 21 (21.0) | 19 (44.2) | 40 (28) | |||
| Gr >3 | 2 (2.2) | 4 (7.5) | 6 (4.2) | 3 (3.0) | 3 (7.0) | 6 (4.2) | |||
| Nausea | <0.001 | 0.001 | 0.514 | ||||||
| Gr 1-2 | 34 (37.8) | 44 (83.0) | 78 (54.6) | 42 (42.0) | 29 (67.4) | 71 (49.7) | |||
| Gr >3 | 1 (1.1) | 0 | 1 (0.7) | 1 (1.0) | 1 (2.3) | 2 (1.4) | |||
| Vomiting | 0.004 | 0.016 | 0.573 | ||||||
| Gr 1-2 | 19 (21.1) | 26 (49.1) | 45 (31.5) | 23 (23.0) | 17 (42.1) | 40 (28) | |||
| Gr >3 | 1 (1.1) | 1 (1.9) | 2 (1.4) | 1 (1.0) | 2 (4.7) | 3 (2.1) | |||
| Fatigue | <0.001 | 0.001 | 0.321 | ||||||
| Gr 1-2 | 23 (25.6) | 41 (77.4) | 64 (44.8) | 39 (39.0) | 31 (72.1) | 70 (49) | |||
| Gr >3 | 0 | 2 (3.8) | 2 (1.4) | 3 (3.0) | 2 (4.7) | 5 (3.5) | |||
| Febrile neutropenia | 0.053 | <0.001 | 1.000 | ||||||
| Gr 3-4 | 1 (1.1) | 4 (7.5) | 5 (3.5) | 0 | 5 (11.6) | 5 (3.5) | |||
| Gr 5 | 1 (1.1) | 1 (1.9) | 2 (1.4) | 1 (1.0) | 1 (2.3) | 2 (1.4) | |||
*: p value: difference between the early and late groups for any toxic event.
**: p value: difference between the oral and IV 5-FU groups for any toxic event.
However, the conclusions from other studies were somehow contradictory, with no obvious benefit on survival or disease control attributed to early AT 9, 26. The limitation of those retrospective studies included a small sample size that may have been inadequate for statistical analysis or an uneven distribution with multiple unmatched patient characteristics. Such factors may have resulted in a biased study. This study highlights that propensity score matching is a feasible method to obtain appropriate results considering retrospective data. In addition, this study revealed that the interval of adjuvant treatment within 8 weeks was one of the major determinants for RFS.
In our pre-matching data, the baseline characteristics between groups were different, and the pre-matched patient number in both group was uneven, with a 3 to 1 ratio in favor of early group, which could produce a statistical bias. After 1:1 matching that equalized the patients' baseline characteristics and sample size, we observed a significant benefit in RFS for patients who commenced AT within 8 weeks post-gastrectomy, whereas the OS benefit was just below the level of significance. Moreover, the DMFS between both groups was more favorable in the early group. The results were totally different from those obtained before matching. When analyzing the clinical factors affecting RFS, we found that early pathologic stage, chemotherapy interval within 8 weeks, and no vascular/lymphatic invasion and perineural invasion resulted in better RFS, and chemotherapy interval remained one of the independent factors affecting RFS after multivariable analysis. These data suggested that initiating AT within 8 weeks post-gastrectomy improves disease control, which in turn produces a positive OS trend.
The preference on early initiation of AT is based on the hypothesis that residual tumor cells will keep proliferating after gastrectomy if no AT is administered, thereby increasing the failure rate because of locoregional and distant metastases. The earlier the AT was applied, the better the reported control rate was. This has already been confirmed in systematic reviews and meta-analysis for other types of cancer such as breast and rectal cancer 23, 24. However, till date, no study, to our knowledge, has analyzed the association between the interval of AT and disease control including LRRFS and DMFS for gastric cancer. In our study, 5-year LRRFS and DMFS rates in the early group were 66.3% and 64.4%, respectively, and were comparable to a previous clinical trial, where AT was initiated within 3-7 weeks after en bloc gastrectomy 27. En bloc gastrectomy removes all the potential locoregional sites of metastasis, but leaves the possible occult micrometastasis at distant sites. Therefore, the major acting site of AT would be located at distant micrometastasis, where gastrectomy cannot approach properly. This is compatible with our results of a significant benefit in DMFS, which showed a significant 30% reduction, but not LRRFS. Moreover, the benefits of AT at distant sites were large and contributed to a significantly better RFS, favoring early AT. This indicates that early initiation of AT for stage II or III gastric cancer might be more effective and timely to eradicate micrometastasis at distant sites.
Although the patients were evenly distributed between the groups considering the baseline characteristics after propensity score matching, there were still some potential factors that could have biased our survival analysis. Post-operative complications and recovery, other than tumor recurrence, were an inevitable bias between the early and late groups. Clinically, late initiation of AT can be caused by several reasons, mainly gastrectomy related, including prolonged recovery after gastrectomy, surgical complications, and poor nutritional intake. Postoperative infections are also important negative prognostic factors 28, 29. These factors could interfere with the analyses for overall survival or disease control rate by causing non-cancer related death or weakening the AT tolerance, increasing treatment toxicities and early AT cessation. Through our data, although we observed a trend in OS favoring early AT, we also noticed that there were more cases of prolonged recovery period or worse surgical complications in the late group. Particularly, five patients needed intensive care after gastrectomy, thereby delaying their AT. Thus, we deducted that worse post-operative general condition could potentially lead to early death, although in the case of these 5 patients, they received and tolerated AT after recovery. This is an inevitable bias when evaluating the initiation time for adjuvant treatment.
However, delayed post-operative recovery did not seem to affect the adverse events due to AT, as the rate of such events were similar between early and late group. Hence, the duration of the recovery time does not affect patient tolerance and response to treatment. Nonetheless, although there were no differences in OS or RFS between oral or IV 5-FU based chemotherapy regimens, we observed that the toxic events were more common and severe in patients who had received IV 5-FU based chemotherapy compared with those who had been prescribed oral 5-FU prodrugs, especially they experienced lower white blood cell and platelet counts and more severe gastrointestinal symptoms. The toxicity profiles we observed in this study were comparable to those reported in previous clinical trials 2, 6, where oral 5-FU prodrugs also had resulted in less toxicity than the IV form 5-FU based chemotherapy. As reported in several clinical trials and meta-analyses of patients who were administered similar IV 5-FU regimens we used 4, 30, 31, grade 3-4 toxicities were as high as 40%, and even reached 56% when combined with radiotherapy; neutropenia was the most common adverse event. Overall, we suggest that patients should start their AT within 8 weeks since the early or late initiation of AT does not affect the toxicities following treatment. Nonetheless, patients who will undergo IV 5-FU based AT should be closely monitored to reduce the severity of toxicities.
Our study had several limitations. First, the retrospective nature of the study may have introduced a selection bias. Proper evaluation of patients' conditions based on retrospectively collected data is challenging. Second, the duration of AT treatment and types of regimens used at either participating institution, which may be key determinants of survival, were not evaluated in detail because of their complexity and diversity in our cohort. A recent S-1 study showed that the duration of AT treatment affected patient outcomes 10. Moreover, to validate our results, randomized trials are inevitably needed for higher-level evidence. However, conducting randomized trials is difficult in reality owing to unstable and diverse clinical circumstances of post-gastrectomy patients and the ethical concern of intentionally delaying AT. In our study, we collected all available data and applied propensity score matching to address the issue of distribution variability/bias between the two groups. This sample size limitation also implied that it was difficult to independently split data into training and validation sets. Based on this reasoning, we did not include the validation process in this study. But further studies to validate our main finding are still needed in the future. However, our results ought to be clinically applicable because we not only investigated a relatively homogenous group of patients with stage II- III gastric cancer but also included patients receiving chemotherapy or chemotherapy and concurrent radiotherapy. The latter is considered a standard adjuvant therapy for stage II or III gastric cancer in the US 32. To the best of our knowledge, our study is the first to evaluate AT timing for gastric cancer after propensity score matching. Given the nature of our study, propensity score matching appears to be a sound method for addressing the question of optimal interval prior to initiating AT.
Conclusion
Clinicians should consider starting AT within 8 weeks after curative gastrectomy. A delay in AT of longer than 8 weeks was found to be associated with higher DMFS and RFS in most patients and might impact survival.
Acknowledgements
This study was supported by Chang Gung Memorial Hospital Research: Grant No. CMRPG3H0471 and CIRPD1D0071-73. The authors would like to thank anonymous reviewers and the editor for their comments, and all the members of the Cancer Center, Chang Gung Memorial Hospital, for their invaluable help.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ferlay J, Soerjomataram I, Dikshit R. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136(5):E359-E386
2. Sakuramoto S, Sasako M, Yamaguchi T. et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. New England Journal of Medicine. 2007;357:1810-20
3. Sasako M, Sakuramoto S, Katai H. et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. Journal of Clinical Oncology. 2011;29:4387-93
4. Bang Y-J, Kim Y-W, Yang H-K. et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. The Lancet. 2012;379:315-21
5. Noh SH, Park SR, Yang H-K. et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. The Lancet Oncology. 2014;15:1389-96
6. Macdonald JS, Smalley SR, Benedetti J. et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. New England Journal of Medicine. 2001;345:725-30
7. Kang SY, Ahn MS, Song GW. et al. Does the timing of adjuvant chemotherapy for gastric cancer influence patient outcome? Acta Oncol. 2015;54:1231-34
8. Park HS, Jung M, Kim HS. et al. Proper timing of adjuvant chemotherapy affects survival in patients with stage 2 and 3 gastric cancer. Ann Surg Oncol. 2015;22(1):224-31
9. Greenleaf EK, Kulaylat AN, Hollenbeak CS, Almhanna K, Wong J. Timing of Adjuvant Chemotherapy and Impact on Survival for Resected Gastric Cancer. Ann Surg Oncol. 2016;23:4203-13
10. Fujitani K, Kurokawa Y, Takeno A. et al. Time to initiation or duration of S-1 adjuvant chemotherapy; which really impacts on survival in stage II and III gastric cancer? Gastric Cancer. 2018;21:446-52
11. Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. The review of economics and statistics. 2002;84(1):151-61
12. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology. 2010;17(6):1471-74
13. Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph Node Staging in Gastric Cancer: Is Location More Important Than Number?: An Analysis of 1,038 Patients. Annals of surgery. 2000;232:62
14. Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Annals of surgical oncology. 2007;14:17
15. Songun I, Putter H, Kranenbarg EM-K, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. The lancet oncology. 2010;11(5):439-449
16. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. American journal of epidemiology. 2006;163:1149-56
17. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical statistics. 2011;10:150-61
18. Nakajima T, Kinoshita T, Nashimoto A. et al. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. British journal of surgery. 2007;94:1468-76
19. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of surgery. 2004;240:205
20. Clavien PA, Barkun J, de Oliveira ML. et al. The Clavien-Dindo classification of surgical complications: five-year experience. Annals of surgery. 2009;250:187-96
21. Trotti A, Colevas AD, Setser A. et al. CTCAE v3. 0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in radiation oncology. 2003;13:176-81
22. Shannon C, Ashley S, Smith I. Does timing of adjuvant chemotherapy for early breast cancer influence survival? Journal of clinical oncology. 2003;21(20):3792-97
23. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335-42
24. Yu K-D, Huang S, Zhang J-X, Liu G-Y, Shao Z-M. Association between delayed initiation of adjuvant CMF or anthracycline-based chemotherapy and survival in breast cancer: a systematic review and meta-analysis. BMC cancer. 2013;13:240
25. Des Guetz G, Nicolas P, Perret G-Y, Morere J-F, Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. European journal of cancer. 2010;46(6):1049-55
26. Ahmed S, Iqbal N, Yadav S. et al. Time to adjuvant therapy and other variables in localized gastric and gastroesophageal junction (GEJ) cancer (IJGC-D-13-00162). Journal of gastrointestinal cancer. 2014;45:284-90
27. Kim TH, Park SR, Ryu KW. et al. Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III-IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. International Journal of Radiation Oncology* Biology* Physics. 2012;84:e585-e592
28. Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Annals of surgical oncology. 2013;20:1575-83
29. Kubota T, Hiki N, Sano T. et al. Prognostic significance of complications after curative surgery for gastric cancer. Annals of surgical oncology. 2014;21:891-98
30. Zhou ML, Kang M, Li GC, Guo XM, Zhang Z. Postoperative chemoradiotherapy versus chemotherapy for R0 resected gastric cancer with D2 lymph node dissection: an up-to-date meta-analysis. World J Surg Oncol. 2016;14(1):209
31. Lee J, Lim DH, Kim S. et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. Journal of clinical oncology. 2011;30:268-73
32. Ajani JA, Bentrem DJ, Besh S. et al. Gastric cancer, version 2.2013: Featured updates to the NCCN Guidelines. Journal of the National Comprehensive Cancer Network. 2013;11:531-46
Author contact
![]() Corresponding author: Bing-Shen Huang, Address: No. 5, Fuxing Street, Guishan District, Taoyuan City, 333. Phone: 886-3-3281200; Fax: 886-3-3280797; Email: beanson.twcom
Corresponding author: Bing-Shen Huang, Address: No. 5, Fuxing Street, Guishan District, Taoyuan City, 333. Phone: 886-3-3281200; Fax: 886-3-3280797; Email: beanson.twcom

 Global reach, higher impact
Global reach, higher impact