3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(5):1189-1196. doi:10.7150/jca.28994 This issue Cite
Research Paper
Transarterial Chemoembolization (TACE) Combined with Sorafenib versus TACE Alone for Unresectable Hepatocellular Carcinoma: A Propensity Score Matching Study
1. Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou 215006, China
2. Department of Interventional Radiology, The Second People's Hospital of Changzhou, Changzhou 213000, China
* These authors contributed equally to this work
Received 2018-8-4; Accepted 2019-1-4; Published 2019-1-29
Abstract
Objective: To compare the outcomes of transarterial chemoembolization (TACE) combined with sorafenib versus TACE alone for treating patients with unresectable hepatocellular carcinoma (HCC).
Methods: This retrospective analysis included all patients receiving either TACE plus sorafenib therapy or TACE alone for unresectable HCC between February 2008 and August 2015 at the First Affiliated Hospital of Soochow University, China. Propensity score matching (PSM) was carried out to reduce bias due to confounding variables. The primary outcome was overall survival (OS), calculated from the date of the first TACE treatment until the date of death of any cause. A multivariate Cox proportional hazards analysis was conducted to examine determinants of OS.
Results: A total of 308 patients were included in the study: 61 receiving TACE plus sorafenib treatment and 247 receiving TACE monotherapy. The PSM cohort included 61 subjects receiving TACE plus sorafenib and 122 subjects receiving TACE alone. In the overall analysis that included all patients, the median OS in the combination group was significantly longer than that in the monotherapy group (29.0 ± 7.2 vs. 14.9 ± 1.1 months; P = 0.008). In the PCM cohort, the median OS was also significantly longer in the combination group (29.0 ± 7.2 vs. 14.9 ± 1.5 months; P = 0.018). Subgroup analysis revealed longer OS in patients receiving combination treatment in both the BCLC-B and BCLC-C subgroups (P < 0.05 for both). Multivariate analyses in the PSM cohort revealed that treatment methods (P = 0.003), number of nodules (P = 0.010), tumor size (P = 0.012), vascular invasion (P = 0.005), and number of TACE (P = 0.029) were independent prognostic factors of OS. The most common adverse events were hand-foot skin reaction (75.4%) and diarrhea (47.5%) in the combination group, and fatigue (19.0%) and liver dysfunction (18.2%) in the monotherapy group. There were no treatment-related deaths in either group.
Conclusion: The combined use of TACE and sorafenib is generally well tolerated and could significantly increase OS of patients with unresectable HCC.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, sorafenib, survival, propensity score
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related deaths worldwide [1]. More than 80% of HCC cases occur in sub-Saharan Africa and Eastern Asia, and China accounts for approximately 50% of all cases [2]. Curative treatments for HCC include surgical resection, liver transplantation, or local ablation therapy for early HCC [3]. However, many patients are diagnosed at an advanced stage of HCC and, therefore, are not candidates for these radical treatment options at the time of diagnosis [4].
Transarterial chemoembolization (TACE) is a major treatment modality for unresectable HCC. The Barcelona Clinic Liver Cancer (BCLC) staging system recommends TACE as a standard treatment for stage B HCC [5]. TACE is also used as an important locoregional treatment for patients with advanced HCC, including those with vascular invasion or metastases [6, 7]. TACE exerts its effects by administering intra-arterial cytotoxic chemotherapeutic agents, followed by embolization, creating vascular occlusion and inducing ischemia and necrosis of the HCC lesion [8]. Two randomized controlled trials (RCTs) have confirmed that TACE improved survival of unresectable HCC patients compared to symptomatic treatment [9, 10].
Sorafenib is currently the only first-line multi-kinase inhibitor approved for use in HCC [11]. Sorafenib inhibits the activity of the Raf serine-threonine kinase and receptor tyrosine kinases, including vascular endothelial growth factor receptor (VEGFR)-1/2/3, platelet-derived growth factor receptor-beta (PDGFR-β), c-KIT, FLT-3, and RET [11, 12]. These kinases are involved in tumor cell signaling, proliferation, angiogenesis and apoptosis. Thus, sorafenib could inhibit tumor growth and neo-angiogenesis. RCTs have shown that it is efficacious for prolonging time to progression (TTP) and median overall survival (OS) of patients with advanced HCC [13, 14].
The combination of TACE with sorafenib is appealing based on a strong scientific rationale. In fact, TACE causes hypoxia within the tumor, resulting in local and systemic increases in VEGF, which may facilitate disease progression and metastasis [15, 16], whereas sorafenib can inhibit the activity of VEGF receptors; thus, the combination therapy may have synergistic effect. Indeed, several prospective studies have shown the safety and potential survival benefits of the use of this combination therapy in patients with unresectable HCC [17-19]. However, a phase III study and a meta-analysis did not find an improvement in OS with the combined therapy [20, 21]. Therefore, whether the combination of TACE and sorafenib in patients with unresectable HCC could improve the survival outcomes remain controversial.
The purpose of this stud was to compare OS of unresectable HCC treated with TACE combined with sorafenib versus TACE monotherapy using propensity score matching (PSM). In addition, we further explored predictors of OS.
Materials and Methods
The study design and patient population
The current retrospective study included all adult patients (18 years or older) with unresectable HCC who were admitted to our department from February 2008 to August 2015. HCC was diagnosed by the practice guidelines of the American Association for the Study of Liver Disease [22].
Patients included in our study met the following criteria: 1) patients were treated with TACE alone or TACE combined with sorafenib instead of any other interventional procedures such as radio-frequency ablation, microwave ablation, iodine-125 seed implantation or percutaneous ethanol injection; 2) Child-Pugh class A or B; 3) Eastern Cooperative Oncology Group performance status (ECOG PS) score of no more than 2; 4) sorafenib treatment at least 5 weeks; 5) patients showed disease progression despite previous surgical treatment. Patients were excluded if they had any of the following reasons: 1) sorafenib was discontinued or the interval between sorafenib administration and initial TACE procedure lasted for more than one month; 2) patients with secondary malignancy; 3) patients who received other targeting agents or immunotherapy.
The study protocol was approved by the Institutional Ethics Review Board of the First Affiliated Hospital of Soochow University (Suzhou, Jiangsu Province, China). Given the retrospective study design, the requirement to obtain informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki on human research.
TACE
The femoral artery was punctured using the Seldinger technique. Angiography of the celiac, hepatic, and superior mesenteric arteries was performed to identify all tumor-feeding arteries by using a 5-Fr catheter. After identification of the target artery, segmental and subsegmental tumor feeding arteries was catheterized using a 2.3-Fr to 2.8-Fr tip microcatheter. An emulsion of chemotherapeutics and iodized oil was slowly injected to the tumor feeding arteries through the microcatheter. The super selective TACE procedure was the preferred modality whenever technically feasible. The treatment regimen was comprised of oxaliplatin (50-100 mg) and pirarubicin (10-40 mg) with lipiodol (2-20 mL). Drug was selected at physician discretion, and the dose of lipiodol was selected by tumor size. Gelatin sponge or polyvinyl alcohol particles (300-500 μm) were injected to embolize tumor-feeding arterioles if necessary, until there was no tumor staining with repeat angiography. In patients with tumor thrombosis in the main portal branch and/or Child-Pugh B liver function, gelatin sponge particles were not used. Contrast agent-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) was performed 6-8 weeks after the procedure. When residual viable tumors were confirmed or new lesions developed in patients with adequate hepatic function, “on demand” TACE were repeated carried out.
Sorafenib therapy
All patients were given detailed information of sorafenib treatment including its efficacy, potential adverse effects, and costs. The use of sorafenib in addition to TACE was recommended by the physicians and the final treatment decision was generally made by the patients or their family members. Sorafenib was administered orally at a dose of 400 mg twice daily within 3 -5 days after the first TACE, and then discontinued the day before each next TACE, and resumed within 3 - 5 days after each repeated TACE. To ensure maximum patient safety, the dose of sorafenib was reduced, or treatment was delayed or temporarily discontinued when we observed grade 3 or 4 toxicity according to the National Cancer Institute Common Toxicity Criteria Adverse Events (CTC AE) version 3.0. Treatment was continued until disease progression, unacceptable toxicities, or death.
Safety assessment
Treatment-emergent adverse events (AEs) were graded by the CTC AE, version 3.0. Vital signs and AEs were monitored throughout the study. Safety assessments were based mainly on the occurrence, frequency, and severity of AEs. For all AEs, where necessary, patients were withdrawn from the study. Safety assessments were analyzed mainly using descriptive statistics.
Follow-up
Subjects underwent monthly follow-up that included complete blood cell count, prothrombin time, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, albumin, and serum alpha-fetoprotein (AFP). Liver contrast-enhanced CT or MRI was performed every 6-8 weeks to evaluate treatment response. If necessary, chest CT and/or a bone scan were also performed to identify extrahepatic metastasis. Tumor response was according to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST) for HCC [23]. February 28, 2017 was the final date of follow-up. OS was calculated the date of the first TACE treatment until the date of death of any cause. Patients who were lost to follow-up were censored at the last date they were known to be alive, and patients who remained alive were censored at the time of data cutoff.
Statistical analysis
Statistical analysis was performed using the SPSS version 22.0 (IBM Corp, Armonk, NY, USA). PSM was carried out, as previously described [24]. Propensity scores for all patients were calculated using a logistic regression model. The covariates in the analysis included sex, age, viral hepatitis, Child-Pugh score, serum AFP levels, BCLC stage, ECOG PS, number of nodules, tumor size, liver cirrhosis, vascular invasion, extrahepatic metastasis, number of TACE procedure, and previous tumor treatment. The nearest neighbor match in accordance with 1:2 ratios balanced the baseline characteristics of the patients. Differences in baseline characteristics of patients of the two groups were compared using Fisher's exact or χ2 test for categorical variables and Student's t-test for continuous variables. OS was analyzed using Kaplan-Meier (KM) analysis followed by a log-rank test. Prognostic factors for OS were performed using univariate and multivariate analyses in the propensity score-matched cohort. Variables that showed statistical significance (P ≤ 0.05) in the univariate analysis were entered into multivariate Cox regression models to look for predictors of efficacy. The outcomes were reported using hazard ratios (HRs) and associated 95% confidence intervals (95% CI). A two-sided P value ≤ 0.05 was taken as statistically significant.
Results
Patient demographic and clinical characteristics
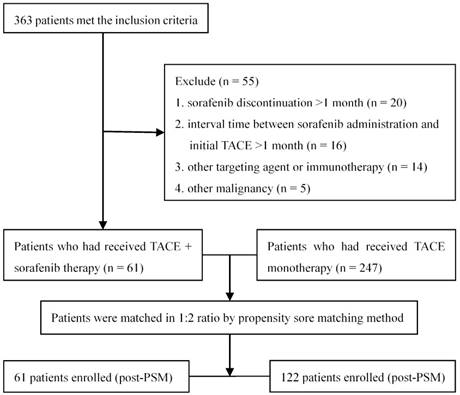
Totally 363 patients with unresectable HCC met the inclusion criteria, of whom 55 were excluded according to the exclusion criteria (Figure 1). A total of 308 patients were included in the data analysis: 61 receiving TACE combined with sorafenib and the remaining 247 receiving TACE alone. One hundred eighty (58.4%) patients had BCLC stage B disease and the remaining 128 (41.6%) had stage C disease. Thirty (49.2%) subjects receiving TACE plus sorafenib had stage B disease and 31 (50.8%) had stage C disease; the median number of TACE sessions was 2 (range: 1-9). Sorafenib therapy was initiated for 56 patients (91.8%) within 5 days after the first TACE session (range 3-5 days). The treatment of the remaining 5 patients (8.2%) was delayed due to TACE-induced adverse effects, but all received sorafenib therapy within 14 days (range 6-14 days) after the completion of TACE. During the treatment, 19 (31.1%) and 5 (8.2%) patients required dose reductions and drug interruptions due to severe sorafenib-related adverse events, respectively. The median duration of sorafenib treatment was 11.7 months (range 2.5-65.6 months). In the 247 subjects receiving TACE alone, the median number of TACE sessions was 3 (range: 1-18).
The study flowchart.
Baseline characteristics of patients before and after propensity score matching.
| Variables | TACE+Sorafenib (n=61) | TACE alone (pre-match, n=247) | P value | TACE alone (matched, n=122) | P value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 48(78.7%) | 209(85.0%) | 0.265 | 102(83.6%) | 0.415 |
| Female | 13(21.3%) | 38(15.0%) | 20(16.4%) | ||
| Age | |||||
| <60y | 39(63.9%) | 148(59.9%) | 0.565 | 71(58.2%) | 0.455 |
| ≥60y | 22(36.1%) | 99(40.1%) | 51(41.8%) | ||
| Viral hepatitis | |||||
| HBV | 50(82.0%) | 192(77.7%) | 0.470 | 93(76.2%) | 0.376 |
| HCV | 5(8.2%) | 13(5.3%) | 9(7.4%) | ||
| No infections | 6(9.8%) | 42(17.0%) | 20(16.4%) | ||
| Child-Pugh score | |||||
| A | 55(90.2%) | 219(88.7%) | 0.738 | 111(91.0%) | 0.857 |
| B | 6(9.8%) | 28(11.3%) | 11(9.0%) | ||
| Serum AFP levels | |||||
| <400ng/ml | 42(68.9%) | 119(48.2%) | 0.014 | 77(63.1%) | 0.443 |
| ≥400ng/ml | 19(31.1%) | 128(51.8%) | 45(36.9%) | ||
| BCLC staging | |||||
| B | 30(49.2%) | 150(60.7%) | 0.101 | 72(59.0%) | 0.207 |
| C | 31(50.8%) | 97(39.3%) | 50(41.0%) | ||
| ECOG PS | |||||
| 0 | 36(59.0%) | 166(67.2%) | 0.237 | 69(56.6%) | 0.751 |
| 1-2 | 25(41.0%) | 81(32.8%) | 53(43.4) | ||
| Number of nodules | |||||
| Single | 40(65.6%) | 127(51.4%) | 0.047 | 77(63.1%) | 0.744 |
| Multiple-diffuse | 21(34.4%) | 120(48.6%) | 45(36.9%) | ||
| Tumor size | |||||
| ≤5cm | 26(42.6%) | 57(23.1%) | 0.002 | 45(36.9%) | 0.453 |
| >5cm | 35(57.4%) | 190(76.9%) | 77(63.1%) | ||
| Liver cirrhosis | |||||
| Yes | 31(50.8%) | 169(68.4%) | 0.010 | 70(57.4%) | 0.400 |
| No | 30(49.2%) | 78(31.6%) | 52(42.6%) | ||
| Vascular invasion | |||||
| Yes | 20(32.8%) | 71(28.7%) | 0.536 | 33(27.0%) | 0.420 |
| No | 41(67.2%) | 176(71.3%) | 89(73.0%) | ||
| Extrahepatic metastasis | |||||
| Yes | 14(23.0%) | 15(6.1%) | 0.000 | 15(12.3%) | 0.063 |
| No | 47(77.0%) | 232(93.9%) | 107(87.7%) | ||
| Number of TACE procedure | |||||
| ≤3 | 48(78.7%) | 169(68.4%) | 0.115 | 93(76.2%) | 0.709 |
| >3 | 13(21.3%) | 78(31.6%) | 29(23.8%) | ||
| Previous tumor treatment (Surgical) | |||||
| Yes | 29(47.5%) | 45(18.2%) | 0.000 | 45(36.9%) | 0.166 |
| No | 32(52.5%) | 202(81.8%) | 77(63.1%) |
TACE: transarterial chemoembolization; HBV: hepatitis B virus; HCV: hepatitis C virus; BCLC: Barcelona Clinic Liver Cancer; ECOG PS: Eastern Cooperative Oncology Group Performance Status.
The demographic and baseline characteristics of the study population are shown in Table 1. The two groups differed significantly in serum AFP levels, number of nodules, tumor size, liver cirrhosis, extrahepatic metastasis, and previous tumor treatment. After matching by the nearest available neighbor method (1:2), based on the number of 61 patients who accepted sorafenib, 122 patients with TACE alone were matched for the analyses. In the PSM cohort, there were no significant differences among the baseline characteristics between the two groups.
OS
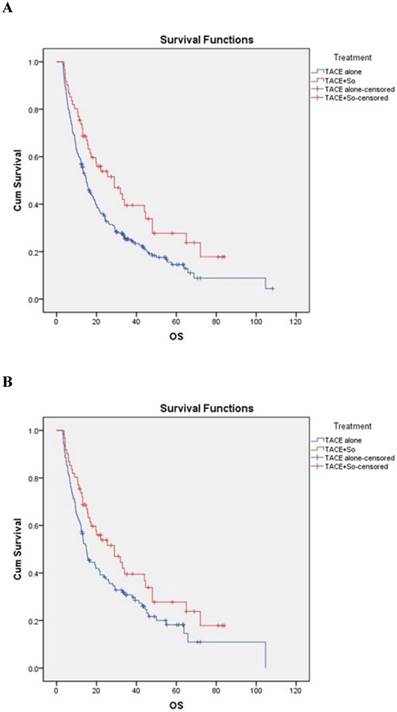
The median OS was 29.0 ± 7.2 (95% CI, 14.945-43.055) months in subjects receiving TACE plus sorafenib and 14.9 ± 1.1 (95% CI, 12.694-17.106) months in subjects receiving TACE alone, strongly favoring the combination treatment (HR = 0.628, 95% CI: 0.445-0.888; P = 0.008; Figure 2A). In the PSM cohort, the median OS was 29.0 ± 7.2 (95% CI, 14.945-43.055) months in the combination group and 14.9 ± 1.5 (95% CI, 12.025-17.775) months in the TACE monotherapy group, also strongly favoring the combination treatment (HR = 0.684, 95% CI: 0.470-0.997; P = 0.018; Figure 2B).
Kaplan-Meier analysis of overall survival (OS) in the combined treatment group and the monotherapy group for all patients (A) and propensity-matched patients (B). Both non-matched and matched models reveal significant differences in OS between the combined treatment group and the monotherapy group (non-matched model: 29.0 ± 7.2 months for the combined treatment group vs. 14.9 ± 1.1 months for the monotherapy group, P = 0.008; matched model: 29.0 ± 7.2 months for the combined treatment group vs. 14.9 ± 1.5 months for the monotherapy group, P = 0.018).
Safety
Treatment-emergent adverse events (AEs) are shown in Table 2. The two most frequent AEs in the TACE alone group were fatigue (19.0%) and liver dysfunction (18.2%). In addition, grade 3/4 liver dysfunction occurred in 8.9% of the patients; no other grade 3/4 AEs were reported. In the TACE plus sorafenib group, hand-foot skin reaction (75.4%), diarrhea (47.5%), and liver dysfunction (32.8%) were the most three most frequent AEs. Grade 3/4 hand-foot skin reaction (18.0%), liver dysfunction (13.1%) and diarrhea (9.8%) were the most common grade 3/4 AEs. There was no treatment-related death in either group.
Adverse events in the combination treatment group and the monotherapy group.
| Adverse events | TACE + sorafenib (n=61) | TACE alone (n=247) | ||
|---|---|---|---|---|
| All grade (n) | Grade 3/4 (n) | All grade (n) | Grade 3/4 (n) | |
| Hand-foot skin reaction | 46 (75.4%) | 11 (18.0%) | 0 | 0 |
| Diarrhea | 29 (47.5%) | 6 (9.8%) | 3 (1.2%) | 0 |
| Hypertension | 10 (16.4%) | 3 (4.9%) | 2 (0.8%) | 0 |
| Alopecia | 19 (31.1%) | 2 (3.3%) | 0 | 0 |
| Gastrointestinal bleeding | 2 (3.3%) | 0 | 5 (2.0%) | 0 |
| Liver dysfunction (AST and/or ALT increase) | 20 (32.8%) | 8 (13.1%) | 45 (18.2%) | 22 (8.9%) |
| Fatigue | 15 (24.6%) | 0 | 47 (19.0%) | 0 |
Subgroup analysis
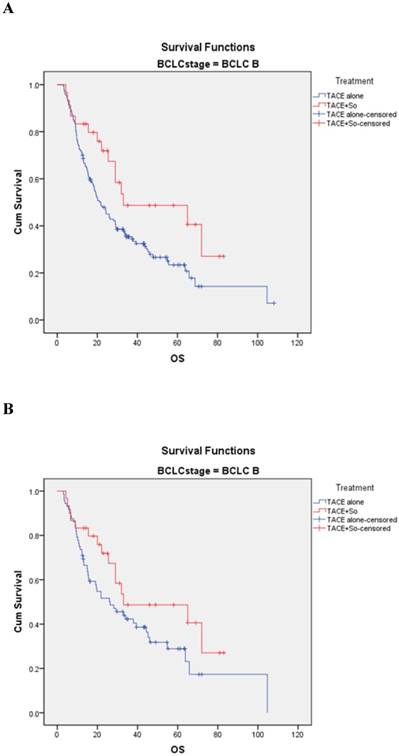
In the BCLC-B subgroup, before matching, the median OS was 33.0 ± 9.8 (95% CI, 18.688-43.312) months in the TACE plus sorafenib group and 21.2 ± 2.3 (95% CI, 16.696-25.704) months in the TACE monotherapy group (HR = 0.547, 95% CI: 0.317-0.943; P = 0.027; Figure 3A). After PSM, the median OS was 33.0 ± 9.8 (95% CI, 18.688-43.312) months in the combination group and 25.3±6.7 (95% CI, 13.135-39.465) months in the monotherapy group (HR=0.620, 95% CI: 0.345-1.114; P = 0.041; Figure 3B).
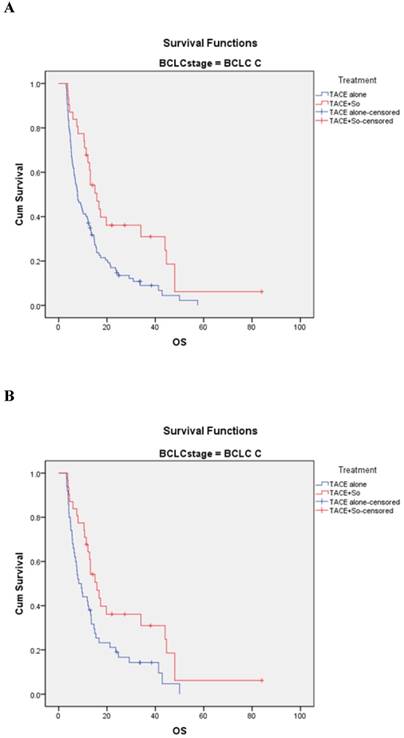
In the BCLC-C subgroup, before matching, the median OS was 15.8 ± 2.0 (95% CI, 11.820-19.780) months in the TACE plus sorafenib group and 7.8 ± 1.1 (95% CI, 5.607-9.993) months in the TACE monotherapy group (HR=0.507, 95% CI: 0.320-0.801; P = 0.003; Figure 4A). After PSM, the median OS was 15.8 ± 2.0 (95% CI, 11.820-19.780) months in the combination group and 8.3 ± 1.4 (95% CI, 5.528-11.072) months in the monotherapy group (HR = 0.544, 95% CI: 0.328-0.902; P = 0.016; Figure 4 B).
Prognostic factors for OS in the PSM cohort
Univariate and multivariate analyses of the factors influencing OS are summarized in Table 3. Univariate log-rank test analysis of the PSM cohort showed that OS was associated with treatment methods, serum AFP levels, BCLC stage, number of nodules, tumor size, liver cirrhosis, vascular invasion, number of TACE, and previous tumor treatment (P ≤ 0.05 for all). Multivariate Cox regression analysis indicated the following independent factors: treatment methods [HR 0.618; 95% CI (0.419-0.912); P = 0.003], number of nodules [HR 2.491; 95% CI (1.705-3.638); P = 0.010], tumor size [HR 2.130; 95% CI (1.343-3.378); P = 0.012], vascular invasion [HR 2.575; 95% CI (1.676-3.957); P = 0.005], and number of TACE [HR 0.621; 95% CI (0.405-0.953); P = 0.029].
Kaplan-Meier analysis of OS in BCLC-B subgroup. (A) Median OS was 33.0 ± 9.8 months in the combined treatment group compared with 21.2 ± 2.3 months in the monotherapy group in the non-matched model (P = 0.027). (B) Median OS was 33.0 ± 9.8 months in the combined treatment group compared with 25.3 ± 6.7 months in the monotherapy group in the matched model (P = 0.041).
Univariate and multivariate analyses of potential prognostic factors for overall survival
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Treatment methods | 0.684 (0.428-0.908) | 0.008 | 0.618 (0.419-0.912) | 0.003 |
| Gender | 1.558 (0.967-2.510) | 0.069 | - | - |
| Age | 1.066 (0.752-1.510) | 0.721 | - | - |
| Viral hepatitis | 0.872 (0.577-1.319) | 0.517 | - | - |
| Child-Pugh score | 0.618 (0.354-1.080) | 0.091 | - | - |
| Serum AFP levels | 1.563 (1.100-2.221) | 0.013 | 1.350 (0.957-1.903) | 0.087 |
| BCLC staging | 2.435 (1.714-3.457) | 0.004 | 1.183 (0.696-2.013) | 0.535 |
| ECOG PS | 1.355 (0.931-1.853) | 0.287 | - | - |
| Number of nodules | 1.950 (1.369-2.778) | 0.026 | 2.491 (1.705-3.638) | 0.010 |
| Tumor size | 2.422 (1.671-3.511) | 0.006 | 2.130 (1.343-3.378) | 0.012 |
| Liver cirrhosis | 1.542 (1.095-2.170) | 0.015 | 1.523 (1.010-2.297) | 0.054 |
| Vascular invasion | 3.097 (2.154-4.451) | 0.011 | 2.575 (1.676-3.957) | 0.005 |
| Extrahepatic metastasis | 1.500 (0.952-2.364) | 0.081 | - | - |
| Number of TACE | 0.663 (0.444-989) | 0.044 | 0.621 (0.405-0.953) | 0.029 |
| Previous tumor treatment | 0.628 (0.440-0.897) | 0.010 | 1.492 (0.928-2.399) | 0.099 |
Treatment methods: TACE + sorafenib vs TACE alone; HR: hazard ratio; 95% CI: 95% confidence interval.
Kaplan-Meier analysis of OS in BCLC-C subgroup. (A) Median OS was 15.8 ± 2.0 months in the combined treatment group compared with 7.8 ± 1.1 months in the monotherapy group in the non-matched model (P = 0.003). (B) Median OS was 15.8 ± 2.0 months in the combined treatment group compared with 8.3 ± 1.4 months in the monotherapy group in the matched model (P = 0.016).
Discussion
This retrospective study demonstrated that the combination treatment with TACE and sorafenib significantly improved OS versus TACE monotherapy in patients with unresectable HCC. This survival advantage was still present even after adjustment of baseline characteristics through PSM.
The survival advantage of combined TACE and sorafenib treatment seen in our study is encouraging. The median OS in our subjects receiving TACE plus sorafenib was numerically longer than 7.5-27 months reported in previous studies [25-27]. Such a discrepancy could reflect differences between the current and previous studies. Firstly, in this study, the median duration of sorafenib treatment was 11.7 months, which is longer than that of the above mentioned studies [25, 27]. It may be the key reason for the success of the combined treatment group. The negative results of the two RCTs, Post-TACE and TACE-2, may be due to the short duration of sorafenib administration [28]. Recently, the preliminary results of the TACTICS trial also supported our viewpoint [29]. Additionally, due to the plasma concentration of VEGF was markedly elevated after TACE treatment [16], patients in our study received timely sorafenib treatment within 3-5 days after the first TACE. Meng et al. [30] demonstrated that earlier administration of sorafenib after the first TACE may lead to a greater survival benefit in patients with HCC.
In subgroup analysis, the median OS of BCLC-B patients was significantly longer in the combined treatment group than in the TACE monotherapy group before and after PSM, suggesting that BCLC stage B HCC patients could benefit from the combination therapy. This finding has also been supported by a recent PSM study [31]. In addition, a meta-analysis of 1254 patients with intermediate stage HCC confirmed that the combination therapy of TACE plus sorafenib can improve OS [32]. Similarly, in the BCLC-C subgroup, the combination therapy of TACE plus sorafenib significantly improved OS versus TACE alone, a finding also confirmed in the PMS cohort. Numerous clinical studies have demonstrated that TACE combined with sorafenib is superior to TACE or sorafenib monotherapy in prolonging OS in advanced HCC patients [33-35]. These findings are further supported by two meta-analyses of RCTs [36, 37].
Our multivariate analysis indicated that the combination treatment with TACE and sorafenib was an independent predictor for OS. In addition, number of nodules, tumor size, vascular invasion, and number of TACE were also significant predictors of OS. These findings are similar to those of a recent study showing TACE plus sorafenib treatment, Child-Pugh class, vascular invasion, tumor number and tumor size as independent predictors of prognosis [38]. Differing results in terms of the number of TACE may be due to differences in patient response to TACE, hepatic reserve function and patient compliance.
Evidence from recently studies have suggested that common AEs are associated with the use of sorafenib, the most common being hand-foot skin reactions, diarrhea, alopecia, and fatigue [39, 40]. Similar safety outcomes were found in the current study, which revealed hand-foot skin reaction and diarrhea to be the most common drug-emergent AEs, followed by alopecia and fatigue. Hypertension and gastrointestinal bleeding were also observed in a small fraction of patients. Most of these AEs in our study were grade 1/2 and well tolerated, which seldom contributed to the discontinuation of therapy.
The current study has some limitations that must be considered. Due to the retrospective nature of this study, there was selection bias in determining treatment modalities. The choice of sorafenib was based on physician discretion and patient financial capability, which introduced a selection bias that could have influenced the difference in survival between the two groups. To minimize the bias, we conducted PSM, which supported the advantage of combination treatment. Secondly, all subjects received treatment at the same research site, and the sample size is relatively small, and the results of which on its own cannot represent the entire population. Thirdly, as BCLC stage B and C HCC include a broad spectrum of tumors, the composition of our sample was mixed. The heterogeneity of our patients precluded a stratified analysis for patients with different extents of disease.
In conclusion, the current study indicated that addition of sorafenib to TACE therapy is generally well tolerated and could increase OS of patients with unresectable HCC. Further multi-center, prospective, randomized, controlled trials are required to confirm our preliminary findings, as well as to identify suitable candidates for treatment.
Abbreviations
TACE: transarterial chemoembolization; HCC: hepatocellular carcinoma; BCLC: Barcelona Clinic Liver Cancer; RCT: randomized controlled trial; VEGFR: vascular endothelial growth factor receptor; TTP: time to progression; OS: overall survival; PSM: propensity score matching; ECOG PS: Eastern Cooperative Oncology Group performance status; CT: computed tomography; MRI: magnetic resonance imaging; CTC AE: Common Toxicity Criteria Adverse Events; AEs: adverse events; AST: aspartate aminotransferase; ALT: alanine aminotransferase; AFP: alpha-fetoprotein; mRECIST: Modified Response Evaluation Criteria in Solid Tumors; HR: hazard ratio; CI: confidence intervals.
Acknowledgements
This study was supported by Jiangsu Province's Key Provincial Talents Program (NO. ZDRCA2016038).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314
2. Zhu RX, Seto WK, Lai CL. et al. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332-339
3. Grandhi MS, Kim AK, Ronnekleiv-Kelly SM. et al. Hepatocellular carcinoma: From diagnosis to treatment. Surg Oncol. 2016;25:74-85
4. Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:4115-4127
5. Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients with Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853
6. Chung GE, Lee JH, Kim HY. et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627-634
7. Luo J, Guo RP, Lai EC. et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413-420
8. Pesapane F, Nezami N, Patella F. et al. New concepts in embolotherapy of HCC. Med Oncol. 2017;34:58
9. Llovet JM, Real MI, Montaña X. et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739
10. Lo CM, Ngan H, Tso WK. et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171
11. Keating GM. Sorafenib: A Review in Hepatocellular Carcinoma. Target Oncol. 2017;12:243-253
12. Mazzoccoli G, Miele L, Oben J. et al. Epidemiology, Clinical Aspects of Hepatocellular Carcinoma and the Role of Sorafenib. Curr Drug Targets. 2016;17:783-799
13. Llovet JM, Ricci S, Mazzaferro V. et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390
14. Cheng AL, Kang YK, Chen Z. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34
15. Wang B, Xu H, Gao ZQ. et al. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523-529
16. Sergio A, Cristofori C, Cardin R. et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921
17. Cabrera R, Pannu DS, Caridi J. et al. The combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;34:205-213
18. Chao Y, Chung YH, Han G. et al. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136:1458-1467
19. Geschwind JF, Kudo M, Marrero JA. et al. TACE Treatment in Patients with Sorafenib-treated Unresectable Hepatocellular Carcinoma in Clinical Practice: Final Analysis of GIDEON. Radiology. 2016;279:630-640
20. Meyer T, Fox R, Ma YT. et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:565-575
21. Li L, Zhao W, Wang M. et al. Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2018;18:138
22. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236
23. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60
24. Haukoos JS, Lewis RJ. The Propensity Score. JAMA. 2015;314:1637-1638
25. Qu XD, Chen CS, Wang JH. et al. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer. 2012;12:263
26. Bai W, Wang YJ, Zhao Y. et al. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J Dig Dis. 2013;14:181-190
27. Wan X, Zhai X, Yan Z. et al. Retrospective analysis of transarterial chemoembolization and sorafenib in Chinese patients with unresectable and recurrent hepatocellular carcinoma. Oncotarget. 2016;7:83806-83816
28. Kudo M, Arizumi T. Transarterial Chemoembolization in Combination with a Molecular Targeted Agent: Lessons Learned from Negative Trials (Post-TACE, BRISK-TA, SPACE, ORIENTAL, and TACE-2). Oncology. 2017;93(Suppl 1):S127-S134
29. Kudo M. Proposal of Primary Endpoints for TACE Combination Trials with Systemic Therapy: Lessons Learned from 5 Negative Trials and the Positive TACTICS Trial. Liver Cancer. 2018;7:225-234
30. Meng XC, Chen BH, Huang JJ. et al. Early prediction of survival in hepatocellular carcinoma patients treated with transarterial chemoembolization plus sorafenib. World J Gastroenterol. 2018;24:484-493
31. Huang Y, Chen B, Liu N. et al. Overall survival in response to sorafenib with transarterial chemoembolization for BCLC stage B hepatocellular carcinoma: propensity score analysis. Int J Clin Pharmacol Ther. 2017;55:498-508
32. Zhang L, Hu P, Chen X. et al. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9:e100305
33. Choi GH, Shim JH, Kim MJ. et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269:603-611
34. Zhu K, Chen J, Lai L. et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib-a retrospective controlled study. Radiology. 2014;272:284-293
35. Hu H, Duan Z, Long X. et al. Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study. PLoS One. 2014;9:e96620
36. Fu QH, Zhang Q, Bai XL. et al. Sorafenib enhances effects of transarterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1429-1440
37. Yang M, Yuan JQ, Bai M. et al. Transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Mol Biol Rep. 2014;41:6575-6582
38. Wu FX, Chen J, Bai T. et al. The safety and efficacy of transarterial chemoembolization combined with sorafenib and sorafenib mono-therapy in patients with BCLC stage B/C hepatocellular carcinoma. BMC Cancer. 2017;17:645
39. Ni JY, Kong J, Sun HL. et al. Prognostic Factors for Survival After Transarterial Chemoembolization Combined with Sorafenib in the Treatment of BCLC Stage B and C Hepatocellular Carcinomas. Acad Radiol. 2018;25:423-429
40. Ye SL, Yang J, Bie P. et al. Safety assessment of sorafenib in Chinese patients with unresectable hepatocellular carcinoma: subgroup analysis of the GIDEON study. BMC Cancer. 2018;18:247
Author contact
![]() Corresponding author: Xiaoli Zhu, M.D., Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, 188 Shizi Road, Suzhou 215006, China. Tel.: 86-0512-67780399, Fax: 86-0512-65222588, E-mail: zhuxiaoliedu.cn
Corresponding author: Xiaoli Zhu, M.D., Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, 188 Shizi Road, Suzhou 215006, China. Tel.: 86-0512-67780399, Fax: 86-0512-65222588, E-mail: zhuxiaoliedu.cn

 Global reach, higher impact
Global reach, higher impact