3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(8):1794-1799. doi:10.7150/jca.30385 This issue Cite
Research Paper
Identification of Optimal Baseline Blood Pressure Predicting Postoperative Digestive Tract Cancer-Specific Mortality in the FIESTA Cohort Involving 6865 Patients
1. Department of Pathology, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital, Fuzhou, Fujian, China.
2. Division of Biological Sciences, University of California, San Diego, La Jolla, CA, USA.
3. Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, Beijing, China.
4. Department of Radiobiology, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital, Fuzhou, Fujian, China.
5. Department of Cardiology, First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China.
*Shared first authors.
Received 2018-10-3; Accepted 2019-2-7; Published 2019-4-21
Abstract
Background and Objectives: Emerging evidence indicates that hypertension is a potential risk and prognostic factor for cancer at many sites. Currently, no data are available on optimal blood pressure target in patients with resectable digestive tract cancer. Here, we did an exploratory analysis in 6865 patients from the FIESTA cohort to identify optimal blood pressure at baseline that can better predict digestive tract cancer-specific mortality risk postoperatively.
Methods and Results: Patients were enrolled between January 2000 and December 2010, with follow-up ending in December 2015. All patients received no preoperative and postoperative chemotherapy or radiotherapy. Data were analyzed using Stata software and R language. Optimal cutting points were determined using survival tree analysis. After a median follow-up of 44.9 months, there were 2808 non-survivors and 4057 survivors. Per 10 mm Hg increment, baseline systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure and mean arterial pressure were associated with the significant risk of digestive tract cancer-specific mortality, even after adjusting for confounding factors (adjusted hazard ratio: 1.06, 1.08, 1.06 and 1.09, 95% confidence interval: 1.04-1.08, 1.04-1.12, 1.03-1.09 and 1.05-1.12, P<0.001, <0.001, <0.001 and <0.001, respectively). Patients with baseline SBP of 176 mm Hg or above and DBP of 100 mm Hg or above had poor survival outcomes (median survival time: 39.6 and 37.1 months, respectively).
Conclusions: We provide evidence for the use of elevated blood pressure (SBP/DBP ≥176/100 mm Hg) before surgery as a powerful harbinger to predict the survival outcomes of digestive tract cancer patients postoperatively.
Keywords: blood pressure, digestive tract cancer, mortality, FIESTA study
Introduction
Hypertension is common and a public health problem gripping both developed and developing countries [1,2]. An emerging body of evidence indicates that hypertension is a potential risk and prognostic factor for cancer at many sites, such as kidney, colon and rectum [3-6]. However, discrepancies exist [7,8], mainly due to population heterogeneity, small sample size, short follow-up interval or diverse cancer sites. Considering that effective blood pressure control may lower cancer risk and improve survival, further evaluation with larger sample size and longer follow-up is needed.
We recently, in an ongoing Fujian prospective investigation of cancer (FIESTA) study have investigated the association of preoperative metabolic syndrome with disease-specific mortality of common digestive tract cancer after radical surgery including esophageal cancer [9], gastric cancer [10] and colorectal cancer [11]. In particular, hypertension (systolic/diastolic blood pressure [SBP/DBP] >140/90 mm Hg), as an integral component of the metabolic syndrome was identified as a significant prognostic factor. The recently released guidelines by the American College of Cardiology/American Heart Association indicate that SBP of 130 to 139 mm Hg or DBP of 80 to 89 mm Hg is defined as stage 1 hypertension [12]. Current evidence, however, is limited on the optimal blood pressure target in patients with resectable digestive tract cancer.
To yield more information, we did an exploratory analysis in 6865 Chinese patients from the FIESTA cohort to identify optimal blood pressure at baseline that can better predict digestive tract cancer-specific mortality risk postoperatively.
Methods
Study patients
A total of 6865 assessable patients were enrolled from Fujian Provincial Cancer Hospital (the current Fujian Cancer Hospital & Fujian Medical University Cancer Hospital) between January 2000 and December 2010, including 2535, 3012 and 1318 patients receiving radical surgery for esophageal cancer, gastric cancer and colorectal cancer, respectively, and all study patients were safely discharged and followed up until December 2015. Detailed information on study design, enrollment, eligibility criteria and follow-up evaluation has been described elsewhere [9-11,13-16].
The FIESTA study was approved by the Ethics Committee of the Fujian Provincial Cancer Hospital, and all study patients who participated in this study provided written informed consents. All patients received no preoperative and postoperative chemotherapy or radiotherapy.
Tissue samples
Primary cancer and adjacent normal tissue samples were collected during the surgery and fixed in 10% neutral-buffered formalin and further paraffin-embedded using standard procedures. All pathological assays were done at the Department of Pathology, Fujian Provincial Cancer Hospital.
Follow-up assessment
The patients were interviewed every six to twelve months by face-to-face interview, or by phone calls or postal mails if they missed appointments at the Out-Patient Department, Fujian Provincial Cancer Hospital. The follow-up started from initial admission after surgery since January 2000 to the date of death attributable to causes other than digestive tract malignancies or the end of patient follow-up visit in December 2015, whichever occurred first. Clinicopathologic characteristics were obtained from medical charts and/or pathological reports.
Clinical outcomes
The primary outcome was death from esophageal, gastric or colorectal cancer. We defined cancer-specific survival time as the time from the date of radical surgery to the date of the death from specific types of digestive tract cancer or the date of the latest follow-up (prior to December 2015), whichever happened first.
Data collection
Each patient was requested to complete a self-designed questionnaire to collect data on age, gender, body weight and height, smoking, drinking and family history of cancer (except non-melanoma skin cancer). Body weight and height were measured when patients were in light clothing and with bare feet. Body mass index (BMI) was calculated as weight divided by height in meters-squared (kg/m2). Blood pressure was measured with mercury sphygmomanometer on three occasions of at least 5-min intervals and the mean of these three readings was recorded at the time of enrollment when receiving radical surgery for digestive tract cancer for the first time. On the basis of SBP and DBP, we produced pulse pressure (PP) and mean arterial pressure (MAP).
Statistical analysis
The optimal blood pressure level was determined by survival tree analysis (STREE, available at the website http://c2s2.yale.edu/software/stree/). Survival tree-based method has been applicable to more general situations based on scientific judgement [17]. The Kaplan-Meier curve and Log-rank test were used to quantify the difference in cumulative survival rates. Proportional hazards assumption was checked by the Weighted Schoenfeld residuals. Association of baseline blood pressure with digestive tract cancer-specific mortality risk was expressed as hazard ratio (HR) and 95% confidence interval (95% CI) before and after adjusting for confounding factors, including age, gender, BMI, smoking, drinking and family cancer history. Three-dimension interactive surface was plotted using the “rgl.surface” order in the “rgl” package, which is a library of functions that offers three-dimensional, real-time visualization functionality to the R programming environment. The core of “rgl” package is a shared library that acts as an interface between R and OpenGL. Data were analyzed using the Stata/SE software version 14.1 (StataCorp, TX, USA), unless otherwise indicated. Study power was estimated by the Power and Sample Size Calculations (PS) software version 3.0.7 [18].
Results
The baseline characteristics of cohort patients by the primary endpoint are provided in Table 1. The median follow-up time was 44.9 months (range: 1.0 to 188.9 months). There were 2808 non-survivors and 4057 survivors as of December 2015. SBP, DBP, PP and MAP were significantly higher in non-survivors than in survivors (P<0.001).
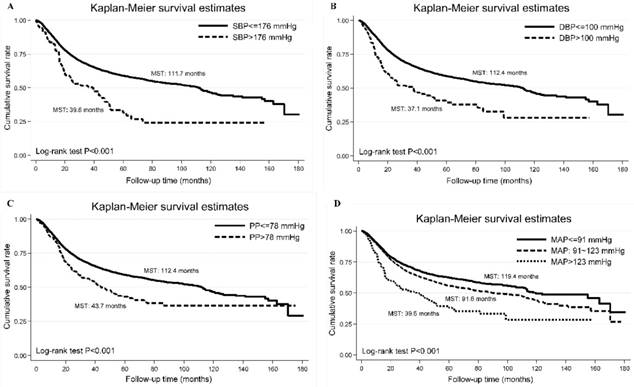
Per 10 mm Hg increment, baseline SBP (adjusted HR: 1.06, 95% CI: 1.04-1.08, P<0.001), DBP (adjusted HR: 1.08, 95% CI: 1.04-1.12, P<0.001), PP (adjusted HR: 1.06, 95% CI: 1.03-1.09, P<0.001) and MAP (adjusted HR: 1.09, 95% CI: 1.05-1.12, P<0.001) were associated with the significant risk of digestive tract cancer- specific mortality, even after adjusting for confounding factors (Table 2). The power to detect the significant prediction of SBP, DBP, PP and MAP was estimated to be 97.5%, 99.9%, 99.4% and 100.0%, respectively. In addition, analysis was conducted after stratifying study patients by age, gender and BMI, respectively, and the effect estimates are presented in Table 2. The risk prediction for digestive tract cancer- specific mortality was more obvious in patients aged ≤ 55 years, with male gender and with BMI ≥ 25 kg/m2.
The mortality risk prediction based on the interaction of SBP and DBP is shown in Supplementary Figure 1.
Using the optimal cutoff points derived by survival tree analysis, the Kaplan-Meier curve of each blood pressure index is shown in Figure 1, and Log-rank test indicate significant difference in cumulative survival rates (P<0.001).
The baseline characteristics of cohort patients.
| Characteristics | Non-survivors (n=2808) | Survivors (n=4057) | P |
|---|---|---|---|
| Age (years) | 58.07 (11.07) | 56.94 (10.69) | <0.001 |
| Males | 74.25% | 70.27% | <0.001 |
| Current/former smoking | 28.59% | 24.93% | 0.001 |
| Current/former drinking | 12.07% | 9.63% | 0.002 |
| Family cancer history | 10.63% | 10.63% | 0.996 |
| Body mass index (kg/m2) | 22.74 (3.16) | 22.61 (3.00) | 0.097 |
| Systolic blood pressure (mm Hg) | 126.63 (20.26) | 123.19 (17.99) | <0.001 |
| Diastolic blood pressure (mm Hg) | 78.25 (11.48) | 76.90 (10.60) | <0.001 |
| Pulse pressure (mm Hg) | 48.38 (14.56) | 46.30 (13.16) | <0.001 |
| Mean arterial pressure (mm Hg) | 94.38 (13.33) | 92.33 (12.01) | <0.001 |
| Tumor-Node-Metastasis stage | <0.001 | ||
| I | 1.93% | 18.41% | |
| II | 14.50% | 33.39% | |
| III | 65.38% | 47.02% | |
| IV | 18.19% | 1.17% |
Data are expressed as either mean (standard deviation) or percentage. P was calculated using the Mann-Whitney test for continuous variables and the Chi-squared test for categorical variables.
The Kaplan-Meier curves of categorized systolic blood pressure (panel A: SBP), diastolic blood pressure (panel B: DBP), pulse pressure (panel C: PP) and mean arterial pressure (panel D: MAP). Abbreviations: MST, median survival time. The vertical coordinate is cumulative survival rate, and the horizontal coordinate is follow-up time in months.
Risk prediction of baseline blood pressure for digestive tract cancer-specific mortality risk overall and upon stratification by age, gender and obesity.
| Baseline blood pressure | Crude HR, 95% CI, P | Adjusted HR, 95% CI, P* |
|---|---|---|
| Overall analysis | ||
| SBP (per 10 mm Hg increment) | 1.07, 1.05-1.09, <0.001 | 1.06, 1.04-1.08, <0.001 |
| DBP (per 10 mm Hg increment) | 1.09, 1.05-1.13, <0.001 | 1.08, 1.04-1.12, <0.001 |
| PP (per 10 mm Hg increment) | 1.08, 1.05-1.11, <0.001 | 1.06, 1.03-1.09, <0.001 |
| MAP (per 10 mm Hg increment) | 1.10, 1.07-1.13, <0.001 | 1.09, 1.05-1.12, <0.001 |
| Subgroup analysis | ||
| Patients aged ≤ 55 years | ||
| SBP (per 10 mm Hg increment) | 1.08. 1.05-1.12, <0.001 | 1.09, 1.05-1.13, <0.001 |
| DBP (per 10 mm Hg increment) | 1.10, 1.04-1.17, 0.001 | 1.11, 1.05-1.18, 0.001 |
| PP (per 10 mm Hg increment) | 1.08, 1.03-1.14, 0.002 | 1.09, 1.03-1.14, 0.002 |
| MAP (per 10 mm Hg increment) | 1.11, 1.06-1.17, <0.001 | 1.12, 1.06-1.18, <0.001 |
| Patients aged > 55 years | ||
| SBP (per 10 mm Hg increment) | 1.05, 1.03-1.08, <0.001 | 1.04, 1.02-1.07, 0.001 |
| DBP (per 10 mm Hg increment) | 1.07, 1.02-1.11, 0.003 | 1.06, 1.01-1.11, 0.010 |
| PP (per 10 mm Hg increment) | 1.05, 1.02-1.09, 0.002 | 1.04, 1.01-1.08, 0.016 |
| MAP (per 10 mm Hg increment) | 1.07, 1.03-1.11, <0.001 | 1.06, 1.02-1.11, 0.002 |
| Males patients | ||
| SBP (per 10 mm Hg increment) | 1.07, 1.05-1.10, <0.001 | 1.06, 1.04-1.09, <0.001 |
| DBP (per 10 mm Hg increment) | 1.08, 1.04-1.12, <0.001 | 1.07, 1.02-1.11, 0.002 |
| PP (per 10 mm Hg increment) | 1.09, 1.06-1.12, <0.001 | 1.08, 1.04-1.11, <0.001 |
| MAP (per 10 mm Hg increment) | 1.10, 1.06-1.14, <0.001 | 1.08, 1.04-1.12, <0.001 |
| Female patients | ||
| SBP (per 10 mm Hg increment) | 1.06, 1.02-1.10, 0.002 | 1.05, 1.00-1.09, 0.033 |
| DBP (per 10 mm Hg increment) | 1.11, 1.04-1.19, 0.002 | 1.10, 1.03-1.18, 0.007 |
| PP (per 10 mm Hg increment) | 1.05, 0.99-1.10, 0.085 | 1.02, 0.96-1.08, 0.473 |
| MAP (per 10 mm Hg increment) | 1.10, 1.04-1.17, 0.001 | 1.09, 1.02-1.16, 0.008 |
| Patients with BMI ≥ 25 kg/m2 | ||
| SBP (per 10 mm Hg increment) | 1.09, 1.04-1.13, <0.001 | 1.07, 1.02-1.12, 0.003 |
| DBP (per 10 mm Hg increment) | 1.07, 1.00-1.15, 0.063 | 1.06, 0.98-1.14, 0.152 |
| PP (per 10 mm Hg increment) | 1.11, 1.05-1.17, <0.001 | 1.08, 1.02-1.15, 0.006 |
| MAP (per 10 mm Hg increment) | 1.11, 1.04-1.18, 0.002 | 1.09, 1.01-1.16, 0.019 |
| Patients with BMI < 25 kg/m2 | ||
| SBP (per 10 mm Hg increment) | 1.06, 1.04-1.08, <0.001 | 1.05, 1.03-1.08, <0.001 |
| DBP (per 10 mm Hg increment) | 1.09, 1.04-1.13, <0.001 | 1.07, 1.03-1.12, 0.001 |
| PP (per 10 mm Hg increment) | 1.06, 1.03-1.10, <0.001 | 1.05, 1.02-1.09, 0.004 |
| MAP (per 10 mm Hg increment) | 1.09, 1.05-1.13, <0.001 | 1.08, 1.04-1.12, <0.001 |
Abbreviations: SBP, systolic blood pressure, DBP, diastolic blood pressure, PP, pulse pressure; MAP, mean arterial pressure; BMI, body mass index; HR, hazard ratio; 95% CI, 95% confidence interval. *P was calculated after adjusting for age, gender, body mass index, smoking, drinking and family cancer history.
Schoenfeld residuals analysis did not show major departures from the proportional hazards assumption. In particular, patients with baseline SBP of 176 mm Hg or above and DBP of 100 mm Hg or above had poor survival outcomes (median survival time: 39.6 and 37.1 months, respectively).
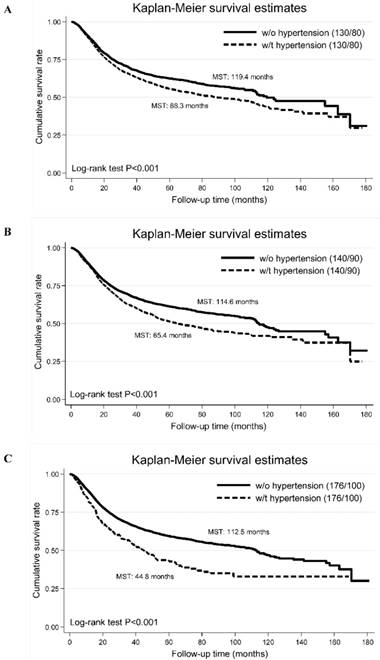
Additionally, we compared the cumulative survival rates of different blood pressure cutoffs (panel A: SBP/DBP >130/80 mm Hg, panel B: 140/90 mm Hg and panel C: 176/100 mm Hg), and found that survival difference was the best for SBP/DBP >176/100 mm Hg (Figure 2).
Discussion
The key finding of this study was the identification of baseline optimal blood pressure cutoff (SBP/DBP: 176/100 mm Hg) in patients with resectable digestive tract cancer that can be used as a powerful harbinger of a poor prognosis. Moreover, the superiority of this optimal blood pressure cutoff over the traditionally accepted (140/90 mm Hg) and recently released (130/80 mm Hg) cutoffs was also demonstrated. This study represents a first step in enriching our understanding that digestive tract cancer patients with SBP/DBP over 176/100 mm Hg presumably need closer monitoring after radical surgery.
Hypertension is increasingly recognized as a risk factor for cancer, and they might share a common pathogenic mechanism [19-21]. A pooled collaborative analysis of 12 Australian and New Zealand cohorts with long-term follow-up suggests that hypertension, both treated and untreated, is associated with a modest increased risk for cancer incidence and mortality [22]. Currently, a majority of studies assessing cancer survival used hypertension as a binary trait, rather than a quantitative trait such as blood pressure. In addition, the blood pressure cutoff (SBP/DBP: 140/90 or 130/80 mm Hg) used to define hypertension might not be suitable to stratify cancer patients. So, seeking an optimal blood pressure cutoff point that can help identify high-risk subgroups of cancer patients is of clinical importance. A literature search has, however, failed to reveal any evidence concerning this aspect. To fill this gap in knowledge, we revisited the FIESTA database and explored in detail the association of quantitative blood pressure with mortality risk of three types of digestive tract cancer. Importantly under the rationales of survival tree analysis, we in a large Chinese cohort of patients with long follow-up, pinpointed an optimal cutoff for SBP at 176 mm Hg and DBP at 100 mm Hg that can divide digestive tract cancer patients into groups with maximal difference in survival intervals. The underlying mechanisms are still speculative but it appears that further investigations are warranted to explore whether elevated blood pressure is an independent predictive factor for cancer survival or an indirect marker of different genetic, demographic or clinicopathologic factors. In support of this claim, Deckers and colleagues in a large population found the interplay between hypertension and genetic defects in the renin-angiotensin-aldosterone system in determining renal cell cancer risk [23]. Irrespective of the mechanism, preoperative high blood pressure can clearly identify digestive tract cancer patients with poorer postoperative survival who could benefit from closer monitoring.
There are several limitations for this study. Firstly, this study was performed in a single hospital, which restricted the generalizability, although it can facilitate consistency of evaluation. Additionally, external validation is necessary. Secondly, due to the difficulty in identifying an external group, we are unable to validate our findings in an independent population. Thirdly, we only had blood pressure recordings at the time of enrollment for the radical surgery, and other recordings at perioperative and postoperative time points, which are not available for us, are of additional interest for comparison. Additionally, we only had smoking status, and other smoking indexes such as smoking pack-year are not available. Fourthly, patients were exclusively enrolled from a southern city in China, which restricted the racial or ethnical extrapolation.
Despite the above limitations, we provide evidence for the use of elevated blood pressure (SBP/DBP ≥176/100 mm Hg) before surgery as a powerful harbinger to predict the survival outcomes of digestive tract cancer patients postoperatively. This study highlights the importance of measuring blood pressure for patients receiving surgery for digestive tract cancer to inform risk assessment, formulate cancer control strategies and prioritize rational planning of health-care resources.
The Kaplan-Meier curves of high blood pressure defined by different blood pressure cutoffs (panel A: the cut-off points of systolic/diastolic blood pressure are 130 and 80 mm Hg; panel B: the cut-off points of systolic/diastolic blood pressure are 140 and 90 mm Hg; panel C: the cut-off points of systolic/diastolic blood pressure are 176 and 100 mm Hg). Abbreviations: MST, median survival time; w/o, without; w/t, with. The vertical coordinate is cumulative survival rate, and the horizontal coordinate is follow-up time in months.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
We thank our colleagues over the years at Fujian Cancer Hospital — particularly Gang Chen, Chao Li, Binying Liang, Xiaohui Chen, Yuzhen Zheng, Qingfeng Zheng, Shuoyan Liu, Zhilian She, Kunshou Zhu, Weidong Zang, Weizhong Ruan, Weimin Fang, Lin Li, Mingqiang Chen, Derong Zhang, Shaofeng Lin, Shunjin Chen, Yigui Chen and Guohong Zhao for performing the surgery, Yanni Gao, Zhenzhou Xiao, Su Lin, Xuehong Liao, Wenhui Jiang, Jieqiong Lin, Xinjing Li, Yi Shi, Xiaojiang Wang, Shanfeng Jin, Hongfei Wang, Wucheng Shen, Weifeng Zhu, Xiaowen Cai, Baozhen Chen, Tongmei Chen, Xueyan Chen and Lifang Chen for collecting the blood/tissue samples and performing the follow-up investigations.
Financial support
This study was supported by the Joint Funds for the Innovation of Science and Technology of Fujian Province (Grant No. 2017Y9090 and 2017Y9082), the Fundamental Research Funds for the Central Universities (Grant No. 3332018170) and the Science and Technology Program of Fujian Province (Grant No. 2018Y2003). The funder of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributors
WN, FP and XZ drafted the protocol; DH, XL, HZ and YX obtained statutory and ethics approvals; DH, XL, HZ and YX contributed to data acquisition; FP, DH, XZ and WN had access to all raw data; RJ, DH, JL, XZ and WN did the data preparation, quality control and analyses, and checked the results; WN and RJ drafted the report. All authors contributed to writing the final report and approved the version to be published.
Human rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cooper RS, Kaufman JS, Bovet P. Global Burden of Disease Attributable to Hypertension. JAMA. 2017;317:2017-8
2. Krzesinski P, Stanczyk A, Piotrowicz K, Gielerak G, Uzieblo-Zyczkowska B, Skrobowski A. Abdominal obesity and hypertension: a double burden to the heart. Hypertens Res. 2016;39:349-55
3. Zhang CJ, Zhang SY, Zhang CD, Lin CR, Li XY, Li QY. et al. Usefulness of bevacizumab-induced hypertension in patients with metastatic colorectal cancer: an updated meta-analysis. Aging (Albany NY). 2018;10:1424-41
4. Nasserinejad M, Baghestani AR, Shojaee S, Pourhoseingholi MA, Najafimehr H, Haghazali M. Diabetes mellitus and hypertension increase the risk of colorectal cancer mortality; a robust Bayesian adjustment analysis. Gastroenterol Hepatol Bed Bench. 2017;10:S44-S7
5. Corrao G, Scotti L, Bagnardi V, Sega R. Hypertension, antihypertensive therapy and renal-cell cancer: a meta-analysis. Curr Drug Saf. 2007;2:125-33
6. Morgado M, Rolo S. Factors influencing medication adherence and hypertension management revisited: recent insights from cancer survivors. Hypertens Res. 2012;35:894-6
7. Leiba A, Kark JD, Afek A, Derazne E, Keinan-Boker L, Shamiss A. et al. Hypertension in adolescence is not an independent risk factor for renal cancer: a cohort study of 918,965 males. J Am Soc Hypertens. 2013;7:283-8
8. Lin CC, Huang KW, Luo JC, Wang YW, Hou MC, Lin HC. et al. Hypertension is an important predictor of recurrent colorectal adenoma after screening colonoscopy with adenoma polypectomy. J Chin Med Assoc. 2014;77:508-12
9. Peng F, Hu D, Lin X, Chen G, Liang B, Zhang H. et al. Analysis of Preoperative Metabolic Risk Factors Affecting the Prognosis of Patients with Esophageal Squamous Cell Carcinoma: The Fujian Prospective Investigation of Cancer (FIESTA) Study. EBioMedicine. 2017;16:115-23
10. Hu D, Peng F, Lin X, Chen G, Zhang H, Liang B. et al. Preoperative Metabolic Syndrome Is Predictive of Significant Gastric Cancer Mortality after Gastrectomy: The Fujian Prospective Investigation of Cancer (FIESTA) Study. EBioMedicine. 2017;15:73-80
11. Peng F, Hu D, Lin X, Chen G, Liang B, Zhang H. et al. Preoperative metabolic syndrome and prognosis after radical resection for colorectal cancer: The Fujian prospective investigation of cancer (FIESTA) study. Int J Cancer. 2016;139:2705-13
12. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269-324
13. Hu D, Peng F, Lin X, Chen G, Liang B, Chen Y. et al. Prediction of three lipid derivatives for postoperative gastric cancer mortality: the Fujian prospective investigation of cancer (FIESTA) study. BMC Cancer. 2018;18:785
14. Peng F, Hu D, Lin X, Chen G, Liang B, Chen Y. et al. An in-depth prognostic analysis of baseline blood lipids in predicting postoperative colorectal cancer mortality: The FIESTA study. Cancer Epidemiol. 2018;52:148-57
15. Peng F, Hu D, Lin X, Liang B, Chen Y, Zhang H. et al. Impact of long-term antihypertensive and antidiabetic medications on the prognosis of post-surgical colorectal cancer: the Fujian prospective investigation of cancer (FIESTA) study. Aging (Albany NY). 2018;10:1166-81
16. Sha H, Hu D, Wu S, Peng F, Xu G, Fan G. et al. Baseline Metabolic Risk Score and Postsurgical Esophageal Cancer-Specific Mortality: The Fujian Prospective Investigation of Cancer (FIESTA) Study. J Cancer. 2018;9:1173-81
17. Zhang H, Singer BH. Analysis of Censored Data: Survival Trees and Random Forests. 1999: pp 93-103.
18. Dupont WD, Plummer WD Jr. Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19:589-601
19. Navin S, Ioffe V. The association between hypertension and prostate cancer. Rev Urol. 2017;19:113-8
20. McIntyre WF, Oqab Z, Hopman WM, Hammad N, Baranchuk A. Hypertension due to antiangiogenic cancer therapy with VEGF inhibitors: is autonomic nervous system toxicity another possible mechanism? Can J Cardiol. 2014;30:1733 e1-2
21. Mogi M, Horiuchi M. Does chronic hypertension prevent cancer progression? Hypertens Res. 2015;38:711-2
22. Harding JL, Sooriyakumaran M, Anstey KJ, Adams R, Balkau B, Brennan-Olsen S. et al. Hypertension, antihypertensive treatment and cancer incidence and mortality: a pooled collaborative analysis of 12 Australian and New Zealand cohorts. J Hypertens. 2016;34:149-55
23. Deckers IA, van den Brandt PA, van Engeland M, van Schooten FJ, Godschalk RW, Keszei AP. et al. Polymorphisms in genes of the renin-angiotensin-aldosterone system and renal cell cancer risk: interplay with hypertension and intakes of sodium, potassium and fluid. Int J Cancer. 2015;136:1104-16
Author contact
![]() Corresponding authors: Wenquan Niu, Ph.D. Address: No.2 Yinghua East Street, Chao Yang District, Beijing 100029, China. Tel & Fax: 86-10-8420 6414. E-mail: niuwenquan_shcncom or niuwenquancom.cn or Feng Peng, M.D. Ph.D. Address: No.20 Chazhong Road, Tai Jiang District, Fuzhou 350005, Fujian, China. Tel: +86-591-8798 1637. Fax: +86-591-8798 1635. E-mail: pengfengfuzhoucom.
Corresponding authors: Wenquan Niu, Ph.D. Address: No.2 Yinghua East Street, Chao Yang District, Beijing 100029, China. Tel & Fax: 86-10-8420 6414. E-mail: niuwenquan_shcncom or niuwenquancom.cn or Feng Peng, M.D. Ph.D. Address: No.20 Chazhong Road, Tai Jiang District, Fuzhou 350005, Fujian, China. Tel: +86-591-8798 1637. Fax: +86-591-8798 1635. E-mail: pengfengfuzhoucom.

 Global reach, higher impact
Global reach, higher impact