3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(17):3933-3940. doi:10.7150/jca.29501 This issue Cite
Research Paper
Surgical Resection plus Radiofrequency Ablation versus Radical Surgery for Hepatocellular Carcinoma: A Propensity Score Matching Analysis
1. Liver Cancer Institute, Zhongshan Hospital, Fudan University; Key Laboratory of Carcinogenesis and Cancer Invasion, Ministry of Education, Fudan University, Shanghai, China
2. Institute of Biomedical Sciences, Fudan University, Shanghai, China
3. State Key Laboratory of Genetic Engineering and Collaborative Innovation Center for Genetics and Development, School of Life Sciences, Fudan University, Shanghai, China
*These authors contributed equally to this work.
Received 2018-8-27; Accepted 2019-5-9; Published 2019-6-24
Abstract
Objective: To evaluate the efficacy and safety of surgical resection plus radiofrequency ablation (SR-RFA) for multifocal hepatocellular carcinoma (HCC) with 2 or 3 nodules compared with surgical resection (SR).
Method: We retrospectively evaluated 824 consecutive HCC patients (SR, n = 754; SR-RFA, n = 70) from January 2009 to December 2015 and performed propensity score matching (PSM) to adjust for patient imbalances at a ratio of 1:4.
Results: At baseline, patients in the SR-RFA group had a smaller tumour size and worse liver function (including more ascites, a higher total bilirubin level, and a longer prothrombin time) than patients in the SR group. However, the two groups had similar overall survival (OS) and recurrence-free survival (RFS) rates (P = 0.209 and P = 0.332). The local recurrence rate of the SR-RFA group was significantly higher than that of the SR group (25.71% and 14.32%, P = 0.011). More patients in the SR-RFA group had postoperative complications (P = 0.003). In the propensity model, there was no intergroup difference in OS or RFS (P = 0.229 and P = 0.311, respectively).
Conclusion: SR-RFA provides a similar long-term survival to that on SR in HCC patients with 2 or 3 nodules, and its application should be carefully considered.
Keywords: hepatocellular carcinoma, radiofrequency ablation, surgical resection, survival rate, tumour recurrence
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third leading cause of cancer-related mortality [1]. It is considered a major public health problem in the Asia-Pacific region and results from virus-related hepatitis.
Liver transplantation, surgical resection (SR), and radiofrequency ablation (RFA) are current curative treatment modalities for HCC. However, the scarcity of donors limits the use of liver transplantation [1]. In recent years, RFA has gained popularity in the treatment of HCC due to its ease of use, safety, and minimal invasiveness. However, RFA has poor local control for patients with multiple nodules or tumours ≥ 5 cm in diameter [2-5]. Thus, SR remains the best hope for a cure, but it is suitable for only 9 - 27% of patients [6]. One important limiting factor in the determination of tumour resectability is the subsequent liver remnant volume. As most cases of HCC (approximately 80%) are associated with chronic hepatitis B virus (HBV) or hepatitis C virus infection and these infections often progress to liver fibrosis or cirrhosis, post-hepatectomy liver failure due to an insufficient future liver remnant is a common and feared complication after liver resection, especially in cases of multifocal HCC [7]. SR might improve the OS and RFS of patients with small HCC but increase complications and hospitalization duration [8, 9].
A strategy of combining RFA with SR (SR-RFA) has been applied in some conventionally unresectable multifocal liver malignancies, such as colorectal liver metastases, and has been proven safe and effective [10-12]. In SR-RFA, the surgeon resects superficial or multifocal tumours confined to one lobe and ablates the nodules that are near major vascular beds or deep within the liver with the intention of preserving more of the liver parenchyma. SR-RFA serves as a new curative treatment for patients with multifocal HCC that is traditionally deemed unresectable.
This study aimed to compare the safety and long-term outcomes of SR-RFA versus SR and to verify the feasibility and practicality of SR-RFA in the treatment of multifocal HCC with 2 or 3 nodules.
Materials and Methods
Patient selection
The study was approved by the Institutional Ethics Committee of Zhongshan Hospital, Fudan University. The medical records of patients who were diagnosed with multifocal HCC between January 2009 and December 2015 were retrospectively reviewed. Only patients who met the following inclusion criteria were enrolled: (1) >18 and ≤75 years of age; (2) multifocal HCC diagnosed by cytologic/ histologic evidence or non-invasive diagnostic measurements recommended by the European Association for the Study of the Liver [1]; (3) Child-Pugh class A; (4) 2 or 3 HCC nodules without macroscopic invasion or extrahepatic metastasis; and (5) provided written informed consent. Patients were excluded if they met any of the following criteria: (1) previous history of anti-cancer treatment of HCC; (2) history of other malignancies; (3) Child-Pugh class B/C; (4) cardiac, pulmonary, cerebral, or renal dysfunction; (5) extrahepatic metastasis or macroscopic vascular invasion; and (6) conversion to liver transplantation during the study period or completely lost to follow-up.
Diagnosis and Definitions
Local recurrence was defined as a recurrent tumour observed in the residual part of the tumour- bearing 3rd-order portal branches after hepatic resection or RFA adjacent to the cut surface of the liver at the time of the initial tumour recurrence; whereas nonlocal recurrence has been defined as the emergence of tumours elsewhere in or outside the liver [13]. Edmondson's grade I-II cancer cells were defined as highly differentiated, whereas Edmondson's grade III-IV were defined as lowly differentiated. Microvascular invasion (MVI) was defined as described by Manuel [14]. Postoperative complications (POC) were defined according to the Clavien-Dindo criteria: grades IIIb, IV, and V were considered severe [15]. Overall survival (OS) was defined as the time from the date of surgery to the date of death, regardless of the cause of death. Recurrence-free survival (RFS) was defined as the time from the date of surgery to the date of first documented disease recurrence by independent radiological or pathological assessment and/or the date of death from any causes, whichever occurred first [3]. Tumour staging in this study was performed according to the China staging classification [16].
Treatment
Surgical approaches
Surgery was performed with the open approach, starting with initial exploration of the abdomen and pelvis to confirm the absence of extrahepatic lesions. Intraoperative ultrasonography was conducted to identify tumour location and number as well as the relationship with the vasculature of the liver. When complete resection of all lesions was not possible, SR-RFA was performed during a single surgical procedure to achieve curative resection.
The SR-RFA technique was described in detail previously [17, 18]. An RFA electrode using the Cool-Tip system (Radionics, Inc., Burlington, MA, USA) was inserted into the target lesion under ultrasound guidance. A single needle or needle cluster was used according to target tumour size. For tumours > 3 cm but < 5 cm, a cluster probe was used. The endpoint of ablation was the complete ablation of visible tumours and margins of at least 0.5-1.0 cm of the surrounding liver parenchyma (internally cooled, 12-18 minutes).
All patients in SR-RFA group were originally enlisted for resection only, but due to various reasons, the operative surgeon adopted RFA as an additional treatment modality with the curative intent of for complete tumour control. The reasons for adoption of combination therapy included bilobar disease (n = 21), proximity to major vessels or the bile duct (n = 19), and small tumours deep in the liver requiring extensive resection (n = 30).
Postoperative treatment
Postoperatively, any patient who met the antiviral therapy criteria of the Asian Pacific Association for the Study of the Liver received lamivudine 100 mg or entecavir 0.5-1.0 mg daily. Adefovir 10 mg was added in case of lamivudine or entecavir resistance [19]. When a patient's liver function recovered at 4 - 6 weeks after SR-RFA or SR, adjuvant transcatheter arterial chemoembolization (TACE) was arranged as described previously [9, 20, 21].
All patients with recurrences after SR-RFA or SR were treated according to tumour size, location, and number as well as liver function. For patients who had tumour recurrence or resectable extrahepatic metastasis after the initial treatment, SR or RFA was performed if it was judged feasible on the basis of liver function reserve and residual liver volume. TACE or sorafenib therapy was applied if the SR and RFA outcomes were unfavourable.
Flowchart of patient selection. HCC, hepatocellular carcinoma; SR-RFA, surgical resection plus radiofrequency ablation; SR, surgical resection.
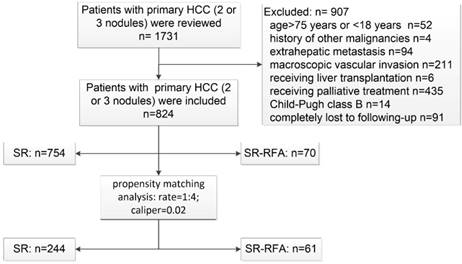
Patient follow-up
Patients were followed up in our clinic once a month in the first postoperative year and once every 3-4 months thereafter. Liver function tests results, serum alpha-fetoprotein (AFP) levels, and haematological parameters were examined. Liver ultrasonography was performed by clinicians blinded to the patients' treatment information. Computed tomography of the chest, abdomen, and pelvis was performed every 6 months. If tumour recurrence in the liver was suspected, contrast-enhanced computed tomography/magnetic resonance imaging or biopsy of the lesions was performed. A bone scan was performed to exclude metastasis as necessary.
Endpoint
The primary endpoint of this study is OS, while the secondary endpoints are RFS and treatment safety.
Propensity score matching analysis
Propensity score matching (PSM) analysis was used to reduce the bias in patient selection to examine the differences between the SR-RFA and SR groups. Variables showing a statistically significant difference or that were associated with patient selection, including age, maximal tumour size, tumour number, degree of cirrhosis, and AFP level, were comprehensively included in the calculation of the propensity score. The calliper value was set at 0.02. Binary logistic regression with selected variables was used to generate a propensity score of 0 to 1. Nearest-neighbour matching without replacement at a ratio of 1:4 was chosen as the matching algorithm.
Statistics
Patients' baseline characteristics were presented as mean ± standard deviation or percentage, as appropriate. The Mann-Whitney U test and Student's t-test were used to compare continuous variables, whereas the χ2 and Fisher's exact test were used to compare categorical variables. OS and RFS rates were examined using the Kaplan-Meier method with log-rank tests. Prognostic factors potentially related to survival included age, tumour number, tumour size, ablated tumour size, tumour capsule, differentiation degree, MVI, cirrhosis, HBV infection, serum bilirubin level, international normalized ratio of prothrombin time (INR), albumin level, and AFP level. Factors with P values <0.10 in univariate analyses were introduced into the multivariate Cox proportional hazards model to determine their independent impacts on OS and RFS. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using a non-parametric log-rank test, the Cox proportional hazards model. All statistical analyses were conducted using SPSS for Windows version 24.0 (IBM, NY, USA). Two-tailed P values <0.05 were considered statistically significant.
Results
Patients
A total of 824 HCC patients who met the criteria were enrolled between January 2009 and December 2015. Among them, 754 received SR and 70 underwent SR-RFA as the primary radical treatment. The patient selection process is detailed in Figure 1. The median follow-up durations were 37 and 31 months, respectively. PSM analysis identified 305 patients at a ratio of 1:4.
Characteristics, recurrence, and survival of all patients
The baseline demographic and clinicopathological data of the 824 patients are displayed in Table 1. The median age of the study population was 55 years (54 years in the SR group, 57 years in the SR-RFA group). The SR-RFA group was associated with a higher serum total bilirubin level, larger INR, and more ascites (All P < 0.050) than the SR group. More patients in the SR-RFA group had cirrhosis (P = 0.004). The maximal tumour diameter was significantly smaller in the SR-RFA group than in the SR group (4.0 ± 2.6 cm and 4.9 ± 3.2 cm, respectively; P = 0.021), but the summed tumour size of the two groups was similar (5.7±2.9 cm and 6.6±3.7 cm, respectively; P= 0.064).
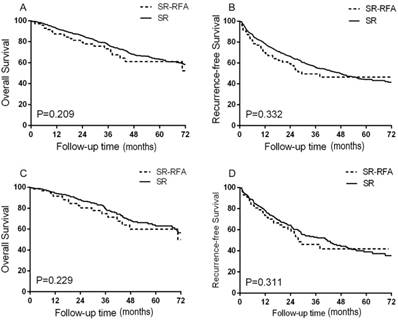
No significant intergroup differences were found in OS or RFS. The median survival time has not yet been reached. The 1-, 3-, and 5-year OS rates were 92.5%, 77.2%, and 63.2% in the SR group and 87.3%, 73.2%, and 61.1% in the SR-RFA group (P = 0.209; Figure 2A). The estimated 1-, 3-, and 5-year RFS rates were 78.6%, 55.6%, and 44.2% in the SR group and 69.9%, 49.5%, and 46.4% in the SR-RFA group (P = 0.332; Figure 2B).
The characteristics of patients selected for the propensity model are shown in Table 2. All variates associated with treatment selection and survival were well-matched (all P > 0.05). OS and RFS did not differ significantly in the propensity model of the SR and SR-RFA groups (Figures 2C, 2D). The 1-, 3-, and 5-year OS rates were 94.3%, 79.8%, and 53.0% for the SR group and 91.6%, 74.8%, and 59.9% for the SR-RFA group (P = 0.229). The 1-, 3-, and 5-year RFS rates were 78.0%, 53.9%, and 39.0% in the SR group and 73.6%, 46.1%, and 41.9% in the SR-RFA group, respectively (P = 0.311).
Complications, perioperative mortality, and recurrence
The SR group had an advantage over the SR-RFA group in terms of POC (P = 0.003) and severe complications (P = 0.016). Sixteen patients (22.9%) in the SR-RFA group had postoperative complications, including 4 grade IV complications; among the 82 patients (10.9%) in the SR group were 2 with grade IIIb and 7 with grade IV complications. The median hospital stay was 8 days (range, 4-34 days) and 8 days (4-37 days) in the SR and SR-RFA groups, respectively (P = 0.990). In the propensity model, the two groups had similar POC. POC occurred in 25 patients of the SR group and 11 of the SR-RFA group (P = 0.092). There were no intergroup differences in the postoperative 90-day mortality rate (1.19% versus 1.43%, respectively; P = 0.591). During the follow-up period, 302 patients (40.1%) of the SR group and 31 patients (44.3%) of the SR-RFA group suffered tumour recurrence (P = 0.490). There were 108 and 18 local recurrences in the SR and SR-RFA group, respectively; patients in the SR-RFA group had a significantly higher local recurrence rate than those in the SR group (25.71% and 14.32%, P=0.011). After the propensity matching, 99 patients in the SR group and 27 patients in the SR-RFA group had tumour recurrence (P= 0.601), while 28 patients in the SR group and 13 patients in the SR-RFA group had local recurrence (P = 0.043).
Clinical Characteristics of All Patients
| Variable | SR (n = 754) | SR-RFA (n = 70) | P Value |
|---|---|---|---|
| Age, yr, mean(SD) | 54(10) | 57(9) | 0.030 |
| Gender | 0.206 | ||
| Male | 673(89.3%) | 59(84.3%) | |
| Female | 81(10.7%) | 11(15.7%) | |
| Tumor Number | 0.054 | ||
| 2 | 611(81.0%) | 50(71.4%) | |
| 3 | 143(19.0%) | 20(28.6%) | |
| Maximal Tumor Size, mean(SD) | 4.9(3.2) | 4.0(2.6) | 0.021 |
| Sum of Tumor Size, mean(SD) | 6.6(3.7) | 5.7(2.9) | 0.064 |
| Ascites | 0.013 | ||
| no | 710(94.2%) | 60(85.7%) | |
| yes | 44(5.8%) | 10(14.3%) | |
| Total bilirubin, μmol/L | 0.031 | ||
| <22.4 | 712(94.4%) | 61(87.1%) | |
| ≥22.4 | 42(5.6%) | 9(12.9%) | |
| Albumin, g/l | 0.068 | ||
| ≥35 | 714(94.7%) | 62(88.6%) | |
| <35 | 40(5.3%) | 8(11.4%) | |
| INR, | 0.000 | ||
| <1.13 | 734(97.3%) | 69(98.6%) | |
| ≥1.13 | 17(2.3%) | 1(1.4%) | |
| Hepatitis B surface antigen | 0.875 | ||
| positive | 652(86.5%) | 61(87.1%) | |
| negative | 102(13.5%) | 9(12.9%) | |
| Alpha-fetoprotein, ng/mL | 0.653 | ||
| <400 | 530(70.3%) | 51(72.9%) | |
| ≥400 | 224(29.3%) | 19(27.1%) | |
| Tumor capsule | 0.186 | ||
| Complete | 342(45.4%) | 26(37.1%) | |
| Incomplete | 412(54.6%) | 44(62.9%) | |
| Tumor Differentiation | 0.505 | ||
| high | 454(60.2%) | 45(64.3%) | |
| low | 300(39.8%) | 25(35.7%) | |
| Microvascular Invasion | 0.345 | ||
| positive | 452(59.9%) | 46(65.7%) | |
| negative | 302(40.1%) | 24(34.3%) | |
| Cirrhosis | 0.004 | ||
| no | 294(39.0%) | 15(21.4%) | |
| yes | 460(61.0%) | 55(78.6%) | |
| Hemorrhage, ml, mean(SD) | 231(222) | 196(221) | 0.142 |
| China staging classification | 0.032 | ||
| Ⅰb | 210(27.9%) | 28(40.0%) | |
| Ⅱa | 544(72.1%) | 42(60.0%) | |
| Tumor location | 0.255 | ||
| I | 21(1.3%) | 1(0.7%) | |
| II | 77(4.7%) | 9(6.7%) | |
| III | 106(6.4%) | 9(6.7%) | |
| IV | 284(17.2%) | 19(14.2%) | |
| V | 281(17.0%) | 28(20.9%) | |
| VI | 330(20.0%) | 26(19.4%) | |
| VII | 284(17.2%) | 31(23.1%) | |
| VIII | 268(16.2%) | 37(27.6%) |
SR, surgical resection; RFA, radiofrequency ablation; SD, standard deviation; INR, international normalized ratio of prothrombin.
Clinical Characteristics of Patients in the Propensity Model
| Variable | SR (n = 244) | SR-RFA ( n= 61) | P Value |
|---|---|---|---|
| Age, yr, mean(SD) | 56(10) | 56(10) | 0.796 |
| Gender | 0.298 | ||
| Male | 216(88.5%) | 51(83.6%) | |
| Female | 28(11.5%) | 10(16.4%) | |
| Tumor Number | 0.727 | ||
| 2 | 191(78.3%) | 49(80.3%) | |
| 3 | 53(21.7%) | 12(19.7%) | |
| Maximal Tumor Size, mean(SD) | 4.2(2.5) | 3.9(2.6) | 0.350 |
| Sum of Tumor Size, mean(SD) | 5.9(3.1) | 5.6(2.9) | 0.565 |
| Ascites | 0.050 | ||
| no | 229(93.9%) | 52(85.2%) | |
| yes | 15(6.1%) | 9(14.8%) | |
| Total bilirubin, μmol/L | 0.062 | ||
| <22.4 | 231(94.7%) | 53(86.9%) | |
| ≥22.4 | 13(5.3%) | 8(13.1%) | |
| Albumin, g/l | 0.110 | ||
| ≥35 | 232(95.1%) | 54(88.5%) | |
| <35 | 12(4.9%) | 7(11.5%) | |
| INR, | 0.573 | ||
| <1.13 | 240(98.4%) | 60(98.4%) | |
| ≥1.13 | 4(1.6%) | 1(1.6%) | |
| Hepatitis B surface antigen | 0.854 | ||
| positive | 218(89.3%) | 54(88.5%) | |
| negative | 26(10.7%) | 7(11.5%) | |
| Alpha-fetoprotein, ng/mL | 0.831 | ||
| <400 | 195(79.9%) | 48(78.7%) | |
| ≥400 | 49(20.1%) | 13(21.3%) | |
| Tumor capsule | 0.480 | ||
| Complete | 96(39.3%) | 21(34.4%) | |
| Incomplete | 148(60.7%) | 40(65.6%) | |
| Tumor Differentiation | 0.810 | ||
| high | 160(65.6%) | 39(63.9%) | |
| low | 84(34.4%) | 22(36.1%) | |
| Microvascular Invasion | 0.808 | ||
| positive | 184(75.4%) | 40(65.6%) | |
| negative | 80(32.8%) | 21(34.4%) | |
| Cirrhosis | 0.090 | ||
| no | 88(36.1%) | 15(24.6%) | |
| yes | 156(63.9%) | 46(75.4%) | |
| Hemorrhage, ml, mean(SD) | 216(223) | 172(202) | 0.160 |
| China staging classification | 0.149 | ||
| Ⅰb | 80(32.8%) | 26(42.6%) | |
| Ⅱa | 164(67.2%) | 35(57.4%) | |
| Tumor location | 0.520 | ||
| I | 6(1.1%) | 0(0.0%) | |
| II | 26(4.8%) | 9(6.7%) | |
| III | 39(7.2%) | 8(6.0%) | |
| IV | 93(17.2%) | 18(13.4%) | |
| V | 89(16.5%) | 26(19.4%) | |
| VI | 102(18.9%) | 20(14.9%) | |
| VII | 94(17.4%) | 24(17.9%) | |
| VIII | 92(17.0%) | 29(21.6%) | |
SR, surgical resection; RFA, radiofrequency ablation; SD, standard deviation; INR, international normalized ratio of prothrombin.
Factors associated with long-term survival
Treatment strategy was not associated with long-term survival in the entity popularity or the propensity model (P = 0.270 and P = 0.231, respectively). In univariate analysis of the OS of all patients, 3 nodules, maximal tumour size ≥ 5 cm, AFP level > 400 ng/mL, low degree of differentiation, and MVI were associated with increased mortality rates (all P < 0.05; Table 3). In the adjusted Cox proportional hazards model, 3 independent prognostic predictors of poor survival were identified: 3 nodules (HR, 1.640; 95% CI, 1.229-2.188; P = 0.001), maximal tumour size ≥ 5 cm (HR, 1.791; 95% CI, 1.378-2.328; P < 0.001), and MVI (HR, 1.645; 95% CI, 1.269-2.132; P < 0.001). In the propensity model, maximal tumour size ≥ 5 cm (HR, 1.730; 95% CI, 1.062-2.817; P = 0.028) and MVI (HR, 1.863; 95% CI, 1.208-2.873; P = 0.005) were identified as independent prognostic predictors of poor survival (Table 4).
RFS was not associated with the surgical approach in the entity popularity or in selected patients (P = 0.258 and P = 0.578, respectively). In the univariate analysis of the RFS of all patients, 3 nodules, maximal tumour size ≥ 5 cm, AFP level ≥ 400 ng/mL, and MVI were associated with an increased rate of recurrence (all P < 0.05; Table 5). In the Cox proportional hazards model, 3 independent predictors of tumour recurrence were identified: 3 nodules (HR, 1.420; 95% CI, 1.118-1.803; P = 0.004), maximal tumour size ≥ 5 cm (HR, 1.311; 95% CI, 1.054-1.630; P = 0.015), and MVI (HR, 1.255; 95% CI, 1.019-4.545; P = 0.032). In the univariate analysis of RFS for patients selected through PSM, maximal tumour size ≥ 5 cm (HR, 1.726; 95% CI, 1.170-2.546; P = 0.006) was the only factor related to RFS (Table 6).
Subgroup analysis
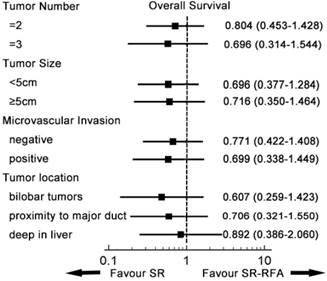
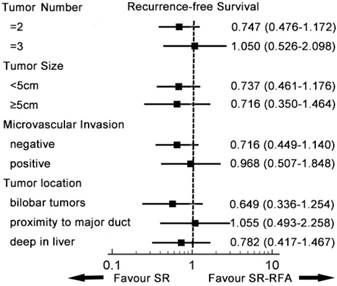
A subgroup analysis was conducted to compare survival from SR-RFA and SR in the entity popularity (Figures 3, 4). Patients were divided into different groups according to independent prognostic factors and tumour location. In all subgroups, SR-RFA and SR had similar effects on OS and RFS (all P > 0.05).
Univariate and Multivariate Analysis for OS in Patients receiving radical treatment
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | CI | P value | HR | CI | P value | |
| SR-RFA | 0.781 | 0.504-1.211 | 0.270 | |||
| Age ≥ 60 yr | 1.141 | 0.857-1.519 | 0.367 | |||
| Sex of Female | 0.912 | 0.619-1.345 | 0.642 | |||
| Tumor number of 3 | 1.588 | 1.191-2.177 | 0.002 | 1.640 | 1.229-2.188 | 0.001 |
| Tumor size≥ 5 cm | 1.949 | 1.508-2.519 | 0.000 | 1.791 | 1.378-2.328 | 0.000 |
| Cirrhosis | 0.976 | 0.895-1.065 | 0.586 | |||
| HBsAg (+) | 0.809 | 0.570-1.148 | 0.236 | |||
| AFP≥ 400 ng/mL | 1.393 | 1.069-1.817 | 0.014 | |||
| Incomplete Tumor capsule | 1.086 | 0.949-1.243 | 0.232 | |||
| Low Differentiate | 1.200 | 1.057-1.364 | 0.005 | |||
| Microvascular Invasion | 1.770 | 1.374-2.281 | 0.000 | 1.645 | 1.269-2.132 | 0.000 |
SR, surgical resection; RFA, radiofrequency ablation; HR: hazard ratio; CI: confidence interval; HBsAg, Hepatitis B surface antigen; AFP, alpha-fetoprotein
Univariate and Multivariate Analysis for OS in Patients receiving radical treatment after PSM
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | CI | P value | HR | CI | P value | |
| SR-RFA | 1.366 | 0.820-2.274 | 0.231 | |||
| Age ≥ 60 yr | 1.337 | 0.864-2.069 | 0.193 | |||
| Sex of Female | 1.004 | 0.546-1.847 | 0.991 | |||
| Tumor number of 3 | 1.201 | 0.734-1.963 | 0.466 | |||
| Tumor size≥ 5 cm | 1.857 | 1.145-3.014 | 0.012 | 1.730 | 1.062-2.817 | 0.028 |
| Cirrhosis | 1.045 | 0.897-1.218 | 0.570 | |||
| HBsAg (+) | 0.600 | 0.326-1.105 | 0.101 | |||
| AFP≥ 400 ng/mL | 1.342 | 0.814-2.211 | 0.249 | |||
| Tumor capsule | 0.995 | 0.801-1.235 | 0.961 | |||
| Low Differentiate | 1.206 | 0.967-1.505 | 0.097 | |||
| Microvascular Invasion | 1.947 | 1.267-2.991 | 0.002 | 1.863 | 1.208-2.873 | 0.005 |
SR, surgical resection; RFA, radiofrequency ablation; HR: hazard ratio; CI: confidence interval; HBsAg, Hepatitis B surface antigen; AFP, alpha-fetoprotein
Univariate and Multivariate Analysis for RFS in Patients receiving radical treatment
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | CI | P value | HR | CI | P value | |
| SR-RFA | 0.814 | 0.569-1.163 | 0.258 | |||
| Age ≥ 60 yr | 1.037 | 0.820-1.311 | 0.762 | |||
| Sex of Female | 1.009 | 0.730-1.396 | 0.955 | |||
| Tumor number of 3 | 1.408 | 1.109-1.787 | 0.005 | 1.420 | 1.118-1.803 | 0.004 |
| Tumor size≥ 5 cm | 1.360 | 1.097-1.685 | 0.000 | 1.311 | 1.054-1.630 | 0.015 |
| Cirrhosis | 1.007 | 0.939-1.079 | 0.855 | |||
| HBsAg (+) | 1.172 | 0.858-1.600 | 0.318 | |||
| AFP≥ 400 ng/mL | 1.310 | 1.058-1.623 | 0.013 | |||
| Tumor capsule | 0.955 | 0.863-1.057 | 0.372 | |||
| Low Differentiate | 1.086 | 0.980-1.204 | 0.117 | |||
| Microvascular Invasion | 1.302 | 1.061-1.598 | 0.012 | 1.255 | 1.019-1.545 | 0.032 |
SR, surgical resection; RFA, radiofrequency ablation; HR: hazard ratio; CI: confidence interval; HBsAg, Hepatitis B surface antigen; AFP, alpha-fetoprotein
Univariate and Multivariate Analysis for RFS in Patients receiving radical treatment after PSM
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | CI | P value | HR | CI | P value | |
| SR-RFA | 1.122 | 0.747-1.685 | 0.578 | |||
| Age ≥ 60 yr | 1.267 | 0.902-1.780 | 0.173 | |||
| Sex of Female | 1.276 | 0.770-2.177 | 0.344 | |||
| Tumor number of 3 | 1.286 | 0.892-1.853 | 0.178 | |||
| Tumor size≥ 5 cm | 1.726 | 1.170-2.546 | 0.006 | 1.726 | 1.170-2.546 | 0.006 |
| Cirrhosis | 1.036 | 0.924-1.163 | 0.543 | |||
| HBsAg (+) | 1.506 | 0.814-2.786 | 0.192 | |||
| AFP≥ 400 ng/mL | 1.035 | 0.690-1.554 | 0.867 | |||
| Tumor capsule | 0.918 | 0.779-1.082 | 0.309 | |||
| Low Differentiate | 1.134 | 0.961-1.348 | 0.134 | |||
| Microvascular Invasion | 1.191 | 0.847-1.674 | 0.316 | |||
SR, surgical resection; RFA, radiofrequency ablation; HR: hazard ratio; CI: confidence interval; HBsAg, Hepatitis B surface antigen; AFP, alpha-fetoprotein
Discussion
This study evaluated the efficiency and safety of SR-RFA for patients with multifocal HCC. Studies have shown that radical surgical treatment is superior to TACE in selected patients [22-25], while only 9-27% of patients are suitable candidates for SR at the time of HCC diagnosis [26]. HCC patients in China usually have poor liver function because of the coexistence of HBV-associated cirrhosis. Poor liver function poses an extra challenge to hepatectomy, which further reduces the already limited liver function, leading to liver failure. In patients with multifocal HCC, multiple partial resections can achieve complete tumour clearance but carry a higher risk of liver failure with doubtful long-term oncological outcomes. A series of studies showed that RFA can achieve similar long-term survival and minor invasiveness compared to SR for tumours limited to 5 cm, although patients in the RFA group had a higher local recurrence rate [27-29].
We combined SR with RFA to treat patients with multifocal HCC that was deemed traditionally unresectable by SR alone due to inadequate functional hepatic reserve. First, if a tumour is small but deep in the liver or bilobar tumours in the liver require a large resection volume for clearance, RFA is a safer option in terms of liver function preservation. Second, if a tumour is near an important anatomical structure such as major vessels or bile ducts, RFA can offer complete tumour clearance, avoiding the need for major hepatectomy. No randomized controlled trials to date have evaluated the effectiveness and safety of SR-RFA in cases of multifocal HCC. A few retrospective studies [6, 11, 12, 30-32] compared SR-RFA with traditional surgical treatment, but the characteristics of patients who received SR-RFA differed naturally from those who received SR. Therefore, a PSM analysis was applied to eliminate patient bias (tumour size, liver function) to increase the validity of our results. In this study, patients selected in the propensity model were well matched at baseline and a further analysis was performed on the base of the model. To our knowledge, this study included the largest number of HCC patients treated with SR-RFA.
If the SR-RFA patients with multiple tumours did not undergo SR-RFA, they would undergo TACE; the OS was only 16.2-22.6 months [33]. A recent study showed that SR-RFA results in better OS and longer time to progression than TACE in HCC patients with stage B Barcelona Clinic Liver Cancer [11]. Here, we used PSM to compare the survival of patients with multifocal HCC treated with SR-RFA versus SR. Our data showed that although the liver reserve function after SR-RFA was insufficient, the OS of patients treated with SR-RFA was similar to that of patients undergoing SR. Our study demonstrated the significance of SR-RFA for the treatment of patients with multifocal HCC, which is otherwise unresectable with traditional resection techniques, and with insufficient liver reserve function.
The higher local recurrence rates of SR-RFA versus SR in this study cannot be overlooked. SR, as the most recommended treatment for HCC, offers the therapeutic possibility of complete eradication of satellite tumour lesions and MVI in the adjacent vasculature. However, a series of studies mentioned increased local recurrence rates among patients treated with RFA versus SR [2, 4, 9, 27]. The application of RFA is the reason for the higher local tumour recurrence rate versus SR. The high tumour recurrence rate is an important factor of HCC that affects long-term survival. Previous randomized controlled studies showed that adjuvant TACE significantly reduced tumour recurrence rates among patients with multifocal HCC after curative hepatectomy [20] as well as patients who had tumours >3 cm [34]. Thus, adjuvant TACE was recommended for SR-RFA patients at 4-6 weeks postoperatively.
Patients treated with SR-RFA had a higher POC rate than did those treated with SR (22.9% versus 10.9%, respectively; P=0.003). The higher POC rate was associated with poorer liver function in the SR- RFA group versus the SR group, but all patients in the SR-RFA group recovered with no serious side effects, and the 90-day mortality rates did not differ (P= 0.591). We further conducted a subgroup analysis of the entity popularity, but the two treatments had similar survival effects. SR is still preferred, considering the higher local tumour recurrence of SR-RFA. In practice, SR is abandoned in many cases with tumour proximity to major vessels or the bile duct and in cases in which large-volume resection is required for small tumours, which increases surgical difficulty. SR-RFA preserved more liver parenchyma and reduced surgical difficulty, while achieving similar long-term survival rates to that on SR. However, considering the higher local tumour recurrence rate of SR-RFA, SR remains the preferred method. The additional role of RFA in SR treatment should be considered for patients with sufficient liver reserve function.
This study has several limitations. First, because of its retrospective nature, it is prone to potential bias, and PSM cannot completely eliminate selection bias. Second, as a single-centre study performed in China, the general population is under-represented. Third, most cases of HCC were related to HBV infection, as 84.7% of patients tested positive for hepatitis B surface antigen; thus, conclusions regarding implications for non-viral HCC require more evidence. Well-designed multi-centre randomized controlled trials are required to further verify the results and the feasibility of SR-RFA.
Survival analysis revealed no difference in OS (A) and RFS (B) between the SR-RFA and SR group in all patients. In the propensity model, OS (C) and RFS (D) did not differ significantly between the two groups. SR-RFA, surgical resection plus radiofrequency ablation; SR, surgical resection; OS, overall survival; RFS, recurrence-free survival.
Subgroup analysis of overall survival. In each subgroup, the overall survival of RFA-SR was similar to that of SR. SR-RFA, surgical resection plus radiofrequency ablation; SR, surgical resection.
Subgroup analysis of recurrence-free survival. In each subgroup, SR-RFA and SR had similar effects on recurrence-free survival. SR-RFA, surgical resection plus radiofrequency ablation; SR, surgical resection.
Conclusion
In conclusion, our study results demonstrate that SR-RFA can achieve parallel effects to those of SR in HCC patients with 2 or 3 nodules before or after PSM, but the higher local tumour recurrence rate and greater POC of SR-RFA cannot be neglected. Thus, the application of SR-RFA should be carefully considered.
Competing Interests
The authors have declared that no competing interest exists.
References
1. EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236
2. Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J. et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-12
3. Lee D, Lee J, Lee J, Kim S, Yoon J, Kim Y. et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900-9
4. Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L. et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59:300-7
5. Qi X, Zhao Y, Li H, Guo X, Han G. Management of hepatocellular carcinoma: an overview of major findings from meta-analyses. Oncotarget. 2016;7(23):34703-34751
6. Itoh S, Morita K, Ueda S, Sugimachi K, Yamashita Y-i, Gion T. et al. Long-term results of hepatic resection combined with intraoperative local ablation therapy for patients with multinodular hepatocellular carcinomas. Ann Surg Oncology. 2009;16:3299-307
7. Van Mierlo KM, Schaap FG, Dejong CH, Olde Damink SW. Liver resection for cancer: new developments in prediction, prevention and management of postresectional liver failure. J Hepatol. 2016;65:1217-31
8. Qi X, Tang Y, An D, Bai M, Shi X, Wang J, Han G, Fan D. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: a meta-analysis of randomized controlled trials. J Clin Gastroenterol. 2014;48(5):450-7
9. Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2018;287(2):461-472
10. Espinosa W, Liu Y, Wang C, Lin C, Wang J, Lu S. et al. Combined resection and radiofrequency ablation versus transarterial embolization for intermediate-stage hepatocellular carcinoma: A propensity score matching study. J Formos Med Assoc. 2018;117:197-203
11. Cheung T, Ng K, Chok K, Chan S, Poon R, Lo C. et al. Combined resection and radiofrequency ablation for multifocal hepatocellular carcinoma: prognosis and outcomes. World J Gastroenterol. 2010;16:3056-62
12. Qiu J, Chen S, Wu H. Long-term outcomes after hepatic resection combined with radiofrequency ablation for initially unresectable multiple and bilobar liver malignancies. J Surg Res. 2014;188:14-20
13. Shindoh J, Makuuchi M, Matsuyama Y, Mise Y, Arita J, Sakamoto Y. et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol. 2016;64:594-600
14. Rodríguez-Perálvarez M, Luong T, Andreana L, Meyer T, Dhillon A, Burroughs A. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325-39
15. Clavien P, Barkun J, de Oliveira M, Vauthey J, Dindo D, Schulick R. et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-96
16. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M. et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer. 2018;7(3):235-60
17. Zhou XD, Tang ZY, Yang BH, Lin ZY, Ma ZC, Ye SL. et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91:1479-86
18. Chen R, Gan Y, Ge N, Chen Y, Wang Y, Zhang B. et al. Transarterial chemoembolization versus radiofrequency ablation for recurrent hepatocellular carcinoma after resection within Barcelona Clinic Liver Cancer Stage 0/A: a retrospective comparative study. J Vasc Interv Radiol. 2016;27:1829-36
19. Sarin S, Kumar M, Lau G, Abbas Z, Chan H, Chen C. et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98
20. Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L. et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24:2074-81
21. Vlachogiannakos J, Papatheodoridis G. Hepatitis B: who and when to treat? Liver Int. 2018;38(Suppl 1):71-8
22. Ciria R, López-Cillero P, Gallardo AB, Cabrera J, Pleguezuelo M, Ayllón MD. et al. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: modern surgical resection as a feasible alternative to transarterial chemoembolization. Eur J Surg Oncol. 2015;41:1153-61
23. Torzilli G, Donadon M, Marconi M, Palmisano A, Fabbro DD, Spinelli A. et al. Hepatectomy for stage B and stage C hepatocellular carcinoma in the Barcelona Clinic Liver Cancer classification: results of a prospective analysis. Arch Surgery. 2008;143:1082-90
24. Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transplantation. 2004;10:S46
25. Kim H, Ahn S, Hong S, Yoon K, Kim H, Choi Y. et al. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br J Surg. 2017;104:1045-52
26. Zhong J, Ke Y, Gong W, Xiang B, Ma L, Ye X. et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329-40
27. Lai E, Fan S, Lo C, Chu K, Liu C, Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg. 1995;221:291-8
28. Feng K, Yan J, Li X, Xia F, Ma K, Wang S. et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794-802
29. Zhang W, Jiang L, Yan L, Yang J, Li B, Wen T. et al. Radiofrequency ablation for HCC patients with multifocal tumours meeting the Milan criteria: a single-center experience. Dig Liver Dis. 2016;48:1485-91
30. Choi D, Lim H, Joh J, Kim S, Kim M, Rhim H. et al. Combined hepatectomy and radiofrequency ablation for multifocal hepatocellular carcinomas: long-term follow-up results and prognostic factors. Ann Surg Oncol. 2007;14:3510-8
31. Eisele R, Zhukowa J, Chopra S, Schmidt S, Neumann U, Pratschke J. et al. Results of liver resection in combination with radiofrequency ablation for hepatic malignancies. Eur J Surg Oncol. 2010;36:269-74
32. Zhang T, Zeng Y, Huang J, Liao M, Wu H. Combined resection with radiofrequency ablation for bilobar hepatocellular carcinoma: a single-center experience. J Surg Res. 2014;191:370-8
33. Bruix J, Sherman M, Llovet J, Beaugrand M, Lencioni R, Burroughs A. et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-30
34. Cheng B, Jia C, Liu C, Fan W, Wang Q, Zhang Z. et al. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299:1669-77
Author contact
![]() Corresponding authors: Dr. Zheng Wang, Liver Cancer Institute and Zhongshan Hospital, Fudan University, Shanghai, 200030, China. Phone number: 86-13817390051, E-mail: wang.zhengsh.cn; wzdoccom or to Dr. Lan Zhang, Liver Cancer Institute and Zhongshan Hospital, Fudan University, Shanghai, 200030, China. Phone number: 86-13918876432, E-mail: zhang.lansh.cn.
Corresponding authors: Dr. Zheng Wang, Liver Cancer Institute and Zhongshan Hospital, Fudan University, Shanghai, 200030, China. Phone number: 86-13817390051, E-mail: wang.zhengsh.cn; wzdoccom or to Dr. Lan Zhang, Liver Cancer Institute and Zhongshan Hospital, Fudan University, Shanghai, 200030, China. Phone number: 86-13918876432, E-mail: zhang.lansh.cn.

 Global reach, higher impact
Global reach, higher impact