3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(14):2644-2654. doi:10.7150/jca.87169 This issue Cite
Research Paper
Doublet or Triplet Antiemetic Prophylaxis for Nausea and Vomiting Induced by Trastuzumab Deruxtecan: an Open-Label, Randomized, and Multicenter Exploratory Phase 2 Study
1. Department of Pharmacy, Gifu University Hospital, 1-1 Yanagido, Gifu, Gifu 501-1194, Japan.
2. Patient Safety Division, Gifu University Hospital, 1-1 Yanagido, Gifu, Gifu 501-1194, Japan.
3. Laboratory of Pharmacy Practice and Social Science, Gifu Pharmaceutical University, 1-25-4 Daigakunishi, Gifu, Gifu 501-1196, Japan.
4. Department of Biostatistics, Yamaguchi University Graduate School of Medicine, 1-1-1 Minamikogushi, Ube, Yamaguchi 755-8505, Japan.
5. Cancer Biostatistics Laboratory, Clinical Research Institute, National Hospital Organization Kyushu Cancer Center, 3-1-1 Notame, Minami-ku, Fukuoka 811-1395, Japan.
6. Institute of Medicine, Breast and Endocrine Surgery, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8575, Japan.
7. Department of Breast Surgery, Gifu University Hospital, 1-1 Yanagido, Gifu, Gifu 501-1194, Japan.
8. Department of Breast Surgery, Yokkaichi Municipal Hospital, 2-2-37 Shibata, Yokkaichi, Mie 510-8567, Japan.
9. Department of Breast Surgery, Asahi University Hospital, 3-23 Hashimoto-cho, Gifu, Gifu 500-8523, Japan.
10. Department of Breast Center, Ehime University Hospital, 454 Shitsukawa, Toon, Ehime 791-0295, Japan.
11. Department of Breast Surgery, Miyagi Cancer Center, 47-1 Aza-nodayama, Medeshima-shiote, Natori, Miyagi 981-1239, Japan.
12. Department of First Surgery, Yamagata University Graduate School of Medicine, 2-2-2 Iida-Nishi, Yamagata, Yamagata, 990-9585, Japan (present address).
13. Department of Surgery, Iwate Medical University, 2-1-1 Idaidori, Yahaba, Shiwa, Iwate 028-3695, Japan.
14. Department of Breast Surgery, Central Japan International Medical Hospital, 1-1 Kenkonomachi, Minokamo, Gifu 505-8510, Japan.
15. Department of Surgery, Gihoku Kosei Hospital, 1187-3 Takatomi, Yamagata, Gifu 501-2105, Japan.
16. Department of Pharmacy, Fukushima Medical University Hospital, 1 Hikariga-oka, Fukushima 960-1247, Japan.
17. Department of Breast Surgery, Gifu Municipal hospital, 7-1 Kashimacho, Gifu, Gifu 500-8513, Japan.
18. Department of Breast Surgery, Gifu Prefectural General Medical Center, 4-6-1 Noishiki, Gifu, Gifu 500-8717, Japan.
19. Department of Breast and Endocrine Surgery, St. Marianna University School of Medicine, 2-16-1 Sugao, Miyamae, Kawasaki, Kanagawa 216-8511, Japan.
20. Department of Pharmacy, Tohoku Rosai Hospital, 4-3-21 Dainohara, Miyagino, Sendai, Miyagi 981-8563, Japan.
21. Department of breast, thyroid, endocrine surgery, University of Tsukuba Hospital, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576, Japan.
22. Department of Pharmacy, Yokkaichi Municipal Hospital, 2-2-37 Shibata, Yokkaichi, Mie 510-8567, Japan.
23. Department of Pharmacy, Asahi University Hospital, 3-23 Hashimoto-cho, Gifu, Gifu 500-8523, Japan.
24. Department of Pharmacy, Miyagi Cancer Center, 47-1 Nodayama, Medeshimashiote, Natori, Miyagi 981-1293, Japan.
25. Laboratory of Advanced Medical Pharmacy, Gifu Pharmaceutical University, 1-25-4 Daigakunishi, Gifu, Gifu, 501-1196, Japan.
Abstract
Background: Trastuzumab deruxtecan is classified as an anticancer agent that poses a moderate emetic risk in the international guidelines for antiemetic therapy. The guidelines recommend emesis prophylaxis using a two-drug combination therapy comprising a 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA) and dexamethasone (DEX). However, the high incidence of nausea and vomiting associated with trastuzumab deruxtecan is problematic. The National Comprehensive Cancer Network guideline version 1.2023 classified trastuzumab deruxtecan as having a high risk of emesis and changed its recommendation to a triplet regimen including a neurokinin-1 receptor antagonist (NK1RA). However, the emetogenic potential of trastuzumab-deruxtecan and the optimal antiemetic prophylaxis are controversial. Hence, this exploratory phase 2 study aimed to assess the efficacy and safety of treatment comprising 5-HT3RA and DEX with or without a NK1RA in preventing trastuzumab deruxtecan-induced nausea and vomiting.
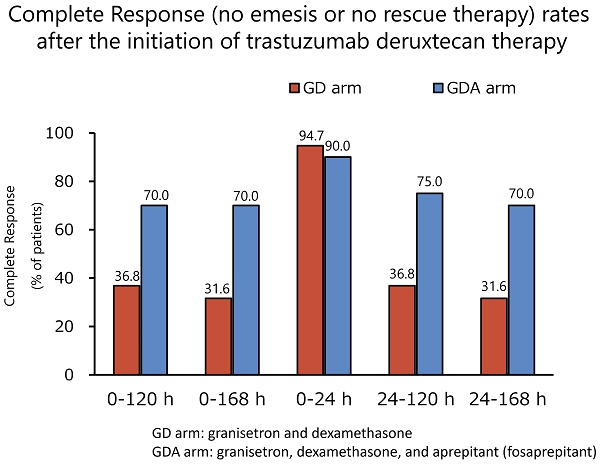
Methods: We conducted an open-label and randomized exploratory phase 2 study at 14 centers in Japan. Patients with breast cancer who were scheduled to receive trastuzumab deruxtecan were enrolled in this study. The patients were randomly assigned to receive granisetron and DEX (arm GD) or granisetron, DEX, and aprepitant (fosaprepitant; arm GDA). The primary endpoint was complete response (CR; no emesis or no rescue therapy) during the overall phase (120 h after the start of trastuzumab deruxtecan).
Results: Between September 2020 and March 2023, 40 patients were randomly assigned to the GD (n = 19) or GDA (n = 21) arm. In the GDA arm, one patient who did not complete the use of the rescue medication listed in the diary was excluded from the efficacy analysis, which included the use of rescue medication. The CR rates during the overall phase were 36.8% and 70.0% in the GD and GDA arms, respectively (odds ratio 0.1334; 95% confidence interval [CI]: 0.0232-0.7672; P = 0.0190), with a difference of 33.2%. No grade 3 or 4 toxicity related to antiemetic therapy was observed.
Conclusions: Patients receiving trastuzumab deruxtecan require triple therapy, including mandatory NK1RA administration.
Keywords: breast cancer, antiemetic regimen, trastuzumab deruxtecan, nausea, vomiting

 Global reach, higher impact
Global reach, higher impact