3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(14):2686-2693. doi:10.7150/jca.86635 This issue Cite
Research Paper
Colon Adenoma After Diagnosis of Immune Checkpoint Inhibitor-mediated Colitis
1. Department of Internal Medicine, The University of Texas Health Science Center, Houston, TX, USA.
2. Department of Gastroenterology, Hepatology, and Nutrition, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
3. Department of Medicine II, University Hospital, Ludwig-Maximilians-University Munich, Munich, Germany.
4. Inflammatory Bowel Disease Center, Cleveland Clinic, Cleveland, OH, USA.
5. Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
6. Department of Hospital Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Abstract
Purpose: While the occurrence of colitis during immune checkpoint inhibitor (ICI) treatment is recognized as a sign of robust immune activation and correlates with better oncological outcomes, the long-term impact of ICI-mediated colitis on the colonic mucosa has not been studied. We thus aim to describe the colonoscopy and histology findings in patients at a follow-up time of ≥ 6 months post initial colitis event.
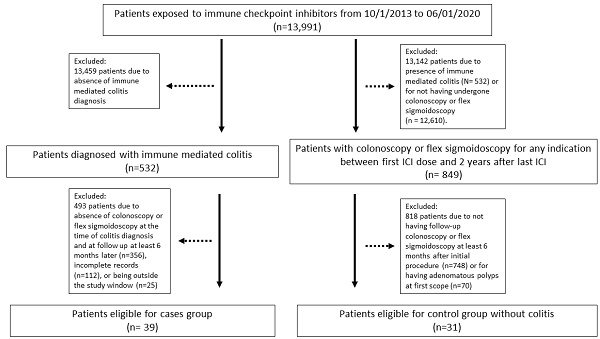
Methods: This retrospective analysis included adult cancer patients diagnosed with ICI colitis at a tertiary cancer center between October 2013 and June 2020. The study group included patients diagnosed with immune mediated colitis who had also undergone a follow up colonoscopy or flex sigmoidoscopy. The control group was patients exposed to ICI without immune mediated colitis. We reported patients' colitis clinical course, treatment, outcomes, and endoscopic and histologic features at diagnosis and at follow-up time of ≥ 6 months.
Results: Total 39 patients met the study criteria, with 82% being male, and 35.8% having melanoma. Most patients received a combination of CTLA-4 and PD-1/L1 inhibitors (82%). On initial endoscopic evaluation, inflammation without ulceration was reported in 76.9% of patients and active inflammation on histologic examination in 79.3% of patients. Most patients (79.4%) received corticosteroids, and 56.4% received add-on selective immunosuppressive therapy. Four patients received fecal microbiota transplantation. On follow-up, new incidence of colonic polyps was reported in 51.2% of patients, including adenomas in 33.3% among the colitis patients with median follow up duration of 12 months. The incidence of adenoma polyps 12 months after the colitis event was significantly higher compared to the control group without colitis based on the time-to-event analysis (p=0.041).
Conclusion: At a median follow up of 12 months after their initial colitis diagnosis, 51.2% of the patients had new incidence of colonic polyps, including a third with adenoma, at a significantly higher incidence than the control group without colitis. Studies with larger sample sizes are needed to further define the long-term impact of colitis and its treatments on colon health and to refine recommendations for surveillance of colonic adenomas and colorectal cancer.
Keywords: immune checkpoint inhibitor, colitis, adenoma, colonoscopy, surveillance

 Global reach, higher impact
Global reach, higher impact