3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(15):2751-2758. doi:10.7150/jca.85748 This issue Cite
Research Paper
Prognostic value of gender and primary tumor location in metastatic colon cancer
1. Department of Innovative Technologies in Medicine and Dentistry, and Center for Advanced Studies and Technology (CAST), G. D'Annunzio University Chieti-Pescara, 66100 Chieti, Italy.
2. Department of Medical, Oral and Biotechnological Sciences, and Center for Advanced Studies and Technology (CAST), G. D'Annunzio University Chieti-Pescara, 66100 Chieti, Italy.
3. Unit of Phase IV Trials, IRCCS Regina Elena National Cancer Institute, 00144 Rome, Italy.
4. Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, 90133 Palermo, Italy.
5. Department of Pharmacy, G. D'Annunzio University, Chieti-Pescara, 66100 Chieti, Italy.
6. Clinical Oncology, S.S. Annunziata Hospital, 66100 Chieti, Italy.
7. Unit of Medical Genetics, Department of Biomedical Sciences-BIOMORF, University of Messina, 98125 Messina, Italy.
8. Medical Oncology, Sandro Pertini Hospital, 00159 Rome, Italy.
9. Laboratory of Biostatistics, Department of Medical, Oral and Biotechnological Sciences, G. D'Annunzio University Chieti-Pescara, 66100 Chieti, Italy.
Received 2023-4-30; Accepted 2023-7-7; Published 2023-9-4
Abstract
Sex might influence prognosis in patients affected by colorectal cancer. We retrospectively studied a cohort of patients affected by metastatic colon cancer (mCC) stratified by sex and primary tumor location. RAS mutational status was also included in the analysis. Overall, 616 patients met the eligibility criteria, 261 women and 355 men. Neither gender, nor RAS mutational status influenced overall survival (OS) in the entire population. As expected, patients with right-sided colon cancer (RCC) had a significant shorter OS compared to those with left-sided colon cancer (LCC) (21.3 vs 33.1 months, p= 0.002). When the analysis was performed stratifying for gender, RCC retained worse prognosis among men (OS 20.5 vs 33.9 months, p= 0.008), but not among women (p= 0.132). Similarly, the presence of RAS mutations had no prognostic effect in women, but was significantly associate with shorter survival in men (OS 29.5 vs 33.7 months, p= 0.046). In addition, when comparing clinical outcome of women or men according to sidedness and RAS mutational status, RCC was associated with dismal prognosis only in men with RAS mutated tumor (OS 17.2 vs 32.3 months, p= 0.008). Our study highlights the importance of gender in the outcome of patients with mCC.
Keywords: Metastatic colorectal cancer (mCRC), Gender, Tumor location, RAS status, Prognosis
Introduction
Colorectal cancer is the third cause of cancer-related death in the world, after lung and prostate cancer in men and after lung and breast cancer in women [1]. In the US, the 5-year survival rate is 90% for people with localized stage, but falls to 14% for those with metastatic disease [2]. Modern first line chemotherapeutic regimens, with the addition of monoclonal antibodies against EGFR (Cetuximab or Panitumumab) in RAS wild-type (wt) tumors or monoclonal antibodies against VEGF (Bevacizumab) in RAS mutated (mut) tumors have improved patient overall survival (OS) [3].
In recent years, epidemiologic studies have revealed that right-sided colon cancers (RCC), i.e. tumors occurring in the cecum, ascending colon or hepatic flexure, are characterized by poorer prognosis compared to left-sided colon cancers (LCC), i.e. tumors of the splenic flexure, descending, sigmoid and rectosigmoid colon [4-6]. Studies on the molecular features of colorectal cancer in relation to primary tumor location have showed important differences: high microsatellite instability (MSI-H), BRAF mutations, and cytosine-guanosine (CpG) island methylation phenotype (CIMP) are frequently observed in RCC [7,8], while chromosomal instability and mutations in the TP53 and APC genes are more common in LCC [9]. In addition, RCC presents a molecular pattern associated with intrinsic resistance to EGFR inhibition as compared with LCC [10-12], and a clear benefit from anti‐EGFR therapies has been reported only in patients with tumors originating in the left side of the colon [13,14]. Consistently, in a previous retrospective study we have observed a more favorable outcome in patients with LCC, but not in those with RCC, treated with anti-EGFR agents compared to those who received Bevacizumab [15]. On the contrary, Bevacizumab significantly improved survival in patients with RCC [16], indicating a predominant involvement of pro-angiogenetic factors in the tumors originating from the right side of the colon.
Along with sidedness, gender may influence colorectal cancer outcome. Compared to women, men seem to have a worse survival rate [17-19]. Levels of circulating estrogens [20], oral contraceptives [21], hormonal replacement therapy [22], diet [23], physical activity [24], and microbiome diversity [25] have been proposed as factors responsible for reduced colorectal cancer incidence and death in women. Nevertheless, compared to men, women are more frequently diagnosed with the more aggressive right-sided proximal tumor [9]. The reason for this difference is not known, but it is plausible the existence of a different biology of RCC in the two sexes. Thus, clinical outcome of women and men with RCC could be different compared with their peers with LCC.
The present study was carried out to investigate gender-associated survival differences in a cohort of patients affected by metastatic colon cancer (mCC) in relation to RAS mutational status and the anatomic location of the primary tumor.
Patients and Methods
Study design and data collection
Patients with newly diagnosed mCC consecutively referred to five Italian cancer centers between January 2010 and December 2020 for first-line therapy were included in this study. For each patient, gender, age, and baseline clinical-pathological features, including tumor histotype, tumor grade, site of metastasis, number of metastatic sites, primary tumor location, RAS mutational status, ECOG performance status, were collected. Information on previous adjuvant chemotherapy, surgery for primary tumor and/or metastasis, were recorded as well. Patient candidates for supportive care after the diagnosis of mCC were excluded from the study.
Clinical Assessment
Overall survival (OS) data were analyzed in the entire population and after stratification of patients by gender, primary tumor location and the presence or absence of RAS mutations. OS was defined as the time from therapy initiation to death or last annotation on clinical records. The date of study cutoff was December 15, 2020.
Statistical Analysis
Descriptive analysis was carried out using mean ± standard deviation or median and interquartile range (IQR) for the quantitative variables and percentages values for the qualitative ones.
Normality distribution was assessed by the Shapiro-Wilk test. Survival analysis was performed by applying the Kaplan-Meier estimator and Log-rank test for equality of survivor functions. The association with clinical features was analyzed with univariate Cox model of proportional hazards (Hazard Ratio - HR and 95% CI) for OS, and the applicability assumption was evaluated by the Schoenfeld test. A p value ≤ 0.05 was retained as the limit of statistical significance. The SPSS version 15.0 statistical software was used to perform all the analyses.
Results
Patients' characteristics
Overall, 616 patients were consecutively diagnosed with mCC and treated with first-line chemotherapy in the five participating Institutions, and were included in the survival analyses. Their clinical and pathological characteristics are summarized in Table 1.
The median age was 66 years (IQR 58-73) and the majority of patients were male (57.6%). Median age was similar in women and men, 64 years and 67 years, respectively. The primary tumor was left-sided in 403 (65.4%) patients, right-sided in the remaining 213 (34.6%). There was a slightly higher prevalence of RAS wt tumors (351 patients, 57%). Most patients, 418 (67.9%), did not received adjuvant chemotherapy after the first diagnosis of colon cancer, 510 (82.8%) had surgery of the primary tumor, and 155 (25.2%) had surgery of metastasis. Three hundred and twenty-three (52.4%) patients presented with multiple metastases. A good performance status (ECOG 0 or 1) was present in 93.4% of cases.
Importantly, all the reported characteristics, including location of primary tumor, were well balanced between men and women, with the exception of RAS mut tumors that were more frequent in women (48% vs 39%, p= 0.023). RAS mut tumors resulted also significantly higher in RCC compared to LCC, 51% and 39%, respectively (p= 0.005) (Table 2), but were equally distributed between the two sex in relation to the sidedness of primary tumor.
Characteristics of the whole population.
| Variable | N = 616 | % |
|---|---|---|
| Median age, ys (IQR)1 | 66 (58-73) | |
| Age (years) | ||
| ≤ 65 | 301 | 48.9 |
| > 65 | 315 | 51.1 |
| Gender | ||
| Female | 261 | 42.4 |
| Male | 355 | 57.6 |
| Sidedness | ||
| Left | 403 | 65.4 |
| Right | 213 | 34.6 |
| Grade | ||
| G1 | 5 | 0.8 |
| G2 | 386 | 62.7 |
| G3 | 140 | 22.7 |
| Unknown | 85 | 13.8 |
| ECOG PS | ||
| 0 | 357 | 58.0 |
| 1 | 218 | 35.4 |
| 2 | 38 | 6.20 |
| 3 | 3 | 0.4 |
| Ras Staus | ||
| Wild-type | 351 | 57.0 |
| Mutated | 265 | 43.0 |
| Previous adjuvant chemo | ||
| No | 418 | 67.9 |
| Yes with oxaliplaton | 121 | 19.6 |
| Yes w/out oxaliplatin | 77 | 12.5 |
| Number of metastasis | ||
| 1 | 293 | 47.6 |
| >1 | 323 | 52.4 |
| Surgery of primary tumor | ||
| No | 106 | 17.2 |
| Yes | 510 | 82.8 |
| Surgery of metastasis | ||
| No | 461 | 74.8 |
| Yes | 155 | 25.2 |
1 IQR, Interquartile Range
Distribution of RAS mutational status according to sidedness and sex.
| Variable | N (%) | Sidedness | Sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left (%) (N= 403) | Right (%) (N= 213) | p-value | Male (N= 355) | Female (N= 261) | p-value | ||||||
| RAS Status | 0.005 | 0.023 | |||||||||
| Wild-type | 351 (57) | 246 (61) | 105 (49) | 216 (61) | 135 (52) | ||||||
| Mutated | 265 (43) | 157 (39) | 108 (51) | 139 (39) | 126 (48) | ||||||
Even treatment approach was similar in the two sexes: independently from gender and tumor location, 70% of patients with RAS wt tumors received anti-EGFR agents (Cetuximab or Panitumumab) in association with the standard chemotherapy backbone, while most patients with RAS mut tumors (71%) received Bevacizumab. Table 3 summarizes the treatment choice in first and second line according to RAS mutational status in the entire cohort.
Type of therapy administered in relation to RAS mutational status.
| Variable | N (%) | RAS Status | ||
|---|---|---|---|---|
| Wild-type (%) (N= 351) | Mutated (%) (N= 265) | p-value | ||
| I line chemotherapy | 0.020 | |||
| + Bevacizumab | 251 (41) | 62 (18) | 189 (71) | |
| + Anti-EGFR* | 245 (40) | 245 (70) | 0 (0) | |
| Chemo alone | 120 (19) | 44 (12) | 76 (29) | |
| II line chemotherapy | 0.517 | |||
| + Anti-VEGF° | 169 (27) | 91 (26) | 78 (29) | |
| + Anti-EGFR* | 72 (12) | 72 (21) | 0 (0) | |
| Chemo alone | 239 (39) | 116 (32) | 123 (46) | |
| Supportive care | 136 (22) | 72 (21) | 64 (25) | |
*Not included in the statistical analysis. °Including Bevacizumab or Aflibercept
Overall Survival
After a median follow-up of 21.4 months, 390 (63.3%) patients were dead. Overall survival was significantly affected by primary tumor location. Median OS was 21.3 months for patients with RCC and 33.1 months for those with LCC (Figure 1).
Cumulative overall survival of the whole population stratified by sidedness of primary tumor.

Neither gender nor RAS mutational status showed prognostic impact in the entire population. Median overall survival (OS) of patients stratified by gender and divided according to tumor sidedness and RAS mutational status are summarized in Table 4.
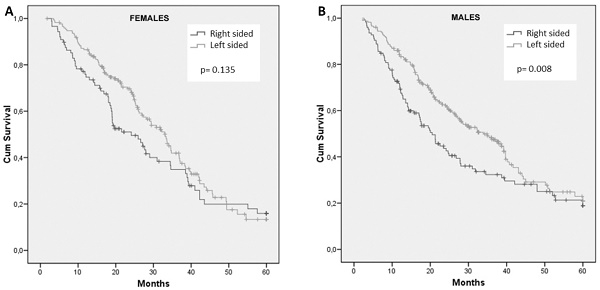
Frequencies of right- and left-sided tumors were comparable between male and female patients. For female patients, survival did not differ with regard to primary tumor site (HR: 1.28; 95% CI, 0.93 to 1.76; p = 0.135) (Figure 2A). In contrast, tumor sidedness influenced survival among male patients. In fact, men with right-sided tumors showed a significantly inferior OS compared to those with left-sided tumor, 20.5 months and 33.9 months, respectively (HR: 1.45; 95% CI, 1.10 to 1.92; p= 0.008) (Figure 2B).
Overall survival in female and male patients according to tumor sidedness and RAS status.
| Variable | Male (N= 355) | Female (N= 261) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | Median (mo) | HR (95% CI) | p-value | N (%) | Median (mo) | HR (95% CI) | p-value | ||
| Sidedness | |||||||||
| Left | 230 (65) | 33.9 | 1.00 | 173 (66) | 33.1 | 1.00 | |||
| Right | 125 (35) | 20.5 | 1.45 (1.10-1.92) | 0.008 | 88 (34) | 24.1 | 1.28 (0.93-1.76) | 0.135 | |
| RAS Status | |||||||||
| Wild-type | 216 (61) | 33.7 | 1.00 | 135 (52) | 31.6 | 1.00 | |||
| Mutated | 139 (39) | 29.5 | 1.30 (1.01-1.70) | 0.046 | 126 (48) | 31.2 | 1.05 (0.77-1.43) | 0.760 | |
| Sidedness in RAS wild-type* | |||||||||
| Left | 147 (68) | 35.0 | 1.00 | 99 (73) | 33.1 | 1.00 | |||
| Right | 69 (32) | 23.5 | 1.25 (0.86-1.84) | 0.244 | 36 (27) | 24.1 | 1.58 (0.98-2.53) | 0.055 | |
| Sidedness in RAS mutated° | |||||||||
| Left | 83 (60) | 32.3 | 1.00 | 74 (59) | 29.4 | 1.00 | |||
| Right | 56 (40) | 17.2 | 1.73 (1.15-2.60) | 0.008 | 52 (41) | 21.5 | 1.03 (0.66-1.61) | 0.886 | |
*Percentage (%) is referred to patients with RAS wild-type tumor
°Percentage (%) is referred to patients with RAS mutated tumor
Similarly, mutation of RAS did not influence survival among women, while was significantly associated with worse prognosis in men (p= 0.046) (Table 4).
When considering survival according to sidedness separately in the group of patients with RAS wt or in those with RAS mut, again no difference was observed among women (Figure 3A and 3B), with a trend toward worse survival in RCC of the RAS wt group (HR: 1.58; 95% CI, 0.98 to 2.53; p = 0.055). Interestingly, men with RCC had a significant reduced survival only in RAS mut tumors (Figure 3D vs 3C). In particular, in male sex, patients with RAS mut tumors had a median OS of only 17.2 months in RCC compared to 32.2 months in LCC (HR: 1.73; 95% CI, 1.15 to 2.60; p = 0.008) (Figure 3D).
Discussion
The results of this retrospective study are in line with the evidence that RCC, compared to LCC, is significantly associated with reduced survival, emphasizing the importance of primary tumor location in defining the prognosis of patients affected by mCC. However, our study revealed that RCC-associated poor outcome is observed in men rather than in women and that this sex difference is particularly evident in RAS mut tumors, indicating a negative impact of RAS mutations in men affected by tumor arising in the right side of the colon.
Despite a lower incidence of RCC [9], men with mCC have a general worse prognosis than women [17-19]. In our cohort median OS was similar in the two sexes, in agreement with other studies that failed to demonstrate differences in survival between men and women [26,27]. The lack of consensus has been attributed to a confounding hormonal effect that is not considered in the different studies, including menopausal status and hormonal replacement therapies [28].
Cumulative overall survival of female (A) and male (B) patients stratified by sidedness of primary tumor.

Cumulative overall survival of females with RAS wild-type (A) or RAS mutant (B) tumors and males with RAS wild-type (C) or RAS mutant (D) tumors stratified by sidedness of primary tumor

RAS mutations have been reported in 40-50% of mCC and, in some studies, were more frequently observed in RCC than LCC [29] and more often in females than males [30]. Consistently, in our cohort, the incidence of RAS mut tumors was 43% and was significantly higher among tumors of the right side of the colon and in females. RAS mutations have been showed to predict a dismal prognosis in patients with mCC [31,32], mainly in the presence of G12C or G12S variants [33], and particularly in patients treated with bevacizumab [34-36]. In our study, RAS mut tumors displayed a worse prognosis, but only in men. The interpretation of this result will be discussed below, on the light of a possible increased effectiveness of bevacizumab in women with RCC.
Concerning sidedness, our findings emphasize the poor outcome of patients with RCC, compared to those with LCC, as reported in several clinical studies [4-6]. The aggressive behavior of RCC has been attributed to a higher occurrence of BRAF mutations [37], PIK3CA mutations [38-40] or CpG island methylation phenotype (CIMP) [41-43], all factors associated with shorter survival [44-47]. However, thus far, no study has focused on sex disparities relative to RCC.
In the present study we report a worse prognosis for men with RCC, when comparing survival separately in the two sexes according to tumor sidedness. This finding strongly suggests the existence of a different biology of RCC in men and women. In some way, adverse prognostic factors could negatively influence the course of cancer arising in the right side of colon in men, but not in woman. However, at the best of our knowledge, no such evidence has been reported yet.
Recently, a unique metabolic phenotype has been described in RCC of women, but not in LCC of women neither in colon cancer of men in both locations [48]. In particular, it has been demonstrated that female RCC displays a high ATP-consuming metabolism supplied by oxidation of fatty acids rather than glycolysis. The increased ATP production seems to be directed to the synthesis of asparagine that is used by tumor cells as an amino acid exchange factor, leading to increased intracellular levels of threonine and serine. These two amino acids, in turn, determine mTOR activation that is eventually responsible for tumor growth and invasiveness [48]. On the bases of this mechanism we would expect that the general poor prognosis of RCC would be even worse in women. Instead, we observed the opposite: compared to LCC, the survival in RCC was shorter only in men.
We could try to explain this apparent paradox by assuming a possible different activity of the anti-angiogenic agent bevacizumab in RCC in the two sexes. This hypothesis is based on the above-described mechanism responsible for mTOR activation which is exclusively expressed in RCC of women [48]. In fact, an increased activity of mTOR is a well-known mechanism promoting angiogenesis [49], and the presence of tumor neo-angiogenesis is a well-established predictive factor of response to bevacizumab [50,51]. In this scenario we could hypothesize that tumors arising in the right side of the colon in women express a pro-angiogenic phenotype and, for this reason, would be more sensitive to bevacizumab. On the contrary, tumors arising in the left side of the colon in woman, as well as those arising in either side of the colon in men, do not have this pro-angiogenic phenotype and, therefore, are less responsive to bevacizumab. Thus, given the intrinsic worse prognosis of RCC, the higher efficacy of bevacizumab in women would counterbalance the basic adverse prognostic factors acting in right-sided tumors. As a result, we did not find difference in survival between LCC and RCC in women. In men, on the contrary, the lack of such a benefit from bevacizumab in RCC resulted in a better outcome for LCC.
In addition, we showed that this gender disparity was more pronounced in patients with RAS mut tumors. In this subgroup, the negative prognostic impact of RAS mutations was clearly evident in males, in spite of a higher incidence of RAS mutations in women. Again, we could interpret this result assuming that women may receive a greater benefit from bevacizumab in RCC, as discussed above, and considering that bevacizumab has been prevalently administered in patients with RAS mut tumors compared to those with RAS wt, who preferentially received anti-EGFR agents. Thus, in a sample enriched with bevacizumab-treated patients, i.e. patients with RAS mut tumors, males showed a shorter survival compared to females and, as expected in this subgroup, male with RCC showed the worst survival curve when the analysis was performed to compare left versus right tumors.
Overall, the findings presented herein may coherently be interpreted by an increased anti-tumor activity of bevacizumab in women with RCC. Notably, data have been provided indicating that the addition of bevacizumab to chemotherapy determines a clinical benefit only in right-sided tumors [16] and that RCC, but not LCC, is endowed with a pro-angiogenic microenvironment characterized by increased expression of endothelial nitric oxide synthase (eNOS), COX2, and ephrin type‐B receptor 4 (EPHB4) [52]. We suggest that these evidences would be influenced by the higher incidence of RCC in women who are the patients who may benefit the most from anti-angiogenic treatments due to the unique metabolic phenotype of cancer arising in this side of the colon.
With the limitations of a retrospective study, the possibility that co-morbidities would have influenced the clinical course of the disease, and the impossibility to evaluate hormonal interference due to lack of information, in our database, about personal medical history, menopausal status, history of hormone replacement therapy, and use of contraceptive agents, the findings we presented are enough robust given the large sample size and the homogenous therapeutic approach for all patients, thus far not influenced by gender nor by tumor sidedness.
Prospective analyses of patient's outcome in relation to gender, sidedness, and RAS mutational status, along with the determination of tumor genetic, epigenetic and metabolic alterations, could help in the near future to identify the different molecular portrait of RCC and LCC, opening to potential novel therapeutic agent to be specifically used for women or for men. We strongly recommend to take sex into account in future colorectal cancer research.
Conclusions
Primary tumor location and gender are important prognostic/predictive factors to consider in studies on mCC. In the present study we found that men affected by tumors arising in the right side of the colon have a shorter survival compared to those with left-sided tumors, especially in the presence of RAS mut. No such difference was observed among women.
Author Contributions
Conceptualization, A.G. and N.T.; methodology P.B.; formal analysis, P.B., S.V. and R.F.; resources, F.S.D.L. and L.F.; data curation, D.B., P.D.M. and A.D.L.; writing—original draft preparation, A.G. and E.C.; writing—review and editing, A.C., P.V., G.C. and N.T.; visualization, L.D.L. and D.B.; supervision, S.A., T.G. and N.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Chieti-Pescara on 04 Feb 2016 (IRB No. 01 2016-02-04).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and the anonymous clinical data used in the analysis.
Data Availability Statement
Data will be made available from the corresponding author on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
2. Viale PH. The American Cancer Society's Facts & Figures: 2020 Edition. J Adv Pract Oncol. 2020;11:135-6
3. Stintzing S, Modest DP, Rossius L. et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17:1426-34
4. Loupakis F, Yang D, Yau L. et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107:dju427
5. Gervaz P, Usel M, Rapiti E. et al. Right colon cancer: Left behind. Eur J Surg Oncol. 2016;42:1343-9
6. Petrelli F, Tomasello G, Borgonovo K. et al. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2017;3:211-9
7. Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403-8
8. Lee GH, Malietzis G, Askari A. et al. Is right-sided colon cancer different to left-sided colorectal cancer? A systematic review. Eur J Surg Oncol. 2015;41:300-8
9. Lee MS, Menter DG, Kopetz S. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J Natl Compr Canc Netw. 2017;15:411-9
10. Cremolini C, Morano F, Moretto R. et al. Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: the PRESSING case-control study. Ann Oncol. 2017;28:3009-14
11. Morano F, Corallo S, Lonardi S. et al. Negative Hyperselection of Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer Who Received Panitumumab-Based Maintenance Therapy. J Clin Oncol. 2019;37:3099-110
12. Missiaglia E, Jacobs B, D'Ario G. et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995-2001
13. Holch JW, Ricard I, Stintzing S. et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87-98
14. Arnold D, Lueza B, Douillard J-Y, Peeters M. et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713-29
15. Grassadonia A, Di Marino P, Ficorella C. et al. Impact of primary tumor location in patients with RAS wild-type metastatic colon cancer treated with first-line chemotherapy plus anti-EGFR or anti-VEGF monoclonal antibodies: a retrospective multicenter study. J Cancer. 2019;10:5926-34
16. Venook AP, Niedzwiecki D, Innocenti F. et al. Impact of primary (1o) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34:3504-4
17. Schmuck R, Gerken M, Teegen E-M. et al. Gender comparison of clinical, histopathological, therapeutic and outcome factors in 185,967 colon cancer patients. Langenbeck's Arch Surg. 2020;405:71-80
18. Yang Y, Wang G, He J. et al. Gender differences in colorectal cancer survival: A meta-analysis. Int J Cancer. 2017;141:1942-49
19. Sant M, Allemani C, Santaquilani M. et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45:931-91
20. Luo G, Zhang Y, Wang L. et al. Risk of colorectal cancer with hysterectomy and oophorectomy: A systematic review and meta-analysis. Int J Surg. 2016;34:88-95
21. Luan N-N, Wu L, Gong T-T. et al. Nonlinear reduction in risk for colorectal cancer by oral contraceptive use: a meta-analysis of epidemiological studies. Cancer Causes Control. 2015;26:65-78
22. Jang Y-C, Huang H-L, Leung CY. Association of hormone replacement therapy with mortality in colorectal cancer survivor: a systematic review and meta-analysis. BMC Cancer. 2019;19:1199
23. Huxley RR, Ansary-Moghaddam A, Clifton P. et al. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171-80
24. Boyle T, Fritschi L, Platell C. et al. Lifestyle factors associated with survival after colorectal cancer diagnosis. Br J Cancer. 2013;109:814-22
25. Johnson CH, Spilker ME, Goetz L. et al. Metabolite and Microbiome Interplay in Cancer Immunotherapy. Cancer Res. 2016;76:6146-52
26. White A, Ironmonger L, Steele RJC. et al. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer. 2018;18:906
27. De Angelis R, Sant M, Coleman MP. et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE-5 - a population-based study. Lancet Oncol. 2014;15:23-34
28. Abancens M, Bustos V, Harvey H. et al. Sexual Dimorphism in Colon Cancer. Front Oncol. 2020;10:607909
29. Bylsma LC, Gillezeau C, Garawin TA. et al. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta-analysis. Cancer Med. 2020;9:1044-57
30. Kwak MS, Cha JM, Cho YH. et al. Clinical Predictors for KRAS Codon 13 Mutations in Patients With Colorectal Cancer. J Clin Gastroenterol. 2018;52:431-36
31. Chen K, Collins G, Wang H. et al. Toh JWT. Pathological Features and Prognostication in Colorectal Cancer. Curr Oncol. 2021;28:5356-83
32. Modest DP, Ricard I, Heinemann V. et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27:1746-53
33. Ottaiano A, Normanno N, Facchini S. et al. Study of Ras Mutations' Prognostic Value in Metastatic Colorectal Cancer: STORIA Analysis. Cancers (Basel). 2020;12:1919
34. Petrelli F, Coinu A, Cabiddu M. et al. KRAS as prognostic biomarker in metastatic colorectal cancer patients treated with bevacizumab: a pooled analysis of 12 published trials. Med Oncol. 2013;30:650
35. Koike J, Ushigome M, Funahashi K. et al. Significance of KRAS mutation in patients receiving mFOLFOX6 with or without bevacizumab for metastatic colorectal cancer. Hepatogastroenterology. 2014;61:2222-26
36. Petrelli F, Coinu A, Cabiddu M. et al. Prognostic factors for survival with bevacizumab-based therapy in colorectal cancer patients: a systematic review and pooled analysis of 11,585 patients. Med Oncol. 2015;32:456
37. Tsai Y-J, Huang S-C, Lin H-H. et al. Differences in gene mutations according to gender among patients with colorectal cancer. World J Surg Oncol. 2018;16:128
38. Sastre J, García-Alfonso P, Viéitez JM. et al. Influence of BRAF and PIK3CA mutations on the efficacy of FOLFIRI plus bevacizumab or cetuximab as first-line therapy in patients with RAS wild-type metastatic colorectal carcinoma and <3 baseline circulating tumour cells: the randomised phase II VISNÚ-2. ESMO open. 2021;6:100062
39. Nosho K, Kawasaki T, Ohnishi M. et al. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia. 2008;10:534-41
40. Phipps AI, Makar KW, Newcomb PA. Descriptive profile of PIK3CA-mutated colorectal cancer in postmenopausal women. Int J Colorectal Dis. 2013;28:1637-42
41. Wiencke JK, Zheng S, Lafuente A. et al. Aberrant methylation of p16INK4a in anatomic and gender-specific subtypes of sporadic colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:501-6
42. Toyota M, Ahuja N, Ohe-Toyota M. et al. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681-6
43. Advani SM, Advani P, DeSantis SM. et al. Clinical, Pathological, and Molecular Characteristics of CpG Island Methylator Phenotype in Colorectal Cancer: A Systematic Review and Meta-analysis. Transl Oncol. 2018;11:1188-201
44. Venderbosch S, Nagtegaal ID, Maughan TS. et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20:5322-30
45. Wang Q, Shi Y-L, Zhou K. et al. PIK3CA mutations confer resistance to first-line chemotherapy in colorectal cancer. Cell Death Dis. 2018;9:739
46. Dahlin AM, Palmqvist R, Henriksson ML. et al. The role of the CpG island methylator phenotype in colorectal cancer prognosis depends on microsatellite instability screening status. Clin Cancer Res. 2010;16:1845-55
47. Li X, Hu F, Wang Y. et al. CpG island methylator phenotype and prognosis of colorectal cancer in Northeast China. Biomed Res Int. 2014;2014:236361
48. Cai Y, Rattray NJW, Zhang Q. et al. Sex Differences in Colon Cancer Metabolism Reveal A Novel Subphenotype. Sci Rep. 2020;10:4905
49. Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neurosci. 2011;4:51
50. Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579-91
51. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039-49
52. Ulivi P, Scarpi E, Chiadini E. et al. Right- vs. Left-Sided Metastatic Colorectal Cancer: Differences in Tumor Biology and Bevacizumab Efficacy. Int J Mol Sci. 2017;18:1240
Author contact
![]() Corresponding author: grassadoniait.
Corresponding author: grassadoniait.

 Global reach, higher impact
Global reach, higher impact