3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(15):2878-2888. doi:10.7150/jca.87626 This issue Cite
Research Paper
Gastrointestinal/genitourinary adverse event after intensity modulated versus three-dimensional primary radiation therapy in the treatment of prostate cancer: systematic review and meta-analysis
1. Department of Radiation Oncology, Hebei Province Cangzhou Hospital of Integrated Traditional and Western Medicine, Cangzhou, Hebei, 061000, China.
2. Key Laboratory of Endocrinology of National Health Commission, Department of Endocrinology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, 100730, China.
†These authors have contributed equally to this work.
Received 2023-6-29; Accepted 2023-8-29; Published 2023-9-11
Abstract
Objective: Prostate cancer (PCa) is one of the most common cancers in the world. The potential benefits of intensity modulated radiation therapy (IMRT) over three-dimensional conformal radiation therapy (3D-CRT) for PCa primary radiation therapy treatment have not yet been clarified. Therefore, this meta-analysis was conducted to assess whether IMRT could improve clinical outcomes in comparison with 3D-CRT in patients diagnosed with PCa.
Materials and methods: Relevant studies were identified through searching related databases till December, 2022. Hazard ratio (HR) or risk ratio (RR) with its corresponding 95% confidence interval (CI) was used as pooled statistics for all analyses.
Results: The incidence of grade 2 or worse acute adverse gastrointestinal (GI) event was analyzed and the pooled data revealed a clear decreasing trend in the IMRT compared with 3D-CRT (RR=0.62, 95% CI: 0.45-0.84, p=0.002). IMRT slightly increased the grade ≥ 2 acute genitourinary (GU) adverse event in comparison with the 3D-CRT (RR=1.10, 95% CI: 1.02-1.19, p=0.015). The IMRT and the 3D-CRT of patients showed no substantial differences in grade ≥ 2 late GI adverse event (RR =0.62, 95% CI: 0.36-1.09, p=0.1). In those included studies, there was no significant difference between IMRT and 3D-CRT in grade 2-4 late GU adverse event (RR =1.08, 95% CI: 0.77-1.51, p=0.65). There was a significant difference in biochemical control favoring IMRT (RR =1.13, 95% CI: 1.05-1.22, p=0.002). IMRT showed modest increase in biochemical control in comparison with 3D-CRT.
Conclusion: In general, based on the above results, IMRT should be considered as a better choice for the treatment of PCa. More randomized controlled trials are needed to determine the subset of patients diagnosed with PCa.
Keywords: IMRT, 3D-CRT, Prostate cancer, Efficacy, Adverse event
Introduction
Prostate cancer (PCa) is among the most prevalent neoplastic diseases worldwide, particularly in Northern America and Western Europe [1]. According to the outcomes of prior investigations, radiation therapy (RT) has gained widespread employment in the treatment of PCa [2, 3]. Several clinical studies have found that increasing the dose is related with better biochemical and overall survival results [4-7]. As the majority of individuals with non-metastatic PCa can endure for over a decade, it is imperative to opt for RT techniques that reduce RT-associated toxicity to enhance their standard of life [8, 9]. Enhanced dosages, on the other hand, may cause enhanced normal tissue toxicity, which includes delayed gastrointestinal (GI) and genitourinary (GU) damage [10].
New RT procedures have arisen as a result of the development of enhanced radiation technologies and have been used in clinical settings. The delivery of a radiation dose that conforms to the target volume of the tumor is made easier by three-dimensional conformal radiation therapy (3D-CRT). [11]. Consequently, the target dose is greatly increased while the impact on normal tissue is simultaneously diminished [12]. The most sophisticated kind of 3D-CRT, intensity modulated radiation therapy (IMRT), which generates non-uniform fields to augment the radiation dosage supplied to the intended target volume while limiting irradiation to the organs at danger, represents the culmination of the development of RT techniques [13]. However, the likelihood of a minor miss represents a potential shortcoming of IMRT. When using IMRT, it is also necessary to take into account the dose homogeneity, the escalation of irradiation doses to bigger volumes of healthy tissues, and longer planning timeframes [14]. The amplified total body exposure and monitor units increase the likelihood of second malignancies when utilizing IMRT, as opposed to conventional RT [15].
Nonetheless, the prospective advantages of IMRT over 3D-CRT for first radiation therapy for treating PCa have not been proven. As a result, this meta-analysis was carried out to determine whether IMRT may improve clinical results in patients with PCa when compared to 3D-CRT.
Materials and methods
Literature search
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria were followed in this meta-analysis [16]. Up until December 2022, we did a literature search utilizing the databases Pubmed, Embase, and Web of Science. The following keywords were used in the search strategy: "prostate cancer [Title/Abstract]", "intensity modulated radiation therapy [Title/Abstract]", "IMRT [Title/Abstract]", "three-dimensional conformal radiation therapy [Title/Abstract]", and "3D-CRT [Title/Abstract]". In addition, we looked at abstracts from major academic conferences. To find possibly eligible articles, the references of the included studies were also evaluated.
Study selection
The selected studies were required to meet the following eligibility criteria: a) all patients were confirmed to have PCa histologically and had not undergone radical prostatectomy; b) the study had clearly defined case selection criteria; c) interventions primarily focused on IMRT and 3D-CRT, and both techniques were conducted within the same study; d) essential data for hazard ratio (HR) or risk ratio (RR) with its corresponding 95% confidence interval (CI) was reported or could be calculated utilizing Tierney's method [17]; e) published in full-text form; f) published in English; g) single prescription dose less than 2.5Gy. Studies were excluded if they fulfilled any of the following criteria: a) insufficient data to estimate HRs or RRs with 95% CIs; b) patients who had undergone pelvic irradiation or radical prostatectomy; c) animal experiments; d) letters, meeting abstracts, or review papers; and e) not presented in English.
Quality assessment of publications
The Newcastle-Ottawa quality assessment scale (NOS) was used in the investigation to rate the effectiveness of both cohort and case-control studies [18]. The NOS scale has three components: defining and selecting case and control groups, comparing case and control groups, and determining exposure. The utmost score is 9 points. In this study, a score of ≥ 7 is established as high-quality research, 4-6 is categorized as medium quality research, and ≤ 3 is characterized as low-quality research. As for the randomized controlled study, it was conducted utilizing the Jadad scale for quality evaluation. The method of creating random grouping sequences, the double-blind procedure, withdrawal, and lack of follow-up are among the grading factors. The total score is 5, with 1-2 indicating bad quality and 3-5 indicating high quality [19].
Data extraction
Two investigators, W.G. and L.Y.Z., used a predetermined process to collect data from the eligible studies. The extracted data included the first author's name, year of publication, study design, duration of follow-up, patient count, radiation dose, planning target volume (PTV), TNM classification, scoring criteria, and androgen deprivation therapy (ADT) details, as well as their corresponding outcomes. For scoring of acute and late radiation damage, Common Terminology Criteria of Adverse Events (CTCAE) or Radiation Therapy Oncology Group (RTOG) Common Toxicity Criteria are typically used. Any inconsistencies or disagreements between the two reviewers were resolved with the help of a third investigator, Y.C.S.
Statistical analysis
The HRs and RRs for clinical results were collected directly from each trial if available, or estimated from raw data using the approach described by Tierney et al. [17]. Cochran's Q test and the Higgins I-squared statistic were used to assess the heterogeneity of the pooled results. The random-effects model was used if the I-squared statistic was larger than 50% and the P-value for heterogeneity was less than 0.1, suggesting significant heterogeneity; otherwise, the fixed-effects model was used. Additionally, sensitivity analyses were carried out to assess the effect of particular research on the total estimate. Begg's funnel plot was also evaluated for possible publication bias. STATA 12.0 software (Stata Corp, College Station, TX, USA) was used for the statistical analysis. A P-value of 0.05 or less was judged to be statistically noteworthy.
Results
Literature search and summary of studies
Initially, the searched keywords yielded an overall number of 2640 articles. After deleting duplicates, 2028 articles remained, of which 1850 were removed after screening titles and abstracts. Following that, full texts and data integrity were thoroughly checked, resulting in the exclusion of an additional 158 studies. Finally, a total of 20 papers were judged eligible and included in the final meta-analysis [20-39]. Figure 1 depicts the selection procedure for our articles. The Jadad scale was used to evaluate randomized controlled trials, with all scores equal to or greater than 3 meeting the inclusion requirements. The remaining retrospective investigations were evaluated using the NOS scale, with all studies scoring 6 or higher (Supplement Table 1).
The overall number of patients included in the meta-analysis was 8645, with patient numbers ranging from 27 to 1571 per study. The basic characteristics of the included studies are shown in Table 1. In the study design, retrospective cohort studies (n = 13) were more common than prospective cohort studies (n = 4). The primary tumor doses were 70-82 Gy in the IMRT group and 66-81 Gy in the 3D-CRT group. The median duration of follow-up ranged from 3 to 120 months. Twelve of the studies included evaluated the acute adverse event of an IMRT group to that of a 3D-CRT group, including acute GI adverse event (n = 10) and acute GU adverse event (n = 12). Furthermore, ten studies evaluated the late adverse event effects of the IMRT group to those of the 3D-CRT group, including late GI (n = 8) and late GU adverse events (n = 9). Furthermore, seven investigations compared the biochemical control of the IMRT and 3D-CRT groups.
Flow chart of the included trials.

Study characteristics.
| Study | Ref. | Year | Study design | Median follow-up (m) (3DCRT/ IMRT) | Number (3DCRT/IMRT) | Total dose/fraction dose (Gy) (3DCRT VS IMRT) | PTV | TNM or risk group | Score criteria | ADT% (3DCRT/IMRT) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ashman JB | [20] | 2005 | Retro. | 30/30 | 27 (14/13) | 75.6/1.8 VS 81/1.8 | Prostatic bed, pelvic nodes, seminal vesicles | T1c-T4 | RTOG toxicity scale | 100/100 | Acute GI, GU |
| Cho JH | [21] | 2008 | Retro. | 3/3 | 50 (35/15) | 70.2/1.8 VS70/2.5 | Prostatic bed | T1-T4N0-1 | RTOG toxicity scale | 44/44 | Acute GI, GU |
| Dolezel M | [22] | 2010 | Pro. | 68.4/37.2 | 232 (94/138) | 74/2 VS78/2 | Prostatic bed, pelvic nodes, seminal vesicles | T1-T3 All risk group | RTOG toxicity scale | 94.7/55 | Acute GI, GU |
| Dolezel M | [23] | 2015 | Pro. | 104/60 | 533 (320/233) | 70-74/2 VS 78-82/2 | Prostatic bed, seminal vesicles | Localized prostate cancer | RTOG toxicity scale, ASTRO Phoenix definitioncity | 40.3/62.3 | Acute GI, GU Late GI, GU BC |
| Jani AB | [24] | 2007 | Pro. | NR/NR | 481(373/108) | 68.5/1.8-2 VS 75/1.8-2 | Prostatic bed, seminal vesicles | T1-T4 | RTOG toxicity scale | 53/51 | Acute GI, GU |
| Kim H | [25] | 2014 | Retro. | 78.6/73.4 | 86 (56/30) | 70/1.8 VS 70/2.5 | Prostatic bed, pelvic nodes, seminal vesicles | T1-T3bN0-N1 | RTOG toxicity scale | 56.7/53.6 | Late GI, GU BC |
| Kupelian PA | [26] | 2002 | Retro. | 25/25 | 282(116/166) | 78/2 VS 70/2.5 | Prostatic bed, pelvic nodes, seminal vesicles | T1-T3 | RTOG toxicity scale, ASTRO Phoenix definitioncity | 72/60 | Acute GU, BC |
| Odrazka K | [27] | 2010 | Retro. | 70.8/36 | 340(228/112) | 70/2 VS 78/2 | Prostatic bed, seminal vesicles | T1-3N0(pN0)M0 | RTOG toxicity scale | 19.7/54.5 | Late GU |
| Sharma NK | [28] | 2007 | Retro. | 86/40 | 293(170/123) | 76/2 VS 76/1.8 | Prostatic bed, pelvic nodes, seminal vesicles | T1-T3Nx-N0M0 | RTOG toxicity scale | 100/100 | Late GI, GU |
| Shimizuguchi T | [29] | 2016 | Retro. | 61.2/54 | 159(70/89) | 76/2 VS 76/2 | Prostatic bed, seminal vesicles | T1-T3N0M0 | CTCAE version 4.0 | 88/90 | Late GI, GU BC |
| Shu HK | [30] | 2001 | Retro. | 30.1/18.7 | 44(26/18) | NR | Prostatic bed, seminal vesicles | T1-T3 | RTOG toxicity scale | 79.5 | Acute GI, GU |
| Sveistrup J | [31] | 2014 | Retro. | 98.4/42 | 503(115/388) | 76/2 VS 78/2 | Prostatic bed, seminal vesicles | High risk | CTCAE version 4.0, ASTRO Phoenix definitioncity | 88/95 | BC |
| Troeller A | [32] | 2014 | Pro. | 106.8/55.2 | 1115(457/658) | 75.6/1.8 VS 75.6/1.8 | Prostatic bed, seminal vesicles | NR | CTCAE version 3.0 | 23.2/19.9 | Late GI |
| Vora SA | [33] | 2007 | Retro. | 60/48 | 416(271/145) | 68.4/NR VS 75.6/NR | Prostatic bed, seminal vesicles | T1b-T3b | RTOG toxicity scale, ASTRO Phoenix definitioncity | 17.6/30.3 | Acute GI, GU Late GI, GU BC |
| Wong WW | [34] | 2009 | Retro. | 120/120 | 584(270/314) | 68.4/1.8-2 VS 75.6/NR | Prostatic bed, seminal vesicles | T1c-T3N0M0 | RTOG toxicity scale, ASTRO Phoenix definitioncity | 17/36 | Acute GI, GU Late GI, GU BC |
| Wortel RC | [35] | 2015 | RCT | 3/3 | 475(215/260) | 78/2 VS 78/2 | Prostatic bed, seminal vesicles | T1-T4 | RTOG toxicity scale | 19.5/66.9 | Acute GI, GU |
| Wortel RC | [36] | 2016 | RCT | 62/57 | 431(189/242) | 78/2 VS 78/2 | Prostatic bed, seminal vesicles | T1b-T4Nx-0Mx-0 | RTOG toxicity scale | 40/66 | Late GI, GU |
| Zelefsky MJ | [37] | 2000 | Retro. | 39/12 | 232(61/171) | 81/1.8 VS 81/1.8 | Prostatic bed, seminal vesicles | T1c-T3 | RTOG toxicity scale | 34/53 | Acute GU |
| Zelefsky MJ | [38] | 2008 | Retro. | 120/78 | 1571(830/741) | 66-81/1.8 VS 81/NR | NR | T1-T3 | CTCAE version 3.0 | 43 | Late GU |
| Matzinger O | [39] | 2009 | RCT | NR/NR | 791(652/139) | 70-78/2 VS 74- 78/2 | NR | cT1b-2cN0M0 | CTCAE version 2.0 | 50 | Acute GI, GU |
BC: Biochemical control; PCa: Prostate cancer; IMRT: Intensity-modulated radiation therapy; 3D-CRT: Three-dimensional conformal radiotherapy; RTOG: Radiation Therapy Oncology Group; GI: gastrointestinal; GU: Genitourinary; RCT: Randomized clinical trials; Retro.: Retrospective study; Pro.: Prospective study; PTV: Planning target volume; NR: Not reported; Ref.: References; ADT: Androgen deprivation therapy. CTCAE: Common Terminology Criteria of Adverse
Events.
Acute GI adverse event
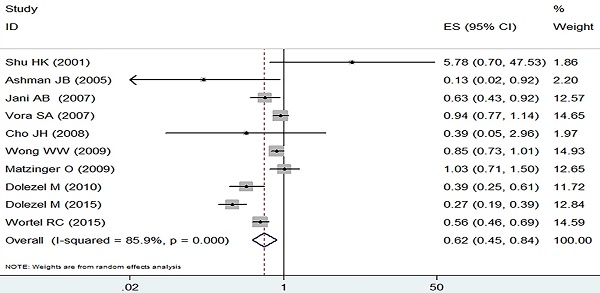
The incidence of grade 2 or worse acute adverse GI event was analyzed by the random effect model due to heterogeneous outcomes (I2=85.9%, p=0.000) and the pooled data revealed a clear decreasing trend in the IMRT compared with 3D-CRT (RR=0.62, 95% CI: 0.45-0.84, p=0.002, Figure 2).
Acute GU adverse event
Analysis by the fixed-effect model (I2=41.4%, p=0.065) showed that IMRT slightly increased the grade ≥ 2 acute GU adverse event in comparison with the 3D-CRT (RR=1.10, 95% CI: 1.02-1.19, p=0.015, Figure 3).
Late GI adverse event
The IMRT and the 3D-CRT of patients showed no substantial differences in grade ≥ 2 late GI adverse event (RR =0.62, 95% CI: 0.36-1.09, p=0.1, Figure 4) and showed a high level of heterogeneity based on the random effect model (I2=94.2%, p=0.000).
Late GU adverse event
With obvious heterogeneity found, the random effect model was employed (I2=81.1%, p=0.000). In those included studies, there was no significant difference between IMRT and 3D-CRT in grade 2-4 late GU adverse event (RR =1.08, 95% CI: 0.77-1.51, p=0.65, Figure 5).
Biochemical control
There was a significant difference in biochemical control favoring IMRT (RR =1.13, 95% CI: 1.05-1.22, p=0.002, Figure 6). IMRT showed modest increase in biochemical control in comparison with 3D-CRT. Random effect model was employed because of the significant heterogeneity (I2=78.6%, p=0.000).
Forest plot for acute GI adverse event.

Forest plot for acute GU adverse event.

Forest plot for late GI adverse event.

Forest plot for late GU adverse event.

Subgroup Analysis
The results of subgroup analysis (based on sample size, median follow-up time, PTV scope and study design) are presented in Table 2-5. In terms of “PTV scope,” IMRT can significantly reduce acute and late GI adverse events with a large PTV scope (Prostatic bed, pelvic nodes and seminal vesicles) (Acute: RR = 0.34, 95% CI: 0.17-0.69, p= 0.003; Late: RR = 0.46, 95% CI: 0.25-0.85, p= 0.013). However, IMRT failed to reduce acute and late GU adverse events with a large PTV scope (Acute: RR = 1.15, 95% CI: 0.86-1.53, p= 0.352; Late: RR = 0.80, 95% CI: 0.32-2.00, p= 0.636). For large sample size studies (n>100), IMRT can significantly reduce acute GI and GU adverse events (Acute GI: RR = 0.62, 95% CI: 0.46-0.84, p= 0.002; Acute GU: RR = 1.12, 95% CI: 1.03-1.22, p= 0.006).
Forest plot for biochemical control.

Subgroup analysis of Acute GI
| Items | No. of studies | RR | 95% CI | P value | |
|---|---|---|---|---|---|
| Sample size | ≤100 | 3 | 0.64 | 0.07-5.74 | 0.692 |
| >100 | 7 | 0.62 | 0.46-0.84 | 0.002 | |
| Study design | Retrospective study | 5 | 0.87 | 0.66-1.15 | 0.333 |
| Prospective | 3 | 0.40 | 0.24-0.67 | 0.001 | |
| RCT | 2 | 0.74 | 0.41-1.35 | 0.330 | |
| Median follow-up(m) | <60 | 6 | 0.61 | 0.38-0.96 | 0.035 |
| ≥60 | 2 | 0.48 | 0.16-1.49 | 0.206 | |
| NR | 2 | 0.81 | 0.50-1.31 | 0.382 | |
| PTV scope | Prostatic bed and seminal vesicles | 6 | 0.65 | 0.45-0.93 | 0.017 |
| Prostatic bed, pelvic nodes and seminal vesicles | 2 | 0.34 | 0.17-0.69 | 0.003 | |
| Prostatic bed | 1 | 0.39 | 0.05-3.00 | 0.366 |
RCT: Randomized clinical trials; NR: Not reported; PTV: Planning target volume; RR: Risk ratio
Subgroup analysis of Acute GU
| Items | No. of studies | RR | 95% CI | P value | |
|---|---|---|---|---|---|
| Sample size | ≤100 | 5 | 0.93 | 0.73-1.19 | 0.568 |
| >100 | 7 | 1.12 | 1.03-1.22 | 0.006 | |
| Study design | Retrospective study | 7 | 1.17 | 1.04-1.33 | 0.011 |
| Prospective | 3 | 1.17 | 0.99-1.38 | 0.06 | |
| RCT | 2 | 0.99 | 0.88-1.13 | 0.927 | |
| Median follow-up(m) | <60 | 8 | 1.00 | 0.90-1.12 | 0.976 |
| ≥60 | 2 | 1.25 | 1.08-1.44 | 0.003 | |
| NR | 2 | 1.15 | 0.98-1.36 | 0.091 | |
| PTV scope | Prostatic bed and seminal vesicles | 7 | 1.09 | 1.00-1.19 | 0.047 |
| Prostatic bed, pelvic nodes and seminal vesicles | 3 | 1.15 | 0.86-1.53 | 0.352 | |
| Prostatic bed | 1 | 0.58 | 0.22-1.53 | 0.273 |
RCT: Randomized clinical trials; NR: Not reported; PTV: Planning target volume; RR: Risk ratio
Subgroup analysis of Late GI
| Items | No. of studies | RR | 95% CI | P value | |
|---|---|---|---|---|---|
| Sample size | ≤100 | 2 | 0.44 | 0.13-1.52 | 0.195 |
| >100 | 6 | 0.66 | 0.36-1.22 | 0.186 | |
| Study design | Retrospective study | 5 | 0.74 | 0.42-1.32 | 0.310 |
| Prospective | 2 | 0.48 | 0.14-1.59 | 0.229 | |
| RCT | 1 | 0.66 | 0.50-0.88 | 0.004 | |
| Median follow-up(m) | <60 | 5 | 0.51 | 0.24-1.08 | 0.078 |
| ≥60 | 3 | 0.89 | 0.70-1.12 | 0.321 | |
| PTV scope | Prostatic bed and seminal vesicles | 6 | 0.64 | 0.34-1.23 | 0.181 |
| Prostatic bed, pelvic nodes and seminal vesicles | 2 | 0.46 | 0.25-0.85 | 0.013 |
RCT: Randomized clinical trials; PTV: Planning target volume; RR: Risk ratio
Subgroup analysis of Late GU
| Items | No. of studies | RR | 95% CI | P value | |
|---|---|---|---|---|---|
| Sample size | ≤100 | 2 | 1.69 | 0.28-10.14 | 0.565 |
| >100 | 7 | 1.06 | 0.75-1.50 | 0.737 | |
| Study design | Retrospective study | 7 | 1.14 | 0.75-1.75 | 0.531 |
| Prospective | 1 | 0.68 | 0.49-0.95 | 0.022 | |
| RCT | 1 | 1.27 | 1.00-1.61 | 0.046 | |
| Median follow-up(m) | <60 | 5 | 0.90 | 0.55-1.47 | 0.670 |
| ≥60 | 4 | 1.30 | 0.76-2.25 | 0.340 | |
| PTV scope | Prostatic bed and seminal vesicles | 6 | 1.10 | 0.77-1.58 | 0.585 |
| Prostatic bed, pelvic nodes and seminal vesicles | 2 | 0.80 | 0.32-2.00 | 0.636 |
RCT: Randomized clinical trials; PTV: Planning target volume; RR: Risk ratio
Sensitivity analysis and publication bias
In the meta-analysis of all outcomes, the Begg's funnel plot did not indicate any statistically significant asymmetry (Figure 7). A sensitivity analysis was carried out to make sure the robustness of the meta-analysis outcome. A statistically stable result was revealed by the sensitivity analysis's findings, which showed that none of the individual studies significantly affected the pooled HR or RR (Figure 8).
Funnel plots evaluating acute GU adverse event.

Sensitivity analysis of the acute GU adverse event.

Discussion
Twenty pertinent articles that evaluated the clinical results of PCa patients who underwent either IMRT or 3D-CRT were included in our meta-analysis. Our study's findings demonstrated that, as contrasted with 3D-CRT, IMRT was linked with a lower incidence of grade ≥ 2 acute GI adverse event and a higher BC. However, when compared to 3D-CRT, IMRT marginally increased the incidence of grade ≥ 2 acute GU adverse event while having identical grade ≥ 2 late GU adverse event. Furthermore, there were no appreciable variations in grade ≥ 2 late GI adverse event across the two treatment regimes. These data imply that IMRT, which has fewer side effects and enhanced PSA relapse-free survival, may be a better therapy option than 3D-CRT for patients with PCa.
Throughout the 1990s, numerous clinical trials proved the safety and efficacy of escalated-dose radiation for the treatment of localized PCa. However, it has been observed that increasing the prescribed dose is linked to an increased risk of late toxicities [40, 41]. As a result, there was a desire for the development and implementation of highly conformal dose delivery systems in order to reduce these toxicities. Treatment technology advanced at the same time, with 3D-CRT replacing two-dimensional treatment [42]. IMRT originated as an evolving version of 3D-CRT during the end of the 1990s [43]. IMRT, a relatively new radiation therapy method, is capable of delivering a dose distribution across a complicated and irregular target volume. Planning studies have shown that IMRT can reduce the dosage to adjacent tissue while maintaining planning target volume coverage [44, 45].
However, there are several drawbacks to IMRT. When compared to 3D-CRT, IMRT exposes a greater volume of healthy tissues to modest doses of radiation, which may increase the risk of second malignancies. However, more complete and clear data are needed to determine the clinical significance of this issue [46]. Furthermore, IMRT is a complex radiation treatment that necessitates a longer delivery time and greater physicist knowledge [47]. IMRT is anticipated to cost around £1100 more than 3D-CRT, owing to more staff time for radiographers, medical experts, and physicists [48]. Regardless, it is critical to assess the cost-effectiveness of IMRT, which may result in more quality-adjusted life-years (QALYs) at a lower overall cost [48]. As a result, it is critical to carefully balance the benefits and dangers of IMRT.
GI and GU adverse events have considerably influenced the quality of life of PCa patients who have received radiation in all published trials. IMRT, according to Worter et al. [35], has significantly reduced acute GU and GI adverse effects. As the study advances, IMRT offers certain advantages in terms of reducing intestine damage when compared to 3D-CRT, but there is no significant difference in urinary tract harm[36]. According to our findings, IMRT is associated with a decrease in grade 2 acute GI adverse event and a little rise in grade ≥ 2 acute GU adverse event. However, no significant differences in grade ≥ 2 late GI and GU adverse events were found. This finding is supported by a study conducted by Yu et al [49], who found that both IMRT and 3D-CRT groups had a decreased incidence of grade ≥ 2 acute GI adverse event and a slightly higher morbidity of grade ≥ 2 acute GU adverse event. Fang and colleagues described observation of 94 patients who underwent IMRT. 13.8% of them suffered from Grade 2 acute GI toxicity while 28.7% experienced that level of GU toxicity. They stated that hypertension increases the risk of acute GI toxicity and ADT, similarly to International Prostate Symptom Score (IPSS), increases acute GU toxicity [50]. In our study, the patients who underwent IMRT received ADT more than 3D-CRT. It might lead to an increase of acute GU adverse event. Grün et al found that the patients who were not able to maintain a partially filled bladder throughout treatment had a significantly higher risk of developing ≥ grade 2 GU acute adverse event [51]. Like other clinical trials there did not appear to be any difference in late genitourinary toxicity by radiation technique [38]. This may be related to the fact that the bladder neck and prostate urethra of 3DCRT and IMRT are inevitably part of the target volume. In addition, variable bladder filling makes the development of models of partial organ irradiation complex. Finally, the expression of late GU adverse event typically is years later than with GI adverse event and our follow-up is too short to identify any latent differences.
Numerous investigations have found that increasing the dose is associated with better biochemical and overall survival outcomes [4-7]. IMRT appears to improve long-term survival in high-risk PCa patients without incurring more adverse events, as opposed to 3D-CRT [52]. Our BC study findings also show a strong rising trend in IMRT when compared to 3D-CRT. Furthermore, following a long period of follow-up, the biochemical recurrence-free survival rate of PCa patients treated with 3D-CRT was significantly lower than that of IMRT-treated patients, particularly in patients with intermediate- to high-risk localized PCa [23, 33].
Aside from the inherent limitations of meta-analyses, our study had several significant drawbacks. For starters, our meta-analysis included a large number of retrospective studies, which may have caused bias when pooling the data. A bigger number of well-designed clinical trials and high-quality prospective studies should be conducted to further validate the findings. Furthermore, because the majority of the patients in our meta-analysis were Caucasian, caution should be exercised when extending the findings of this study to other ethnic communities.
In conclusion, our study found that IMRT is linked with a decrease in grade ≥ 2 acute GI adverse event and an improvement in biochemical control when compared to 3D-CRT, but a little rise in grade ≥ 2 acute GU adverse event. These findings show that IMRT should be considered as the best therapeutic approach for PCa. However, additional randomized controlled trials are required to determine the exact subset of PCa patients who might benefit the most from this treatment.
Abbreviations
IMRT: Intensity modulated radiation therapy; 3D-CRT: Three-dimensional conformal radiation therapy; PCa: Prostate cancer; GI: Gastrointestinal; GU: Genitourinary; HR: Hazard ratio; RR: Risk ratio; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines; CI: Confidence interval; PTV: Planning target volume; QALYs: Quality-adjusted life-years; NOS: Newcastle-Ottawa quality assessment scale; TNM: Tumor-node-metastasis; CTCAE: Common Terminology Criteria of Adverse; RTOG: Radiation Therapy Oncology Group.
Supplementary Material
Supplementary tables and information.
Acknowledgements
Funding
Cangzhou Science and Technology Project Self-financing Project (213106092).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: Wei Guo, Yun-Chuan Sun.
Data curation: Wei Guo, Li-Yuan Zhang.
Formal analysis: Wei Guo, Li-Yuan Zhang.
Project administration: Yun-Chuan Sun.
Methodology: Yun-Chuan Sun, Li-Yuan Zhang.
Resources: Xiao-Ming Yin.
Supervision: Yun-Chuan Sun.
Writing - original draft: Wei Guo, Li-Yuan Zhang.
Writing - review & editing: Wei Guo, Yun-Chuan Sun, Li-Yuan Zhang.
Availability of data and materials
All data are available from the references provided.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249
2. Lo Greco MC, Marletta G, Marano G, Fazio A, Buffettino E, Iudica A. et al. Hypofractionated Radiotherapy in Localized, Low-Intermediate-Risk Prostate Cancer: Current and Future Prospectives. Medicina (Kaunas). 2023;59:1144
3. Chinniah S, Stish B, Costello BA, Pagliaro L, Childs D, Quevedo F. et al. Radiation Therapy in Oligometastatic Prostate Cancer. Int J Radiat Oncol Biol Phys. 2022;114:684-692
4. Groen VH, Haustermans K, Pos FJ, Draulans C, Isebaert S, Monninkhof EM. et al. Patterns of Failure Following External Beam Radiotherapy With or Without an Additional Focal Boost in the Randomized Controlled FLAME Trial for Localized Prostate Cancer. Eur Urol. 2022;82:252-257
5. Kerkmeijer LGW, Groen VH, Pos FJ, Haustermans K, Monninkhof EM, Smeenk RJ. et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J Clin Oncol. 2021;39:787-796
6. Gómez-Aparicio MA, Valero J, Caballero B, García R, Hernando-Requejo O, Montero Á. et al. Extreme Hypofractionation with SBRT in Localized Prostate Cancer. Curr Oncol. 2021;28:2933-2949
7. Potters L, Rana Z, Lee L, Cox BW. Outcomes of a Dose-Escalated Stereotactic Body Radiation Phase 1 Trial for Patients With Low- and Intermediate-Risk Prostate Cancer Int J Radiat Oncol Biol Phys. 2019; 104:334-342.
8. Ohri N, Dicker AP, Showalter TN. Late toxicity rates following definitive radiotherapy for prostate cancer. Can J Urol. 2012;19:6373-6380
9. Daskivich TJ, Chamie K, Kwan L, Labo J, Palvolgyi R, Dash A. et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117:2058-2066
10. Hirata T, Suzuki O, Otani K, Miyake A, Tamari K, Seo Y. et al. Increased toxicities associated with dose escalation of stereotactic body radiation therapy in prostate cancer: results from a phase I/II study. Acta Oncol. 2023;62:488-494
11. Zelefsky MJ, Leibel SA, Gaudin PB, Kutcher GJ, Fleshner NE, Venkatramen ES. et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:491-500
12. Fiveash JB, Hanks G, Roach M, Wang S, Vigneault E, McLaughlin PW. et al. 3D conformal radiation therapy (3DCRT) for high grade prostate cancer: a multi-institutional review. Int J Radiat Oncol Biol Phys. 2000;47:335-342
13. Marta GN, Silva V, De Andrade Carvalho H, De Arruda FF, Hanna SA, Gadia R. et al. Intensity-modulated radiation therapy for head and neck cancer: systematic review and meta-analysis. Radiother Oncol. 2014;110:9-15
14. Damast S, Wolden S, Lee N. Marginal recurrences after selective targeting with intensity-modulated radiotherapy for oral tongue cancer. Head Neck. 2012;34:900-906
15. Buwenge M, Scirocco E, Deodato F, Macchia G, Ntreta M, Bisello S. et al. Radiotherapy of prostate cancer: impact of treatment characteristics on the incidence of second tumors. BMC Cancer. 2020;20:90
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-94
17. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16
18. Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605
19. Arab A, Mehrabani S, Moradi S, Amani R. The association between diet and mood: A systematic review of current literature. Psychiatry Res. 2019;271:428-37
20. Ashman JB, Zelefsky MJ, Hunt MS, Leibel SA, Fuks Z. Whole pelvic radiotherapy for prostate cancer using 3D conformal and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:765-771
21. Cho JH, Lee CG, Kang DR, Kim J, Lee S, Suh CO. et al. Positional reproducibility and effects of a rectal balloon in prostate cancer radiotherapy. J Korean Med Sci. 2009;24:894-903
22. Dolezel M, Odrazka K, Vaculikova M, Vanasek J, Sefrova J, Paluska P. et al. Dose escalation in prostate radiotherapy up to 82 Gy using simultaneous integrated boost: direct comparison of acute and late toxicity with 3D-CRT 74 Gy and IMRT 78 Gy. Strahlenther Onkol. 2010;186:197-202
23. Dolezel M, Odrazka K, Zouhar M, Vaculikova M, Sefrova J, Jansa J. et al. Comparing morbidity and cancer control after 3D-conformal (70/74 Gy) and intensity modulated radiotherapy (78/82 Gy) for prostate cancer. Strahlenther Onkol. 2015;191:338-346
24. Jani AB, Gratzle J, Correa D. Influence of intensity-modulated radiotherapy on acute genitourinary and gastrointestinal toxicity in the treatment of localized prostate cancer. Technol Cancer Res Treat. 2007;6:11-15
25. Kim H, Kim JW, Hong SJ, Rha KH, Lee CG, Yang SC. et al. Treatment outcome of localized prostate cancer by 70 Gy hypofractionated intensity-modulated radiotherapy with a customized rectal balloon. Radiat Oncol J. 2014;32:187-197
26. Kupelian PA, Reddy CA, Carlson TP, Altsman KA, Willoughby TR. Preliminary observations on biochemical relapse-free survival rates after short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy/fraction) for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:904-912
27. Odrazka K, Dolezel M, Vanasek J, Vaculikova M, Zouhar M, Sefrova J. et al. Late toxicity after conformal and intensity-modulated radiation therapy for prostate cancer: impact of previous surgery for benign prostatic hyperplasia. Int J Urol. 2010;17:784-790
28. Sharma NK, Li T, Chen DY, Pollack A, Horwitz EM, Buyyounouski MK. Intensity Modulated Radiation Therapy Reduces Gastrointestinal Toxicity in Patients Treated with Androgen Deprivation Therapy for Prostate Cancer. Int J Radiat Oncol Biol Phys. 2007;69:S10
29. Shimizuguchi T, Nihei K, Okano T, Machitori Y, Ito K, Karasawa K. A comparison of clinical outcomes between three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for prostate cancer. Int J Clin Oncol. 2017;22:373-379
30. Shu HK, Lee TT, Vigneauly E, Xia P, Pickett B, Phillips TL. et al. Toxicity following high-dose three-dimensional conformal and intensity-modulated radiation therapy for clinically localized prostate cancer. Urology. 2001;57:102-107
31. Sveistrup J, Af Rosenschold PM, Deasy JO, Oh JH, Pommer T, Petersen PM. et al. Improvement in toxicity in high risk prostate cancer patients treated with image-guided intensity-modulated radiotherapy compared to 3D conformal radiotherapy without daily image guidance. Radiat Oncol. 2014;9:44
32. Troeller A, Yan D, Marina O, Schulze D, Alber M, Parodi K. et al. Comparison and limitations of DVH-based NTCP models derived from 3D-CRT and IMRT data for prediction of gastrointestinal toxicities in prostate cancer patients by using propensity score matched pair analysis. Int J Radiat Oncol Biol Phys. 2015;91:435-443
33. Vora SA, Wong WW, Schild SE, Ezzell GA, Halyard MY. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:1053-1058
34. Wong WW, Vora SA, Schild SE, Ezzell GA, Andrews PE, Ferrigni RG. et al. Radiation dose escalation for localized prostate cancer: intensity-modulated radiotherapy versus permanent transperineal brachytherapy. Cancer. 2009;115:5596-5606
35. Wortel RC, Incrocci L, Pos FJ, Lebesque JV, Witte MG, Van Der Heide UA. et al. Acute toxicity after image-guided intensity modulated radiation therapy compared to 3D conformal radiation therapy in prostate cancer patients. Int J Radiat Oncol Biol Phys. 2015;91:737-744
36. Wortel RC, Incrocci L, Pos FJ, Van Der Heide UA, Lebesque JV, Aluwini S. et al. Late Side Effects After Image Guided Intensity Modulated Radiation Therapy Compared to 3D-Conformal Radiation Therapy for Prostate Cancer: Results From 2 Prospective Cohorts. Int J Radiat Oncol Biol Phys. 2016;95:680-689
37. Zelefsky MJ, Fuks Z, Happersett L, Lee HJ, Ling CC, Burman CM. et al. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother Oncol. 2000;55:241-249
38. Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A. et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124-1129
39. Matzinger O, Duclos F, Van Den Bergh A, Carrie C, Villa S, Kitsios P. et al. Acute toxicity of curative radiotherapy for intermediate- and high-risk localised prostate cancer in the EORTC trial 22991. Eur J Cancer. 2009;45:2825-2834
40. Kim S, Kong JH, Lee Y, Lee JY, Kang TW, Kong TH. et al. Dose-escalated radiotherapy for clinically localized and locally advanced prostate cancer. Cochrane Database Syst Rev. 2023;3:CD012817
41. Groen VH, Zuithoff NPA, van Schie M, Monninkhof EM, Kunze-Busch M, de Boer HCJ. et al. Anorectal dose-effect relations for late gastrointestinal toxicity following external beam radiotherapy for prostate cancer in the FLAME trial. Radiother Oncol. 2021;162:98-104
42. Dearnaley DP, Khoo VS, Norman AR, Meyer L, Nahum A, Tait D. et al. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet. 1999;353:267-272
43. Sheets NC, Goldin GH, Meyer AM, Wu Y, Chang Y, Sturmer T. et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611-1620
44. Fathy MM, Hassan BZ, El-Gebaly RH, Mokhtar MH. Dosimetric evaluation study of IMRT and VMAT techniques for prostate cancer based on different multileaf collimator designs. Radiat Environ Biophys. 2023;62:97-106
45. Ling CC, Burman C, Chui CS, Kutcher GJ, Leibel SA, LoSasso T. et al. Conformal radiation treatment of prostate cancer using inversely-planned intensity-modulated photon beams produced with dynamic multileaf collimation. Int J Radiat Oncol Biol Phys. 1996;35:721-730
46. Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83-88
47. Van De Werf E, Lievens Y, Verstraete J, Pauwels K, Van den Bogaert W. Time and motion study of radiotherapy delivery: Economic burden of increased quality assurance and IMRT. Radiother Oncol. 2009;93:137-140
48. Hummel S, Simpson EL, Hemingway P, Stevenson MD, Rees A. Intensity-modulated radiotherapy for the treatment of prostate cancer: a systematic review and economic evaluation. Health Technol Assess. 2010;14:1-108
49. Yu T, Zhang Q, Zheng T, Shi H, Liu Y, Feng S. et al. The Effectiveness of Intensity Modulated Radiation Therapy versus Three-Dimensional Radiation Therapy in Prostate Cancer: A Meta-Analysis of the Literatures. PLoS One. 2016;11:e0154499
50. Fang P, Mick R, Deville C, Both S, Bekelman JE, Christodouleas JP. et al. A case-matched study of toxicity outcomes after proton therapy and intensity-modulated radiation therapy for prostate cancer. Cancer. 2015;121:1118-27
51. Grün A, Kawgan-Kagan M, Kaul D, Badakhshi H, Stromberger C, Budach V. et al. Impact of bladder volume on acute genitourinary toxicity in intensity modulated radiotherapy for localized and locally advanced prostate cancer. Strahlenther Onkol. 2019;195:517-525
52. Jacobs BL, Zhang Y, Skolarus TA, Wei JT, Montie JE, Miller DC. et al. Comparative effectiveness of external-beam radiation approaches for prostate cancer. Eur Urol. 2014;65:162-168
Author contact
![]() Corresponding authors: Yun-Chuan Sun, Department of Radiation Oncology, Hebei Province Cangzhou Hospital of Integrated Traditional and Western Medicine, Cangzhou Hebei, 061000, China. Email: doctorsunyccom. Li-Yuan Zhang, Key Laboratory of Endocrinology of National Health Commission, Department of Endocrinology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, 100730, China. Email: pumchzlypumc.edu.cn.
Corresponding authors: Yun-Chuan Sun, Department of Radiation Oncology, Hebei Province Cangzhou Hospital of Integrated Traditional and Western Medicine, Cangzhou Hebei, 061000, China. Email: doctorsunyccom. Li-Yuan Zhang, Key Laboratory of Endocrinology of National Health Commission, Department of Endocrinology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, 100730, China. Email: pumchzlypumc.edu.cn.

 Global reach, higher impact
Global reach, higher impact