3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(18):3397-3403. doi:10.7150/jca.89082 This issue Cite
Research Paper
Pharmacological effects of Niraparib and its combination with angiogenic inhibitor in ovarian cancer
1. Hunan Key Laboratory of Oncotarget Gene, Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan 410013, PR China.
2. Key Laboratory of Carcinogenesis and Invasion, Chinese Ministry of Education, Cancer Research Institute, School of Basic Medicine, Central South University, Changsha, Hunan 410078, PR China.
3. Early Clinical Trial Center, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan 410013, PR China.
# These authors contribute equally to the paper.
Abstract
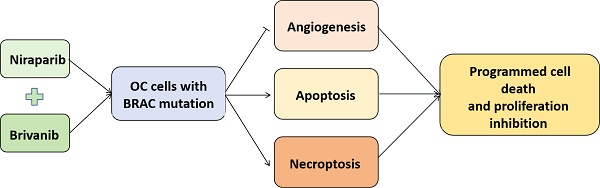
Background: Ovarian cancer (OC) represents the seventh most lethal female tumors worldwide. The combination of PARP inhibitor (PARPi) and angiogenic inhibitor has been shown to be effective as a first-line or second-line maintenance regimen to synergistically exert antitumor effects, which prompts us to further evaluate the therapeutic effect of the combination of PARP inhibitor Niraparib and anti-angiogenic Brivanib on OC.
Method:3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay were applied to evaluate the anti-proliferative effect of Niraparib, Brivanib, or the combination treatment on OC cells. The Annexin V-FITC/PI apoptotic assay was adopted to detect cell apoptosis. Tumor xenograft experiment and immunohistochemical (IHC) analysis were performed to evaluate the effect of single or combination treatment on the tumorigenicity of OC in vivo.
Results: Our current findings revealed that OC cells harboring BRAC1/2 mutations were more sensitive to Niraparib treatment compared to those with BRAC wild-type, and the addition of Brivanib enhanced programmed cell death (PCD) to sensitize OC cells with BRAC mutations to Niraparib treatment in vitro and in vivo.
Conclusion: Our work illustrates that the combination regimen of PARPi and angiogenic inhibitor treatment should be beneficial for the OC patients with BRAC mutations, at least partially owing to the induction of multiple forms of programmed cell death (PCD).
Keywords: Ovarian cancer, Niraparib, Brivanib, Regulated cell death

 Global reach, higher impact
Global reach, higher impact