3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(5):1366-1377. doi:10.7150/jca.89831 This issue Cite
Research Paper
Dioscin inhibits non-small cell lung cancer cells and activates apoptosis by downregulation of Survivin
1. Department of Thoracic Surgery, Hunan Chest Hospital, Changsha 410013, Hunan, China.
2. Department of Radiology, The Third Xiangya Hospital of Central South University, Changsha, Hunan 410013, China.
3. Department of Ultrasound, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430022, China.
# Equal contribution.
Received 2023-9-4; Accepted 2023-12-22; Published 2024-1-20
Abstract
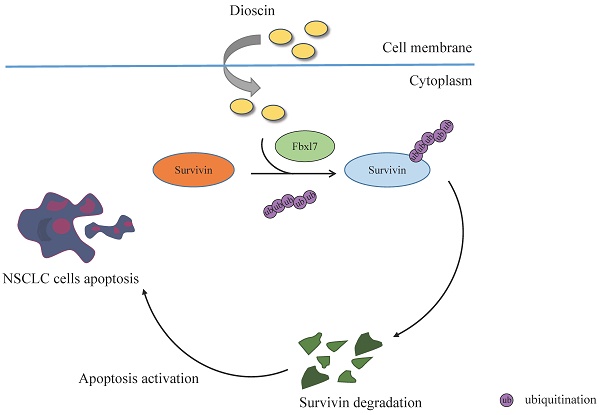
Human malignancies exhibit elevated levels of survivin, and have been linked to poor prognosis. Targeting survivin expression is a promising therapeutic strategy against cancer cells. Natural compounds have become a hot topic in research due to their non-toxic, non-invasive, and efficient treatment of multiple diseases. In this current investigation, it was discovered that Dioscin, as a natural compound, exerted profound antitumor activity against NSCLC cell lines, inhibiting NSCLC cell viability and promoting apoptosis. Further mechanistic studies showed that Dioscin promoted ubiquitination-mediated survivin degradation via strengthening the interaction between survivin and the E3 ubiquitin ligase Fbxl7. Furthermore, Dioscin exhibited a strong tumor suppressive effect in xenograft tumor models, and Dioscin treatment led to a notable decrease in tumor volume and weight. Based on our findings, Dioscin is expected to be a potential antitumor agent for non-small cell lung cancer treatment.
Keywords: NSCLC, Survivin, Dioscin, Ubiquitination
Introduction
Lung cancer is the most common cause of cancer deaths worldwide, with reports indicating that approximately 1.8 million people die from lung cancer each year [1]. Approximately 85% of lung cancers are Non-small-cell lung cancer (NSCLC), whose incidence increases yearly [2]. While numerous studies have demonstrated that smoking constitutes the foremost risk factor in developing NSCLC cancer, other risk factors include genetic history, toxic substance exposure, etc. [3], the mechanism of NSCLC tumorigenesis is not yet clear. According to the stage of lung cancer, histology, genetic alterations, and individual patient differences, the treatments for NSCLC generally include radiotherapy, surgery, immunotherapy, chemotherapy, and molecular targeted therapy. In recent years, we have made remarkable strides in targeted therapy and immunotherapy for NSCLC [3, 4]. However, early diagnosis of NSCLC, control of metastatic spread, and prevention of therapeutic resistance remain the main challenges to control and eradicate the disease [5]. Therefore, elaboration of the pathogenesis of NSCLC and the discovery of new antitumor targets and therapeutic approaches remains major obstacles to be solved.
Survivin, belonging to the family of inhibitory apoptosis proteins (IAPs), stands out as one of the most significant oncogene proteins identified up to this point [6]. It exhibits high expression levels in various tumor tissues, such as lung cancer, pancreatic cancer, breast cancer, colorectal cancer (CRC), and liver cancer, and exerts a key role in proliferation, apoptosis, angiogenesis, and therapy resistance [7-14]. Survivin can bind to caspases to inhibit apoptosis, regulate microkinetics in the G2/M phase of proliferating cells to promote mitosis, and form chromosome passenger complexes (CPC) with CDCA8/Borealin, AURKB (Aurora B kinase) and INCENP (inner centromere protein) [15]. Recent studies have shown that survivin could be a biomarker and potential prognostic indicator for breast cancer (BC) [16, 17]. Survivin overexpression in head and neck squamous carcinoma cells (HNSCC) exhibits treatment resistance [18, 19]. Targeting survivin can make cervical cancer cells more sensitive to radiation therapy [20]. Thus, survivin plays a critical regulatory role in both apoptosis and mitosis. Consequently, all these biological characteristics endow survivin as an attractive and promising therapeutic target against cancer cells [21-23].
Dioscin is a natural plant-derived steroidal saponin, which has a good curative effect on metabolic disorders, organ damage, viral and fungal infections, and multiple malignant tumor cells, and it has gained immense attention and popularity in preclinical research [24]. Notably, Dioscin was found to suppress tumorigenesis of colitis-associated CRC by modulating myeloid-derived suppressor cells (MDSCs) and macrophage polarization [25]. Additionally, Dioscin has demonstrated the capability to inhibit osteosarcoma stem-cell-like characteristics and tumor growth by repressing the Akt/GSK3/β-catenin signaling pathway [26]. Accumulating studies have elucidated the potent antitumor pharmacological activity of Dioscin in human cancers, which can be attributed to DNA damage, apoptosis, the induction of cell cycle arrest, and reprogramming glycolysis [24, 27, 28]. However, the potential antitumor mechanism of Dioscin on NSCLC has not been comprehensively clarified. Thus, the primary objective of this study is to explore the pharmacological activity of Dioscin on NSCLC cells and delve into the underlying mechanism responsible for its antitumor effect.
Materials and methods
Chemical reagents and cell culture
293T, human NSCLC cell lines containing H1975, HCC827, and A549, and normal non-tumor cells HBE, these cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Following ATCC protocols, all cells were maintained in a humidified incubator (37°C, 5% CO2) and underwent mycoplasma analysis every two months. Chemicals, incorporating SDS, NaCl, Tris base, DMSO, and lipofectamine™ 2000 (#11668019), were gained through Sigma-Aldrich (St. Louis, MO). Dioscin, cycloheximide (CHX) and MG132 were sourced from Selleck Chemicals (Houston, TX). The RPMI-1640 and DMEM culture media, along with Fetal Bovine Serum (FBS) and penicillin-streptomycin (P/S), were sourced from Invitrogen (Grand Island, NY) to support the cell culture process. Primary antibodies targeting ki67 (#ab16667, IHC: 1:300) and FbxL7 (#ab59149, IB: 1:1000) were obtained from Abcam (Cambridge, United Kingdom) for use in this study, cytochrome C (#11940, IB: 1:1000), survivin (#2808, IB: 1:1000, IP: 1:200, IHC: 1:200), ubiquitin (#3936, IB: 1:1000), β-actin (#3700, IB: 1:10000), α-tubulin (#3873, IB: 1:5000), VDAC1 (#4661, IB: 1:2000), Bax (#14796, IB: 1:1000), cleaved-caspase 3 (#9664, IB: 1:1000, IF: 1:400) were obtained from Cell Signaling Technology, Inc. (Beverly, MA). We obtained the Flag-survivin construct from Origene (RC205935).
MTS assays
After counting, NSCLC cells were plated in 96-well plates with a density of 3×103 cells per well and cultured overnight. Cells were exposed to various doses of Dioscin for 24, 48, and 72 hours. Finally, cell viability was assessed following standard protocols using MTS reagent (#G3580, Madison, WI).
Anchorage-independent cell growth
The methodology for the Anchorage-independent cell growth assay was executed following the previously established protocol [29]. Briefly, NSCLC cells (8×103) were cultured in 6-well plates containing 0.6% Basal Medium Eagle agar supplemented with 10% FBS and different concentrations of Dioscin. Subsequently, the cells were cultured under controlled conditions at 37°C with 5% CO2 for 14 days to support their growth.
Immunofluorescence (IF)
The immunofluorescence staining was conducted as previously described [30]. Cells were placed on chamber slides and treated with 4% paraformaldehyde for 20 minutes. After permeabilization in 0.5% Triton X-100 for an additional 20 min, cells were incubated in 5% BSA for 1 h. Afterward, the cells were exposed to cleaved-caspase 3 antibodies and incubated overnight at 4°C. Afterward, the cells underwent a PBS wash and were incubated with Alexa Fluor 568 dye-labeled anti-rabbit IgG. Finally, DAPI (P36935, Thermo Fisher Scientific) was used for nuclear staining for 30 min. Fluorescence images were acquired utilizing the confocal microscope system (Nikon C1si; NIKON Instruments Co.).
Flow cytometry
The procedure for apoptosis analysis using flow cytometry was carried out following the previously established method [31]. In summary, NSCLC cells underwent a 24-hour exposure to Dioscin. Subsequently, 1×106 cells were suspended in 500 μl of binding buffer, followed by the addition of 5 μl of Annexin V-FITC and 10 μl of PI, mixed thoroughly, and incubated for 5 minutes at room temperature and protected from light. Finally, the apoptotic cells were analyzed by FACS.
Western blotting (WB)
WB was implemented as formerly depicted [32]. Shortly, Whole-cell extract (WCE) was formulated by RIPA buffer supplemented with proteinase inhibitors and concentrated through BCA protein evaluation. The entire 20 μg WCE blended with loading buffer seethed at 95 °C for 5 min. Following this, the next steps included SDS-PAGE electrophoresis and electroblotting. Seal the membranes with 5% non-fat milk for 1 hour at room temperature, then incubate the corresponding antibody at 4 °C overnight. Subsequently, membranes were treated with secondary antibodies for 1 h at ambient temperature. The interest protein was discernible by chemiluminescence for immunoblot (IB) analysis.
Ubiquitination analysis
The Ubiquitination examination was implemented as formerly depicted [33]. NSCLC cells were treated with Dioscin and lysed with modified RIPA buffer. The cell lysates were sonicated and heated to 95°C for 15 min, then were mixed with RIPA buffer containing 0.1% SDS for dilution and centrifuged at 16000×g for 15min. The supernatants were incubated overnight with survivin antibody conjugated protein A-Sepharose beads at 4 °C with rotation. Subsequently, the beads underwent a washing process and boiled in 2×loading buffer for 5 min. The protein supernatant was collected after centrifugation at 3000 rpm for 1 min, and then subjected to western blotting assay. The ubiquitination level of survivin was detected by incubating with ubiquitin (Ub) antibody.
Co-immunoprecipitation (Co-IP) assays
We conducted Co-IP assays following previously established methods [34]. Cells were cultured in the medium of RPMI 1640 and 10% FBS and treated with Dioscin or DMSO control for 24 hours. Cells were reaped in IP lysis buffer (#87788, Thermo Scientific) as same as the manufacturer's manual. Following the guidelines provided by the manufacturer, we assessed the protein concentration using the BCA protein assay kit. Protein A/G-agarose beads were pre-cleared and immunoprecipitated with 2 μg of survivin antibody at 4°C overnight. The next day, the cell lysates were left to incubate with the above beads at 4°C for 2 hours. SDS-PAGE separated immunocomplexes and co-immunoprecipitated proteins were measured by using related antibodies.
Immunohistochemical (IHC) Staining
Tumor tissues were collected, fixed, and subjected to IHC analysis following established protocols [35]. The tissue sections underwent a process of deparaffinization using xylene, rehydrated with ethanol, and then placed in 10 mM citrate buffer and boiled for 10 min to achieve antigen retrieval. Following washing with ddH2O three times, the slides underwent treatment with a 3% H2O2 solution in methanol for 10 minutes to deactivate peroxidase activity. Followed by blocking with 50% goat serum albumin in PBS at ambient temperature for 1 h, the slides underwent an overnight primary antibody incubation at 4°C. Then, tissue slides were washed thrice with PBS and treated with a secondary antibody for 45 min at 37°C. Finally, positive staining was observed using the 3,3'-diaminobenzidine (DAB) substrate. Hematoxylin was applied as a counterstain.
Subcellular Fraction Isolation
A549 cells were treated with Dioscin for 24 hours. According to the standard instructions of the Kit, subcellular Protein Fractionation Kit (Thermo Fisher Scientific, Inc. #78840) and Mitochondria Isolation Kit (Thermo Fisher Scientific, Inc. #89874) were used to prepare proteins from different cellular compartments.
Cycloheximide assay
Dioscin treatment was applied to HCC827 cells for 24 hours, and CHX was added to the culture medium at a final concentration of 20 ug/ml. Cell lysates were collected at 0, 2, 4, and 8 h after CHX treatment. The half-life of survivin was detected by WB assay.
siRNA transfection
A549 cells were seeded in 6-well plates and transfected with FBXL7 siRNA (sc-62306, Santa Cruz Biotechnology, Inc.) and Ctrl siRNA (sc-37007, Santa Cruz Biotechnology, Inc.) using HiPerFect transfection reagent (Cat. 301705, Qiagen, Inc.) following the Kit protocols. Cells were incubated with siRNA transfection reagent for 48 h, and WB assay was then performed to confirm that FBXL7 was knocked down.
Blood assay
Blood was gathered from mice through a heart prick using EDTA-coated tubes. Red blood cell (RBC) and white blood cell (WBC) counts, along with alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, were analyzed at the laboratory of Central South University, China.
In vivo tumorigenicity assessment
The maintenance and experimentation of all mice were authorized by the Institutional Animal Care and Use Committee (IACUC) of Central South University (Changsha, China). Briefly, A549 (2×106) cells were s.c.inoculated onto the right side of 6-week-old female thymus-free nude mice to create the xenograft mouse model. Tumor volume and mouse body weight were detected every two days. Upon the tumor volume attaining 100 mm3, the mice with established tumors were randomly divided into two cohorts (n=6). The compound-treated group was given an intraperitoneal injection of Dioscin dissolved in 70% corn oil+30% PEG400 (10 mg/kg every 2 days), and the control group received the vehicle control treatment. Tumor volume was determined according to the following formula: length×width×width/2. Upon reaching the experimental endpoint, mice were euthanized, and the xenografts were removed, weighed, and then processed for immunohistochemical analysis.
Statistics assay
GraphPad Prism 5 was used for statistical analysis of tentative data. The discrepancy between the test groups was appraised by one-way ANOVA or t-test, and the statistical meaning benchmark was p under 0.05. All mensurable experimental data exhibited qua mean ± sd.
Results
Dioscin inhibits NSCLC cells in vitro
In preclinical research, the remarkable therapeutic effect of the natural product Dioscin (Fig. 1A) makes it a potential antitumor agent [24]. Here, our findings demonstrated that Dioscin exerted a dose-dependent inhibitory impact on the cell viability of A549, HCC827, and H1975 cells (Fig. 1B-D). After 5 μM Dioscin treatment for 72 h, the viability of all three groups of NSCLC cells decreased by more than 90%. Notably, the results indicated that the cell viability of immortalized non-tumor cells HBE1 was not notably affected following treatment with Dioscin (Fig. 1E). Next, the anchorage-independent cell growth assay was performed to detect the anti-tumor activity of Dioscin, and findings pointed out that the formation of colonies of NSCLC cells in soft agar was significantly hindered after exposure to Dioscin. And the inhibitory effect of Dioscin on the aforementioned three tumor cells was enhanced in a dosage-related manner (Fig. 1F). These data above suggest that Dioscin exerts robustly antitumor activity against NSCLC cells in vitro.
Dioscin induces apoptosis in NSCLC cells
It has been verified that Dioscin inhibited tumor growth and is partly attributed to induce apoptosis [36]. We then examined whether apoptosis signaling was activated in NSCLC cells following treatment with Dioscin. As shown in Fig 2A, we found that the protein expression of cleaved-Caspase 3 (c-Caspase 3) increased in A549 and HCC827 cells after treatment with Dioscin (Fig. 2A). Next, we extracted subcellular fractions from Dioscin-treated A549 cells. The findings indicated an elevation in the migration of cytochrome C from mitochondria to the cytoplasm, along with an increase in Bax protein levels within mitochondria, as the concentration of Dioscin rose (Fig. 2B). Moreover, IF results showed that Dioscin treatment promoted apoptosis in HCC827 and A549 cells, as the number of c-Caspase 3 positive cells increased substantially (Fig. 2C). We next conducted Annexin V/PI staining to measure the effect of Dioscin on the induction of apoptosis in A549 and HCC827 cells. The results demonstrate that the application of Dioscin for 24 h triggered significant apoptosis dose-dependently (Fig. 3A and 3B).
Dioscin promotes survivin degradation in NSCLC cells
To further investigate the underlying mechanism of Dioscin-induced apoptosis in NSCLC cells, we first examined survivin, one of the most critical anti-apoptotic proteins. Interestingly, IB results demonstrated that the expression level of survivin decreased with increasing Dioscin concentration in A549 and HCC827 cells (Fig. 4A). Furthermore, the protein level of survivin was restored following treatment with MG132, a proteasome inhibitor, even in the presence of Dioscin in NSCLC cells (Fig. 4B). As depicted in Fig. 4C, the results manifested that the half-life of survivin was obviously reduced in HCC827 cells treated with Dioscin, suggesting that Dioscin-induced survivin downregulation was related to its stability change. Subsequently, the ubiquitination level of survivin was determined. The results indicated that the ubiquitination of survivin was dose-dependently increased by Dioscin treatment in HCC827 cells (Fig. 4D). Moreover, our data indicated a mutation at K90/91, which has a pivotal function in maintaining the stability of survivin[37], reduced Diosicn-induced survivin ubiquitination in HCC827 cells (Fig. 4E). These results illustrated that Dioscin induced-survivin ubiquitination and degradation might be associated with apoptosis activation.
Fbxl7-mediated ubiquitination is required for Dioscin-induced survivin degradation
Previous reports indicate that E3 ligase Fbxl7 is an essential regulator of survivin protein, regulating its expression via inducing substrate protein ubiquitination and proteasome degradation [38, 39]. To investigate whether E3 ligase Fbxl7 is involved in Dioscin-induced survivin ubiquitination. Co-IP assay was conducted and the result showed that Dioscin treatment increased the interaction between Fbxl7 and survivin protein in NSCLC cells (Fig. 5A). Expectedly, knockdown Fbxl7 using siRNA impaired Dioscin-induced survivin ubiquitination in NSCLC cells, suggesting that Dioscin-induced survivin ubiquitination and degradation necessitate the presence of the E3 ligase Fbxl7 (Fig. 5B). We further explored the crucial role of E3 ligase Fbxl7 in the antitumor effect of Dioscin on NSCLC cells. Firstly, the apoptosis of cells was detected by western blotting assay. It was found that the knockdown of Fbxl7 neutralized the upregulation of c-Caspase 3 protein level induced by Dioscin (Fig. 5C). Consistently, the IF results manifested that the number of c-Caspase 3 positive cells was dramatically reduced after knockdown of Fbxl7 (Fig. 6A and B). Notably, cell viability (Fig. 6C and D) and colony formation (Fig.6E) of NSCLC cells were significantly restored in Fbxl7 knockdown cells treated with Dioscin. Overall, our data suggest that Fbxl7 is responsible for survivin degradation and apoptosis activation in Dioscin-treated NSCLC cells.
Dioscin inhibits NSCLC cells in vitro. (A)The molecular configuration of Dioscin. (B-D) Assessment of cellular viability in A549 (B), HCC827 (C), and H1975 (D) cell lines following exposure to Dioscin treatment through MTS assay. ***p<0.001. (E) MTS assay analysis of the cell viability of HBE1. **p<0.01. (F) Analysis of colony formation in A549, HCC827, and H1975 cells using an anchorage-independent cell growth assay. Scale bar, 1 mm.**p<0.01, ***p<0.001. ns, not statistically significant.

Dioscin induces apoptosis in NSCLC cells. (A) A549 and HCC827 cells were exposed to varying concentrations of Dioscin for 24 hours. Subsequently, the whole-cell extract (WCE) was utilized for immunoblotting analysis. (B) Dioscin treatment was administered to A549 cells for 24 hours, followed by the isolation of subcellular fractions and subsequent immunoblotting analysis. (C) Immunofluorescence (IF) analysis of c-Caspase 3 in HCC827 cells and A549 cells subjected to varying concentrations of Dioscin treatment. Scale bar, 10 µm. ***p<0.001.

Dioscin promotes apoptosis in vitro. (A and B) Detection of apoptosis in A549 cells and HCC827 cells treated with different concentrations of Dioscin by flow cytometry. ***p<0.001.

Dioscin promotes survivin degradation in NSCLC cells. (A) A549 and HCC827 cells were treated with Dioscin for 24 h. The WCE underwent IB analysis. (B) Dioscin treatment was administered to A549 and HCC827 cells for 24 hours, followed by an 8-hour incubation with MG132 (20 µM). The WCE was subjected to IB analysis. (C) Dioscin treatment was applied to HCC827 cells for 24 hours, and subsequently, cells were exposed to various time points of CHX incubation. The whole-cell extract (WCE) was then used for immunoblotting analysis. (D) HCC827 cells were treated with Dioscin for 24 h, and incubated with MG132 (20 µM) for 8 h. The WCE underwent analysis for survivin ubiquitination. (E) Following transfection with different constructs for 48 hours, HCC827 cells were exposed to Dioscin treatment for 24 hours and subsequently the cells were subjected to an 8-hour incubation with MG132 (20 µM). Survivin ubiquitination analysis was performed on the whole cell extract (WCE).

Dioscin suppresses tumor growth of NSCLC cells in vivo
To investigate the anti-tumor efficacy of Dioscin in vivo, xenograft mouse models were generated. The results manifested that Dioscin markedly suppressed tumor development of A549-derived xenograft tumors (Fig. 7A-7C), showing that the tumor volume (Fig. 7A and B) and tumor weight (Fig. 7C) were markedly reduced with Dioscin treatment, in which the tumor volume in the vehicle-treated group was more than 500 mm3, while in the Dioscin-treated group was less than 200 mm3 (Fig. 7A). Additionally, IHC data showed that the levels of Ki67 and survivin protein expression were significantly reduced in Dioscin-treated xenograft tumors (Fig. 7D). Furthermore, the in vivo tolerance of Dioscin was evaluated by blood routine analysis. It was shown that there was no significant difference in the RBC and WBC counts, AST and ALT levels between control and Dioscin-treated mice (Fig. 7E). In conclusion, these data indicate that Dioscin, a naturally occurring compound, demonstrates excellent tolerance and effectively inhibits tumor growth in vivo within NSCLC cells.
Fbxl7-mediated ubiquitination is required for Dioscin-induced survivin degradation. (A) After a 24-hour exposure to Dioscin, HCC827 cells were subsequently incubated with MG132 (20 µM) for 8 hours. Co-immunoprecipitation (Co-IP) analysis was performed on the whole cell extract (WCE). (B) HCC827 cells were transfected with Ctrl siRNA sequence and Fbxl7 siRNA sequence for 48 h, treated with or without Diosicn for 24 h, followed by an 8-hour incubation with MG132 (20 µM), respectively. The WCE underwent analysis to study survivin ubiquitination. (C) HCC827 cells underwent transfection with Ctrl siRNA sequence and Fbxl7 siRNA sequence for 48 h, treated with or without Diosicn for 24 h, respectively, and subjected to IB analysis.

Discussion
Dioscin (C45H72O16), a natural steroid saponin, is found in many plants and has antioxidant, anti-inflammatory and hypolipidemic, anti-obesity, hepatoprotective, and anti-tumor effects, thus protecting the body from cancer, gastrointestinal diseases, cerebrovascular diseases, and organ toxicity [40, 41]. Previous studies have demonstrated that Dioscin exhibits potent antitumor effects, a property that is mainly accomplished through its regulatory effects on cell cycle, autophagy, cell migration, apoptosis, reactive oxygen radicals (ROS) generation, proliferation, Ca2+ release and DNA damage, as well as its mechanistic effects on relevant signaling pathways and multiple target proteins in the growth and metastasis of cancer [41-43]. In addition, epithelial-mesenchymal transition (EMT) and angiogenesis, which have critical roles in tumor invasion and progression, can also be regulated by Dioscin[24]. However, its antitumor effects and potential mechanism of action on NSCLC have not been comprehensively elucidated. This study found that Dioscin significantly suppressed the malignant phenotypic properties, such as colony formation ability and cell viability. Our data identify a novel anti-tumor mechanism of Dioscin, suggesting that targeting survivin is a promising alternative strategy for treating NSCLC.
Survivin, as a crucial protumor factor in tumorigenesis, development, and poor prognosis, is an important biomarker and a promising target in tumor therapy [44, 45]. Therefore, exploring the regulatory mechanism of survivin in tumor cells remains pivotal scientific research. Ubiquitin is one of the crucial ways of protein post-translational modification and is involved in mediating protein biological function and expression. At present, some ubiquitin enzymes and deubiquitinases have been proven to be closely related to the intracellular regulation of survivin[46]. Arora et al. found that E3 activity of X-linked inhibitor of apoptosis (XIAP) directly promoted survivin ubiquitination and played a critical role in XAF1-mediated survivin degradation [47]. FBXL7, another E3 ubiquitin ligase, had been verified to be a survivin-interacting protein, which induced ubiquitin-proteasome degradation of survivin[38]. Consistently, in the present study, the interaction between survivin and E3 ligase Fbx17 was increased after treatment with Dioscin, which accounted for Dioscin-induced survivin ubiquitination and degradation. Several deubiquitination enzymes, such as CSN5, USP1, USP19, and STAMBPL1, prevent the ubiquitin degradation process of survivin and stabilize its expression, leading to tumor progression and therapeutic resistance [48-50]. Increasingly, researchers are devoted to discovering an effective survivin-targeting agent and moving it into clinical therapy.
Dioscin induces NSCLC cells apoptosis in an Fbxl7-dependent manner. (A and B) Immunofluorescence (IF) assessment of c-Caspase 3 in HCC827 (A) and A549 (B) cells transfected with Ctrl siRNA sequence and Fbxl7 siRNA sequence for 48h and subsequently exposed to Dioscin treatment or left untreated for 24 hours. Scale bar, 10 μm.***p<0.001. (C-D) HCC827 (C) and A549 (D) cells transfected with ctrl siRNA sequence and Fbxl7 siRNA sequence for 48 h and subsequently exposed to Dioscin treatment or left untreated for 24 hours, cell viability was assessed using the MTS assay. (E) HCC827 cells transfected with ctrl siRNA sequence and Fbxl7 siRNA sequence for 48 h and subsequently exposed to Dioscin treatment or left untreated for 24 hours, colony formation ability by anchorage-independent cell growth assay. ***p<0.001.

Dioscin suppresses in vivo tumor growth of NSCLC cells. (A-C) The tumor volume (A), The image of tumor mass (B), and tumor weight (C) in A549-derived xenograft tumors subjected to either vehicle control or Dioscin. ***p<0.001. (D) Analysis of Ki67 and survivin expression through IHC staining in A549-derived xenograft tumors. Scale bar, 25 μm. ***p<0.001. (E) Blood analysis of RBC, WBC, AST, and ALT levels in tumor-bearing mice with vehicle or Dioscin treatment. ns, not statistically significant.

Summarily, there are five main categories of targeted survivin cancer therapeutics in the current: survivin immunotherapy, survivin mRNA inhibitors, survivin transcription inhibitors, survivin homodimerization and survivin-partner protein interaction inhibitors [51-53]. However, developing a novel survivin-targeting agent with well-tolerance and effective antitumor activity remains challenging. YM155 is the first discovered small molecule survivin inhibitor, and it can significantly downregulate survivin expression by disrupting the survivin transcription factor Sp1, ILF3, and p54nrb in cancer cells [54-56]. Plescia et al. designed a cell-permeable peptidomimetic shepherdin, which can destabilize its client protein survivin by binding to the ATP pocket of Hsp90 to induce tumor cell apoptosis [57]. Natural products have attracted wide attention recently because of their low toxicity and sustainably obtained properties. A large number of preclinical studies have proved that natural compounds exert an antitumor effect by targeting a variety of pro-tumor proteins, such as survivin[58-60]. Xanthohumol significantly reduced survivin expression by regulating phosphorylation at Thr34 and facilitating ubiquitination, inhibiting oral squamous cell carcinoma (OSCC) cell growth [35]. Dihydromyricetin, a natural product derived from ampelopsis grossedentata, exhibits profound antitumor activity against NSCLC cells by downregulating the epidermal growth factor receptor (EGFR)/Akt/survivin signal pathway [61]. In the early stage of the research, we have confirmed that Dioscin exhibits obvious antitumor biological activity. Further mechanistic study illustrated that Dioscin administration remarkably activated the apoptosis signaling by facilitating the interaction between survivin and E3 ligase Fbx17 to promote survivin ubiquitination and degradation, which complements the new pharmacological activity of Dioscin in the treatment of human diseases.
Conclusions
This study shows that the natural compound Dioscin could downregulate survivin expression to inhibit NSCLC cells by promoting Fbxl7-induced survivin ubiquitination and degradation. Our discoveries imply that Dioscin holds potential as a viable therapeutic candidate for NSCLC.
Acknowledgements
Funding
This work was supported by the Scientifc Research Program of Hunan Provincial Health Committee (202203085243).
Data Availability Statement
The datasets used and analyzed in this study are available from the corresponding authors upon request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
2. Friedlaender A, Addeo A, Russo A, Gregorc V, Cortinovis D, Rolfo CD. Targeted Therapies in Early Stage NSCLC: Hype or Hope? Int J Mol Sci. 2020 21
3. Alexander M, Kim SY, Cheng H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung. 2020;198:897-907
4. Alduais Y, Zhang H, Fan F, Chen J, Chen B. Non-small cell lung cancer (NSCLC): A review of risk factors, diagnosis, and treatment. Medicine (Baltimore). 2023;102:e32899
5. Kumar M, Sarkar A. CURRENT THERAPEUTIC STRATEGIES AND CHALLENGES IN NSCLC TREATMENT: A COMPREHENSIVE REVIEW. Exp Oncol. 2022;44:7-16
6. Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917-21
7. Sanomachi T, Suzuki S, Togashi K, Seino S, Yoshioka T, Kitanaka C. et al. Brexpiprazole Reduces Survivin and Reverses EGFR Tyrosine Kinase Inhibitor Resistance in Lung and Pancreatic Cancer. Anticancer Res. 2019;39:4817-28
8. Kováčová Z, Hodorová I. Carbonic Anhydrase IX and Survivin in Colorectal Adenocarcinoma Cells: Slovakian Population Study. Biology (Basel). 2021 10
9. Ma S, Li X, Ran M, Ji M, Gou J, Yin T. et al. Fabricating nanoparticles co-loaded with survivin siRNA and Pt(IV) prodrug for the treatment of platinum-resistant lung cancer. Int J Pharm. 2021;601:120577
10. Jha K, Shukla M, Pandey M. Survivin expression and targeting in breast cancer. Surg Oncol. 2012;21:125-31
11. Wanandi SI, Limanto A, Yunita E, Syahrani RA, Louisa M, Wibowo AE. et al. In silico and in vitro studies on the anti-cancer activity of andrographolide targeting survivin in human breast cancer stem cells. PLoS One. 2020;15:e0240020
12. Dai DJ, Lu CD, Lai RY, Guo JM, Meng H, Chen WS. et al. Survivin antisense compound inhibits proliferation and promotes apoptosis in liver cancer cells. World J Gastroenterol. 2005;11:193-9
13. Kapiris I, Nastos K, Karakatsanis A, Theodosopoulos T, Karandrea D, Kondi Pafiti A. et al. Survivin expression in hepatocellular carcinoma. Correlation with clinicopathological characteristics and overall survival. J buon. 2019;24:1934-42
14. Sun X, Angelastro JM, Merino D, Zhou Q, Siegelin MD, Greene LA. Dominant-negative ATF5 rapidly depletes survivin in tumor cells. Cell Death Dis. 2019;10:709
15. Lin TY, Chan HH, Chen SH, Sarvagalla S, Chen PS, Coumar MS. et al. BIRC5/Survivin is a novel ATG12-ATG5 conjugate interactor and an autophagy-induced DNA damage suppressor in human cancer and mouse embryonic fibroblast cells. Autophagy. 2020;16:1296-313
16. Veiga GLD, Silva R, Pereira EC, Azzalis LA, Alves B, Gehrke FS. et al. The role of Survivin as a biomarker and potential prognostic factor for breast cancer. Rev Assoc Med Bras (1992). 2019;65:893-901
17. Fitzpatrick C, Bendek MF, Briones M, Farfán N, Silva VA, Nardocci G. et al. Mitochondrial ncRNA targeting induces cell cycle arrest and tumor growth inhibition of MDA-MB-231 breast cancer cells through reduction of key cell cycle progression factors. Cell Death Dis. 2019;10:423
18. Frassanito MA, Saltarella I, Vinella A, Muzio LL, Pannone G, Fumarulo R. et al. Survivin overexpression in head and neck squamous cell carcinomas as a new therapeutic target (Review). Oncol Rep. 2019;41:2615-24
19. Zhang L, Zhang W, Wang YF, Liu B, Zhang WF, Zhao YF. et al. Dual induction of apoptotic and autophagic cell death by targeting survivin in head neck squamous cell carcinoma. Cell Death Dis. 2015;6:e1771
20. Zhou J, Guo X, Chen W, Wang L, Jin Y. Targeting survivin sensitizes cervical cancer cells to radiation treatment. Bioengineered. 2020;11:130-40
21. Rafatmanesh A, Behjati M, Mobasseri N, Sarvizadeh M, Mazoochi T, Karimian M. The survivin molecule as a double-edged sword in cellular physiologic and pathologic conditions and its role as a potential biomarker and therapeutic target in cancer. J Cell Physiol. 2020;235:725-44
22. Dai D, Liang Y, Xie Z, Fu J, Zhang Y, Zhang Z. Survivin deficiency induces apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells. Oncol Rep. 2012;27:621-7
23. Lee SH, Lee JY, Jung CL, Bae IH, Suh KH, Ahn YG. et al. A novel antagonist to the inhibitors of apoptosis (IAPs) potentiates cell death in EGFR-overexpressing non-small-cell lung cancer cells. Cell Death Dis. 2014;5:e1477
24. Bandopadhyay S, Anand U, Gadekar VS, Jha NK, Gupta PK, Behl T. et al. Dioscin: A review on pharmacological properties and therapeutic values. Biofactors. 2022;48:22-55
25. Xun J, Zhou S, Lv Z, Wang B, Luo H, Zhang L. et al. Dioscin modulates macrophages polarization and MDSCs differentiation to inhibit tumorigenesis of colitis-associated colorectal cancer. Int Immunopharmacol. 2023;117:109839
26. Liu W, Zhao Z, Wang Y, Li W, Su Q, Jia Q. et al. Dioscin inhibits stem-cell-like properties and tumor growth of osteosarcoma through Akt/GSK3/β-catenin signaling pathway. Cell Death Dis. 2018;9:343
27. Liu YX, Xu BW, Niu XD, Chen YJ, Fu XQ, Wang XQ. et al. Inhibition of Src/STAT3 signaling-mediated angiogenesis is involved in the anti-melanoma effects of dioscin. Pharmacol Res. 2022;175:105983
28. Zhou L, Yu X, Li M, Gong G, Liu W, Li T. et al. Cdh1-mediated Skp2 degradation by dioscin reprogrammes aerobic glycolysis and inhibits colorectal cancer cells growth. EBioMedicine. 2020;51:102570
29. Li M, Liu H, Zhao Q, Han S, Zhou L, Liu W. et al. Targeting Aurora B kinase with Tanshinone IIA suppresses tumor growth and overcomes radioresistance. Cell Death Dis. 2021;12:152
30. Liu W, Li W, Liu H, Yu X. Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int J Biol Sci. 2019;15:2497-508
31. Li W, Yu X, Tan S, Liu W, Zhou L, Liu H. Oxymatrine inhibits non-small cell lung cancer via suppression of EGFR signaling pathway. Cancer Med. 2018;7:208-18
32. Li M, Gao F, Zhao Q, Zuo H, Liu W, Li W. Tanshinone IIA inhibits oral squamous cell carcinoma via reducing Akt-c-Myc signaling-mediated aerobic glycolysis. CELL DEATH & DISEASE. 2020 11
33. Yu X, Zhou L, Liu W, Liu L, Gao F, Li W. et al. Skp2 stabilizes Mcl-1 and confers radioresistance in colorectal cancer. Cell Death Dis. 2022;13:249
34. Li M, Gao F, Zhao Q, Zuo H, Liu W, Li W. Tanshinone IIA inhibits oral squamous cell carcinoma via reducing Akt-c-Myc signaling-mediated aerobic glycolysis. Cell Death Dis. 2020;11:381
35. Li M, Gao F, Yu X, Zhao Q, Zhou L, Liu W. et al. Promotion of ubiquitination-dependent survivin destruction contributes to xanthohumol-mediated tumor suppression and overcomes radioresistance in human oral squamous cell carcinoma. J Exp Clin Cancer Res. 2020;39:88
36. Yao Y, Cui L, Ye J, Yang G, Lu G, Fang X. et al. Dioscin facilitates ROS-induced apoptosis via the p38-MAPK/HSP27-mediated pathways in lung squamous cell carcinoma. Int J Biol Sci. 2020;16:2883-94
37. Dong X, Liu W, Li X, Gan Y, Zhou L, Li W. et al. Butein promotes ubiquitination-mediated survivin degradation inhibits tumor growth and overcomes chemoresistance. Sci Rep. 2022;12:20644
38. Kamran M, Long ZJ, Xu D, Lv SS, Liu B, Wang CL. et al. Aurora kinase A regulates Survivin stability through targeting FBXL7 in gastric cancer drug resistance and prognosis. Oncogenesis. 2017;6:e298
39. Liu Y, Lear T, Iannone O, Shiva S, Corey C, Rajbhandari S. et al. The Proapoptotic F-box Protein Fbxl7 Regulates Mitochondrial Function by Mediating the Ubiquitylation and Proteasomal Degradation of Survivin. J Biol Chem. 2015;290:11843-52
40. Zhong Y, Liu J, Sun D, Guo T, Yao Y, Xia X. et al. Dioscin relieves diabetic nephropathy via suppressing oxidative stress and apoptosis, and improving mitochondrial quality and quantity control. Food Funct. 2022;13:3660-73
41. Ding Q, Zhang W, Cheng C, Mo F, Chen L, Peng G. et al. Dioscin inhibits the growth of human osteosarcoma by inducing G2/M-phase arrest, apoptosis, and GSDME-dependent cell death in vitro and in vivo. J Cell Physiol. 2020;235:2911-24
42. Lv L, Zheng L, Dong D, Xu L, Yin L, Xu Y. et al. Dioscin, a natural steroid saponin, induces apoptosis and DNA damage through reactive oxygen species: a potential new drug for treatment of glioblastoma multiforme. Food Chem Toxicol. 2013;59:657-69
43. Zhao X, Xu L, Zheng L, Yin L, Qi Y, Han X. et al. Potent effects of dioscin against gastric cancer in vitro and in vivo. Phytomedicine. 2016;23:274-82
44. Fäldt Beding A, Larsson P, Helou K, Einbeigi Z, Parris TZ. Pan-cancer analysis identifies BIRC5 as a prognostic biomarker. BMC Cancer. 2022;22:322
45. Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46-54
46. Han S, Wang R, Zhang Y, Li X, Gan Y, Gao F. et al. The role of ubiquitination and deubiquitination in tumor invasion and metastasis. Int J Biol Sci. 2022;18:2292-303
47. Arora V, Cheung HH, Plenchette S, Micali OC, Liston P, Korneluk RG. Degradation of survivin by the X-linked inhibitor of apoptosis (XIAP)-XAF1 complex. J Biol Chem. 2007;282:26202-9
48. Chaithongyot S, Naumann M. Helicobacter pylori-induced reactive oxygen species direct turnover of CSN-associated STAMBPL1 and augment apoptotic cell death. Cell Mol Life Sci. 2022;79:86
49. Chandrasekaran AP, Tyagi A, Poondla N, Sarodaya N, Karapurkar JK, Kaushal K. et al. Dual role of deubiquitinating enzyme USP19 regulates mitotic progression and tumorigenesis by stabilizing survivin. Mol Ther. 2022;30:3414-29
50. Woo SM, Kim S, Seo SU, Kim S, Park JW, Kim G. et al. Inhibition of USP1 enhances anticancer drugs-induced cancer cell death through downregulation of survivin and miR-216a-5p-mediated upregulation of DR5. Cell Death Dis. 2022;13:821
51. Li F, Aljahdali I, Ling X. Cancer therapeutics using survivin BIRC5 as a target: what can we do after over two decades of study? J Exp Clin Cancer Res. 2019;38:368
52. Martínez-García D, Manero-Rupérez N, Quesada R, Korrodi-Gregório L, Soto-Cerrato V. Therapeutic strategies involving survivin inhibition in cancer. Med Res Rev. 2019;39:887-909
53. Albadari N, Li W. Survivin Small Molecules Inhibitors: Recent Advances and Challenges. Molecules. 2023 28
54. Cheng Q, Ling X, Haller A, Nakahara T, Yamanaka K, Kita A. et al. Suppression of survivin promoter activity by YM155 involves disruption of Sp1-DNA interaction in the survivin core promoter. Int J Biochem Mol Biol. 2012;3:179-97
55. Yamauchi T, Nakamura N, Hiramoto M, Yuri M, Yokota H, Naitou M. et al. Sepantronium bromide (YM155) induces disruption of the ILF3/p54(nrb) complex, which is required for survivin expression. Biochem Biophys Res Commun. 2012;425:711-6
56. Voges Y, Michaelis M, Rothweiler F, Schaller T, Schneider C, Politt K. et al. Effects of YM155 on survivin levels and viability in neuroblastoma cells with acquired drug resistance. Cell Death Dis. 2016;7:e2410
57. Plescia J, Salz W, Xia F, Pennati M, Zaffaroni N, Daidone MG. et al. Rational design of shepherdin, a novel anticancer agent. Cancer Cell. 2005;7:457-68
58. Budi HS, Farhood B. Tumor microenvironment remodeling in oral cancer: Application of plant derived-natural products and nanomaterials. Environ Res. 2023;233:116432
59. Svolacchia F, Brongo S, Catalano A, Ceccarini A, Svolacchia L, Santarsiere A. et al. Natural Products for the Prevention, Treatment and Progression of Breast Cancer. Cancers (Basel). 2023 15
60. Dong X, Liu W, Li X, Gan Y, Zhou L, Li W. et al. Author Correction: Butein promotes ubiquitination-mediated survivin degradation inhibits tumor growth and overcomes chemoresistance. Sci Rep. 2023;13:1780
61. Li X, Zhou L, Wang R, Zhang Y, Li W. Dihydromyricetin suppresses tumor growth via downregulation of the EGFR/Akt/survivin signaling pathway. J Biochem Mol Toxicol. 2023;37:e23328
Author contact
![]() Corresponding author: Dr. Gaoyan Deng, E-mail: 478376814com.
Corresponding author: Dr. Gaoyan Deng, E-mail: 478376814com.

 Global reach, higher impact
Global reach, higher impact