3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(10):3024-3033. doi:10.7150/jca.94539 This issue Cite
Research Paper
Neoadjuvant targeted immunotherapy followed by surgical resection versus upfront surgery for hepatocellular carcinoma with macrovascular invasion: A multicenter study
1. Department of Liver Surgery, Peking Union Medical College Hospital, PUMC and Chinese Academy of Medical Sciences, Dongcheng, Beijing 100730, China.
2. 4+4 Medical Doctor Program, PUMC and Chinese Academy of Medical Sciences, Dongcheng, Beijing 100730, China.
3. Department of Hepatobiliary Surgery, the 302nd Hospital of Chinese PLA, Fengtai, Beijing, 100039, China.
4. Department of Hepatobiliary Surgery, Jiangxi Provincial Cancer Hospital, Nanchang, Jiangxi, 330029, China.
5. Department of Hepatobiliary Surgery, the Fourth Affiliated Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050011, China.
*Xiangan Wu, Yuxin Wang, Sen Wang and Ye Chen contributed equally to this manuscript.
Received 2024-1-21; Accepted 2024-3-17; Published 2024-4-8
Abstract
Background: This study aimed to investigate the safety and efficacy of preoperative targeted immunotherapy followed by surgical resection for hepatocellular carcinoma (HCC) patients with macrovascular invasion.
Method: Clinical information of HCC patients with macrovascular invasion was collected from four medical centers. These patients were divided into two cohorts: the upfront surgery group (n=40) and the neoadjuvant group (n=22). Comparisons between the two groups were made with appropriate statistical methods.
Results: HCC Patients with macrovascular invasion in the neoadjuvant group were associated with increased incidence of postoperative ascites (72.73% vs. 37.5%, P=0.008), but shorter postoperative hospital stay (10 days vs. 14 days, P=0.032). Furthermore, targeted immunotherapy followed by surgical resection significantly reduced the postoperative recurrence rate at both 3 months and 1 year (9% versus 28.9%, 32.1% versus 67.9%, respectively; P=0.018), but increased the postoperative nononcologic mortality rate within 1 year (20.1% vs. 2.8%; P= 0.036).
Conclusion: For HCC patients with macrovascular invasion, preoperative targeted immunotherapy significantly decreased the postoperative tumor recurrence rate while maintaining relative safety, but such a treatment may also result in chronic liver damage and increased risk of nononcologic mortality.
Keywords: targeted immunotherapy, surgery, complications, the recurrence rate
Background
Among all malignant tumors, primary liver cancer ranks 6th in incidence and 3rd in mortality [1]. For early-stage hepatocellular carcinoma (HCC), radical surgery is the primary treatment with a 5-year survival rate of 60-70% [2]. However, more than 50% of HCC patients are in the Barcelona Clinic Liver Cancer (BCLC) stage C and D at the time of diagnosis. Late diagnosis deprives most patients of surgical opportunities and leads to a high mortality rate of HCC [3]. According to the diagnostic criteria of the 2019 edition of the Chinese clinical guidelines for primary liver cancer, invasion of the intrahepatic vessels, including the portal vein and hepatic vein, defines China Liver Cancer (CNLC) stage Ⅲa and progressive HCC, equivalent to the partial BCLC stage C HCC [4, 5]. Surgical resection for such patients leads to a better prognosis, with overall survival (OS) of 8.9-33 months compared to 4-6 months of untreated patients [6]. Therefore, radical surgical resection is currently recommended for HCC patients with macrovascular invasion, but high postoperative recurrence still impairs the prognosis of some patients [7-9]. Preoperative adjuvant therapy for HCC patients with macrovascular invasion may increase the rate of radical surgical resection, reduce the risk of postoperative recurrence, and prolong OS [10-12]. For instance, Xubiao Wei et al. found that neoadjuvant radiotherapy (RT) provided significantly better postoperative survival outcomes than surgery alone for resectable HCC with portal vein tumor thrombosis (PVTT) [13]. Jianping Zhao et al. suggested the neoadjuvant drug-eluting bead transarterial chemoembolization (D-TACE) and tislelizumab therapy were safe and benefited to the pathological results and prognosis for patients with resectable or borderline resectable HCC [14].
In recent years, targeted immunotherapy has shown great advantages in treating HCC, leading to an improved conversion rate of unresectable HCC, diminishing postoperative recurrence and prolonging OS [15-17]. For example, a phase Ib clinical trial showed that the combination of lenvatinib and pembrolizumab for unresectable HCC achieved a median OS of 22 months and an objective response rate (ORR) of 46%, despite grade≥3 treatment-related adverse events (AEs) occurring in approximately 67% of patients, which were mostly manageable [18]. The IMbrave150 global multicenter phase Ⅲ trial showed that the combination of atezolizumab and bevacizumab, compared to single-agent sorafenib, significantly benefited the survival of patients with unresectable HCC, with a 12-month survival rate of 67.2% versus 54.6% and an improved median progression-free survival (PFS) of 6.8 months versus 4.3 months. Common adverse effects included hypertension, proteinuria, liver dysfunction, diarrhea, and decreased appetite [19]. A study of 38 patients with advanced HCC with extrahepatic oligometastasis by Yang et al. reported that the combination of tyrosine kinase inhibitors (TKIs), immune checkpoint inhibitors (ICIs) and at least one local therapeutic modality enableed successful tumor downstaging and subsequent surgical resection in 9 patients (23.7%), with a low recurrence rate during the follow-up period. All patients had varying degrees of AEs, with approximately 55.6% being grade 3 AEs [20]. Other ICIs, such as durvalumab, and nivolumab, have also been shown to significantly prolong OS in advanced HCC, with manageable adverse reactions [21, 22].
Targeted immunotherapy has achieved great success in the treatment of advanced HCC. Previous studies reported prolonged OS, tumor downgrading, lower risk of postoperative recurrence, and tolerable AEs associated with targeted immunotherapy [23-25]. HCC with macrovascular invasion is an advanced-stage malignancy that may benefit from targeted immunotherapy and subsequent surgical resection [26], but the comparison between surgery following neoadjuvant targeted immunotherapy and upfront surgery in terms of short-term postoperative complications and long-term prognosis remains unclear. Therefore, this multicentered study aimed to investigate the safety and efficacy of targeted immunotherapy followed by surgical resection for HCC with macrovascular invasion, taking upfront surgery as control.
Patients and Methods
Patients
This study conducted a retrospective analysis of clinical data collected from HCC patients with macrovascular invasion who underwent hepatic surgery between January 2019 and May 2022 at four medical institutions: Peking Union Medical College Hospital, Beijing 302 Hospital, Jiangxi Provincial Cancer Hospital, and the Fourth Affiliated Hospital of Hebei Medical University. Enrollment criteria included: a) adults aged 18 and 70 years with a clinical or pathological diagnosis of HCC; b) evidence of tumor thrombus in the portal or hepatic vein ascertained through imaging or postoperative pathology, corresponding to CNLC stage IIIa or a subset of BCLC stage C HCC. The HCC diagnostic and staging guidelines were in accordance with Chinese standards [4, 5]. c) technically and oncologically resectable HCC; d) HCC patients with macrovascular invasion who meet the general condition and liver function requirements for surgery according to the preoperative assessment and have undergone complete tumor resection. Exclusion criteria were as follows: a) HCC previously treated with other antitumor therapies excluding combined targeted immunotherapy; b) HCC with distant extrahepatic metastases; c) recurrent HCC; d) a history of other malignancies.
Informed consent was obtained from all enrolled patients, and approval was obtained from the ethics committees of the four medical centers mentioned above. Demographic, surgical, pathological and postoperative information were collected from enrolled patients. The dimensions and multiplicity of tumors were determined by the last preoperative imaging. A major liver resection was defined as surgical removal of three or more liver segments. The treatment interval refers to the duration between the cessation of targeted or immunotherapy agents and the date of surgery; typically, ICIs are discontinued 4 weeks prior to surgical intervention.
Treatments
Patients were divided into two treatment cohorts: the upfront surgery group and the neoadjuvant group. The former underwent immediate surgery, while the latter received targeted immunotherapy, potentially supplemented with additional adjuvant treatments, such as TACE, Hepatic Artery Infusion Chemotherapy (HAIC), and radiothrapy (RT), prior to surgery. All neoadjuvant treatments were based on the combination of antiangiogenic agents and ICIs, but the specific regimens were personalized for each patient. Tumors were monitored monthly by enhanced Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) during the preoperative period. The Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and modified RECIST (mRECIST) criteria were used to assess treatment responses. Shrinkage or stabilization of the tumors suggested surgery, which would be individualized according to each patient's condition [27].
During the surgery, Pringle's maneuver was employed to obstruct the hepatic blood inflow, thereby minimizing intraoperative bleeding. Postoperative complications were stratified according to the Clavien-Dindo (CD) classification [28], with principal complications including postoperative hemorrhage, liver dysfunction, bile leakages, pulmonary infections, alterations in plasma albumin levels, bilirubin concentrations, and prothrombin time (PT). Complications were considered severe if classified as grade 3 or above according to the CD classification.
Enrolled patients were followed-up until June 2022. The primary endpoint of was recurrence-free survival (RFS), which was defined as the interval from the date of surgery to the detection of tumor recurrence by radiological examination. The secondary endpoint was OS, defined as the period from surgery to death, loss of follow-up or the end of the follow-up period. Postoperative complications were recorded during the postoperative hospital stay. Patients underwent regularly followed-ups, with monthly check-ups in the first six months after the surgery, quarterly for the subsequent two years, and biannually thereafter. Monitoring for postoperative recurrence involved alpha-fetoprotein (AFP) blood tests, hepatobiliary ultrasounds, and CT or MRI scans. Tumor recurrence was confirmed by a rise in AFP levels or visible lesions on imaging.
Statistics
Continuous variables were analyzed using median values and ranges with the Mann-Whitney U test, while categorical variables were expressed as percentages and analyzed with the chi-square test. Competing risk models were employed to explore the effect of upfront surgery versus neoadjuvant therapy on postoperative RFS. P <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics 22.0, GraphPad Prism 8, and R version 4.13.
Results
Characteristics
From January 2019 to May 2022, a cohort of 62 HCC patients with macrovascular invasion who underwent liver resection in the four participating medical centers was identified as eligible for the study. Of these, 40 underwent immediate surgery, and 22 received surgical resection following targeted immunotherapy. The average duration of preoperative adjuvant therapy was 4 months (95% confidence interval [CI] 3.0-4.9), with a median of 18 days elapsing between treatment conclusion and surgery (95% CI: 9.5-27.0) (Table 4). The baseline clinical characteristics of the patients in both groups are shown in Table 1. More than 90% of all patients were males of similar age, with 83.9% having a history of hepatitis B virus (HBV) infection. Fifteen percent of the upfront surgery group and 50% of those receiving neoadjuvant therapy were on regular preoperative antiviral treatment. All patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, and 93.5% presented with a preoperative liver function of Child-Pugh A. Median baseline levels of total bilirubin, albumin, and PT were within the normal limits for both groups, and elevated AFP was observed in over 60% of patients. Radiological examinations revealed that both groups had predominantly solitary tumors of similar size. The primary type of tumor thrombus was PVTT, and the main surgical procedure was anatomical hepatictomy. There was a higher proportion of minor hepatic resections within the upfront surgery group, whereas major hepatic resections were more common in the neoadjuvant group.
Baseline characteristics of the study cohorts
| Parameters | Total (n=62) | Upfront surgery (n=40) | Neoadjuvant (n=22) | P-value |
|---|---|---|---|---|
| Gender, n (%) | 0.908 | |||
| Male | 56 (90.3) | 36 (90.0) | 20 (90.9) | |
| Female | 6 (9.7) | 4 (10.0) | 2 (9.1) | |
| Age, median (IQR), years | 55.0 (45.8, 61.3) | 56.0 (48.2, 62.8) | 53.0 (43.0, 57.0) | 0.102 |
| HBV, n (%) | 52 (83.9) | 34 (85.0) | 17 (77.27) | 0.446 |
| Antivirus, n (%) | 17 (27.4) | 6 (15.0) | 11 (50.0) | 0.003 |
| Liver cirrhosis, n (%) | 50 (80.6) | 32 (80.0) | 18 (81.8) | 0.862 |
| ECOG-PS, n (%) | 0.082 | |||
| 0 | 39 (63.0) | 22 (55.0) | 17 (77.3) | |
| 1 | 23 (37.0) | 18 (45.0) | 5 (22.7) | |
| Child-Pugh stage, n (%) | 0.650 | |||
| Grade A | 58 (93.5) | 37 (92.5) | 21 (95.5) | |
| Grade B | 4 (6.5) | 3 (7.5) | 1 (4.5) | |
| Basic bilirubin, median (IQR), umol/L | 13.3 (10.9, 19.1) | 15.1 (11.1, 21.9) | 12.3 (10.4,15.23) | 0.109 |
| Basic albumin, median (IQR), g/L | 39.0 (37.0, 41.1) | 39.1 (37.1 42.0) | 38.2 (36.0, 41.0) | 0.247 |
| Basic prothrombin time, median (IQR), s | 12.1 (11.3, 13.0) | 12.3 (11.4, 13.3) | 11.9 (11.3, 12.4) | 0.164 |
| AFP level, n (%) | 0.929 | |||
| ≤ 20 ng/L | 23 (37.1) | 15 (37.5) | 8 (36.4) | |
| >20 ng/L | 39 (62.9) | 25 (62.5) | 14 (63.6) | |
| Tumor diameter, median (IQR), cm | 7.1 (5.0, 9.4) | 6.8 (5.0, 9.5) | 7.3 (5.0, 9.3) | 0.906 |
| Tumor number, n (%) | 0.645 | |||
| Single | 39 (62.9) | 26 (65.0) | 13 (59.1) | |
| Multiple | 23 (37.1) | 14 (35.0) | 9 (40.9) | |
| Tumor thrombus, n (%) | 0.437 | |||
| Portal vein | 54 (87.1) | 35 (87.5) | 19 (86.4) | |
| Hepatic vein | 6 (9.7) | 3(7.5) | 3 (13.6) | |
| Both | 2 (3.2) | 2 (5.0) | 0 (0.0) | |
| Anatomic resection, n (%) | 46 (74.2) | 28 (70.0) | 18 (81.8) | 0.309 |
| Hepatectomy, n (%) | 0.176 | |||
| Minor | 38 (61.3) | 27 (67.5) | 11 (50.0) | |
| Major | 24 (38.7) | 13 (32.5) | 11 (50.0) |
IQR, interquartile range; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; HBV, hepatitis B virus; AFP, alpha-fetoprotein.
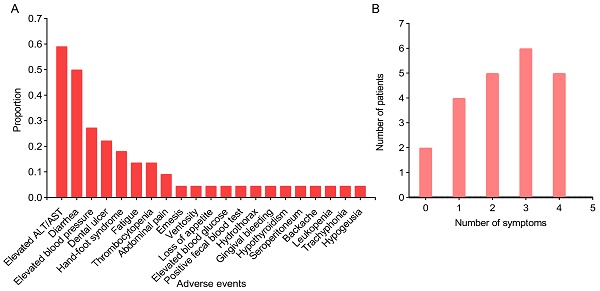
In this study, six preoperative adjuvant targeted immunotherapy regimens were utilized (Table 4): Antiangiogenic+ICI for two patients, Antiangiogenic+ICI+HAIC for four patients, Antiangiogenic+ICI+TACE for eight patients, Antiangiogenic+ICI+TACE+HAIC for one patient, Antiangiogenic+ICI+RT for three patients, and Antiangiogenic+ICI+TACE+RT for four patients. The combination of Antiangiogenic + ICI with TACE or HAIC represented the most employed therapeutic protocol, used in 13 out of 22 cases. Upon preoperative evaluation, 21 out of 22 patients attained preoperative partial remission (PR), and 1 out of 22 exhibited stable disease (SD). The postoperative pathology indicated that pathologic complete response (PCR) occurred in 3 patients. Comparison between the six regimens revealed that the antiangiogenic+ICI+HAIC arm had the highest proportion of PCR (2/4). The most common AE of targeted immunotherapy was aminotransferase (ALT) elevation (59.1%), followed closely by diarrhea (50%) (Figure 1A). Other common AEs included elevated blood pressure, dental ulcer, hand-foot syndrome, fatigue, thrombocytopenia, abdominal pain, emesis, and ventosity, etc. The variety and frequency of symptoms exhibited considerable interpatient variability: some individuals presented with no symptoms or a singular symptom, whereas others experienced multiple complications concurrently (Figure 1B). All AEs were tolerable and manageable with supportive therapy and symptomatic relief, resulting in no treatment discontinuations or transitions to alternative antitumor therapies.
Information regarding the surgical procedures and short-term postoperative outcomes is summarized in Table 2. The median operative time and hilar occlusion duration for the upfront surgery cohort were 240.0 minutes (interquartile rage [IQR] 202.5-300.0) and 21.5 minutes (IQR 12.3-30.8). respectively. In contrast, the neoadjuvant group recorded longer median times of 292 minutes (IQR 232.5-343.8) for surgery and 31.5 minutes (IQR 0.0-62.3) for hilar occlusion; however, these differences did not attain statistical significance. Interestingly, the neoadjuvant group exhibited lower median intraoperative blood loss [300.0 ml (IQR 175.0-575.0 ml) versus 400 ml (IQR 200.0-600.0 ml)], yet a greater proportion of these patients required perioperative blood transfusions (63.6% versus 45%). This heightened demand for blood products in the neoadjuvant group may be attributable to preoperative adjuvant therapy-related hepatic function compromise. Neoadjuvant group patients also experienced a significantly higher incidence of postoperative massive ascites (72.73% versus 37.5%; P=0.008). There was no significant difference in postoperative serum albumin, total bilirubin, and PT levels across both groups, possibly because of the benefits of supportive measurements, such as exogenous albumin supplementation and plasma transfusions, etc.
Overview of adverse events (A) and distribution of symptoms (B) in patients with preoperative targeted immunotherapy. A, the x-axis represents the various adverse events, and the y-axis represents the proportion of adverse events to the total cases; B, the x-axis represents the number of symptoms one has experienced, and the y-axis represents the number of patients.

Characteristics of surgical and postoperative features
| Outcomes | Total (n=62) | Upfront surgery (n=40) | Neoadjuvant (n=22) | P-value |
|---|---|---|---|---|
| Operative time, median (IQR), min | 267.5 (210.0, 300.0) | 240.0 (202.5, 300.0) | 292.0 (232.5, 343.8) | 0.182 |
| Hilar occlusion, median (IQR), min | 22.5 (10.3, 34.0) | 21.5 (12.3, 30.8) | 31.5 (0.0, 62.3) | 0.220 |
| Intraoperative blood loss, median (IQR), ml | 400.0 (200.0, 600.0) | 400.0 (212.5.0, 600.0) | 300.0 (175.0, 575.0) | 0.270 |
| Perioperative transfusion, n (%) | 32 (51.6) | 18 (45.0) | 14(63.6) | 0.160 |
| Bilirubin, median (IQR), μmol/L | 31.5 (23.9, 41.6) | 31.4 (23.9, 43.2) | 31.6 (25.9, 40.4) | 1.000 |
| Albumin, median (IQR), g/L | 32.8 (29.5, 35.4) | 31.8 (29.2, 34.8) | 34.5 (30.2, 36.0) | 0.233 |
| Prothrombin time, median (IQR), s | 14.0 (12.3, 15.7) | 13.4 (11.9, 15.7) | 14.7 (13.1, 15.5) | 0.369 |
| Clavien-Dindo classification, n (%) | 0.699 | |||
| grade 0/I | 40 (64.5) | 27 (67.5) | 13 (59.1) | |
| grade II | 14 (22.6) | 9 (22.5) | 5 (22.7) | |
| grade III | 5(8.1) | 3 (7.5) | 2 (9.1) | |
| grade IV | 2(3.2) | 1 (2.5) | 1 (4.5) | |
| grade V | 1(1.6) | 0 (0.0) | 1 (4.5) | |
| Major complications, n (%) | 8(12.9) | 4 (10.0) | 4 (18.2) | 0.438 |
| Bile leakage, n (%) | 6 (9.7) | 3 (7.5) | 3 (13.64) | 0.434 |
| Ascites, n (%) | 31 (50.0) | 15 (37.5) | 16 (72.73) | 0.008 |
| Postoperative hospital stay, median (IQR), days | 12.0 (8.0, 15.0) | 14.0 (9.0, 15.0) | 10.0 (7.8, 13.0) | 0.032 |
| 90-days-mortality, n (%) | 5 (8.1) | 3 (7.5) | 2 (9.1) | 0.826 |
| Tumor differentiation, n (%) | 0.637 | |||
| Poor | 25 (40.3) | 17 (42.5) | 8 (36.4) | |
| Moderately/Well | 37 (59.7) | 23 (57.5) | 14 (63.6) | |
| Satellite foci, n (%) | 17 (27.4) | 14 (35.0) | 3 (13.6) | 0.071 |
| Cutting edge, n (%) | 0.650 | |||
| R0 | 58 (93.5) | 37 (92.5) | 21 (95.5) | |
| R1 | 4 (6.5) | 3 (7.5) | 1 (4.5) | |
| Survival analysis | ||||
| RFS, mean (95%CI), month | 11.5 (8.2, 14.9) | 9.7 (6.3, 13.2) | 9.6 (6.8, 12.5) | 0.275 |
| OS, mean (95%CI), month | 28.2 (23.8, 32.6) | 28.2 (23.0, 33.4) | 13.5 (11.2, 15.7) | 0.858 |
IQR, interquartile range; RFS, recurrence free survival; OS, overall survival.
Neoadjuvant treatments based on targeted immunotherapy
| Preoperative regimens | Cases | Duration, mean, months | Interval, mean, Days | PR | SD | PCR |
|---|---|---|---|---|---|---|
| Antiangiogenic+ICI | 2 | 6.3 | 11 | 2/2 | 0/2 | 0/2 |
| Antiangiogenic+ICI+HAIC | 4 | 3.8 | 28 | 4/4 | 0/4 | 2/4 |
| Antiangiogenic+ICI+TACE | 8 | 3.7 | 18 | 7/8 | 1/8 | 1/8 |
| Antiangiogenic+ICI+TACE+HAIC | 1 | 4.8 | 12 | 1/1 | 0/1 | 0/1 |
| Antiangiogenic+ICI+RT | 3 | 3.5 | 12 | 3/3 | 0/3 | 0/3 |
| Antiangiogenic+ICI+TACE+RT | 4 | 4.2 | 19 | 4/4 | 0/4 | 0/4 |
| Total | 22 | 4.0 (3.0, 4.9) | 18 (9.5, 27.0) | 21/22 | 1/22 | 3/22 |
Duration, the time from the beginning to the end of preoperative adjuvant treatment; Interval, the time from the end of preoperative treatment to the date of surgery; PR, partial remission; SD, stable disease; PCR, pathologic complete response; ICI, immune checkpoint inhibitors; HAIC, hepatic arterial infusion chemotherapy; TACE, transarterial chemoembolization; RT, radiotherapy.
More reassuringly, the neoadjuvant group demonstrated CD classifications (P=0.669), rates of major postoperative complications (7.5% vs. 18.2%; P=0.204), and incidences of postoperative biliary leakages (7.5% vs. 13.64%; P=0.434) that were comparable to those of the upfront surgery group. The neoadjuvant group experienced a shorter median postoperative hospital stay [10 days (IQR 7.8-13.0) versus 14 days (IQR 9.0-15.0); P=0.032]. The prevalence of satellite focus was lower in the neoadjuvant group (13.6% vs. 35%), although these findings did not achieve statistical significance, possibly a result of preoperative adjuvant therapy deactivating peritumoral microsatellite focus. Both groups exhibited similar tumor differentiation statuses and proportions of positive resection margins.
The median follow-up durations were 9 months (IQR 5.0-19.5) for the upfront surgery cohort and 8.5 months (IQR 5.8-13.8) for the neoadjuvant cohort. Given instances of nononcologic mortality among these patients, a multifactorial competing risks model was deployed to discern clinical factors influencing patient prognosis (Table 3). Preoperative adjuvant therapy followed by surgery markedly decreased the hazard of tumor recurrence (Hazard ratio [HR]=0.39, 95% CI 0.15-0.98; P=0.046), whereas larger tumor size (HR=1.13, 95% CI 1.01- 1.27; P=0.028) and R1 resection status (HR=4.61, 95%CI 1.55-13.75; P=0.006) were associated with a heightened hazard. Furthermore, neoadjuvant targeted immunotherapy preceding surgery resulted in substantially reduced postoperative tumor recurrence rates after both 3 months and 1 year (9% vs. 28.9% and 32.1% vs. 67.9%, respectively; P=0.018) (Figure 2A). However, the neoadjuvant cohort demonstrated a higher rate of 1-year nononcologic mortality (20.1% vs. 2.8%; P=0.036). There were no statistically significant differences in RFS and OS between the groups (Table 2; figures 2B, C). One patient from the neoadjuvant group experienced biliary leakage with subsequent biliary stricture, developed a secondary biliary infection, and ultimately succumbed to secondary pulmonary embolism and pulmonary heart disease. Additionally, a separate patient from the same group suffered from postoperative bile leakage and biliary stricture, endured recurrent biliary tract infections, and passed away from infectious shock. A further patient in the neoadjuvant group, notwithstanding an excellent PCR, faced multiple postoperative complications necessitating ongoing medical management, these included liver function impairment, massive thoracoabdominal effusion, gastrointestinal bleeding, liver abscess, gastrobiliary fistula, biliary stricture, and thrombocytopenia, that led to recurrent hospital admissions and substantially diminished quality of life.
A competing risk model of RFS
| Parameters | HR | 95% CI | P-value |
|---|---|---|---|
| Treatment groups (Neoadjuvant versus upfront surgery) | 0.39 | 0.15 to 0.98 | 0.046 |
| Antivirus treatment (yes versus no) | 0.83 | 0.26 to 2.65 | 0.750 |
| AFP (>20 ng/L versus ≤ 20 ng/L) | 0.76 | 0.36 to 1.61 | 0.470 |
| Tumor diameter | 1.13 | 1.01 to 1.27 | 0.028 |
| Tumor number (multiple versus single) | 1.58 | 0.67 to 3.77 | 0.300 |
| Anatomic resection (yes versus no) | 0.45 | 0.17 to 1.18 | 0.100 |
| Tumor differentiation (Moderately/Well versus poor) | 1.18 | 0.44 to 3.13 | 0.740 |
| Satellite foci (yes versus no) | 0.98 | 0.45 to 2.13 | 0.960 |
| Resection margin (R1 versus R0) | 4.61 | 1.55 to 13.75 | 0.006 |
AFP, alpha-fetoprotein.
Survival analysis between the upfront surgery group and neoadjuvant group. (A) Cumulative incidence of postoperative tumor recurrence and nononcologic mortality between the two groups, 1 refers to tumor recurrence, and 2 refers to nononcologic mortality. (B) Kaplan-Meier Curve for RFS between the two groups. (C) Kaplan-Meier Curve for OS between the two groups. RFS, recurrence-free survival, OS, overall survival.

Discussion
The Chinese guidelines [4] and National Comprehensive Cancer Network (NCCN) guidelines concur that surgical resection, whether performed immediately (upfront surgery) or following preoperative adjuvant therapy, is the primary treatment for HCC with macrovascular invasion. The advent of immunotherapy has revolutionized the adjuvant treatment of HCC. Preoperative targeted immunotherapy allowed more patients with advanced disease to attempt radical surgical resection, thereby greatly prolonging the OS of advanced HCC [29, 30]. Evidence from several cohorts illustrates that the combination of targeted therapy and immune therapy, optionally augmented by additional interventions, has yielded improved ORR of 30.0%-80.6% and increased conversion surgery rates of 10.0%-42.4% in patients with advanced HCC [24, 26, 31-33]. However, little attention has been paid to the difference in postoperative outcomes between upfront surgery and surgical resection after neoadjuvant targeted immunotherapy. In this study, we compared the rate of perioperative complications, RFS and OS among HCC patients with macrovascular invasion who received upfront surgery or surgical resection subsequent to preoperative targeted immunotherapy.
HCC with macrovascular invasion is an aggressive tumor that presents formidable challenges to surgical resection due to its size and invasion on adjacent structures. Optimistically, the objectives of preoperative adjuvant therapy include tumor shrinkage and downgrading, thereby reducing the difficulty of subsequent surgery. However, therapy-induced liver function impairment and perihepatic adhesion, along with increased vessel fragility and subsequent intraoperative bleeding, pose additional challenges. As Zhou et al. reported, preoperative TACE alone did not improve surgical outcomes, but instead increased surgical difficulty and higher risk of postoperative liver failure [34]. In our study, the upfront surgery group and neoadjuvant group had similar operative time, hepatic hilar occlusion time, and intraoperative blood loss, suggesting that the benefits and additional problems of preoperative adjuvant therapy seem to balance out, with no significant impact on the difficulty of subsequent surgery.
Preoperative adjuvant therapy resulted in increased incidence and volume of postoperative ascites, but perioperative blood transfusion rates, postoperative bilirubin, albumin, PT, bile leakages, and other major complications were comparable between the two groups, suggesting that although preoperative adjuvant therapy may induce some liver damage, the essential and synthetic functions of the liver remain generally preserved. Conversely, postoperative hospital stay was shortened for patients who received preoperative adjuvant therapy, indicating that the targeted immunotherapy-associated ascites had minor effects on postoperative recovery. Moreover, advancements in medical technology and enhanced postoperative care have greatly improved the recovery process, thereby reducing the length of postoperative hospital stays.
Our research suggests that preoperative targeted immunotherapy appears to potentially increase the risk of serious postoperative biliary complications, although there is no statistically significant difference in the incidence of biliary leakages between the two groups. Two patients from the neoadjuvant cohort experienced postoperative bile leakages that further led to life-endangering complications, while another developed a postoperative gastro-biliary fistula followed by a biliary stricture that significantly impaired the post-surgery quality of life. Mechanistically, preoperative adjuvant therapy, particularly immunotherapy, has been shown to damage hepatocytes and bile ducts, thereby compromising postoperative recovery [35-37]. Severe biliary complications can critically impact patients' quality of life and potentially be life-threatening. In our study, bile leakages and subsequent complications partially contributed to the nononcologic mortality rate of neoadjuvant cohort, thus influencing the observed discrepancies in postoperative nononcologic mortality rates between the two cohorts. However, the robustness of this conclusion must be approached with caution, considering the limited sample size of our study and the presence of statistically nonsignificant trends.
Further Kaplan-Meier survival analysis revealed no significant difference in RFS and OS between the two groups. However, given the presence of nononcologic mortalities in both cohorts, we performed a competing risk survival analysis and found that preoperative adjuvant therapy significantly reduced the postoperative recurrence rate in HCC patients with macrovascular invasion, but also increased the risk of postoperative nononcologic mortality. The significant decrease in postoperative recurrence rate in neoadjuvant group patients could be attributable to the suppressive effects of the adjuvant therapy on satellite focus and oncologic microvascular thrombus. The increased incidence of nononcologic mortality in the neoadjuvant group may be related to liver function and biliary system damage by preoperative targeted immunotherapy. Two cases in the neoadjuvant group died from complications related to liver failure, while another two died due to further complications induced by postoperative bile leakages. In contrast, only one case in the upfront surgery group experiecnced nononcologic mortality due to postoperative liver failure. Barbier et al. performed preoperative adjuvant sorafenib therapy for advanced HCC and found that preoperative sorafenib treatment also increased the incidence of postoperative nononcologic mortality: four patients in the sorafenib group died due to postoperative liver failure, while only one patient in the control group died due to postoperative liver failure [38]. Many literatures suggest that antiangiogenic drugs, ICIs, RT, and many other interventions can lead to elevated transaminases and bile duct injury [39, 40]. Hepatoprotective drugs offered some protection, leading to normal values from biochemical tests. However, therapeutic damage to hepatic tissue and function may persist over extended periods, as illustrated by the ongoing postoperative complications observed in certain participants within this study.
Based on the aforementioned results and analysis, we believe that preoperative targeted immunotherapy is both safe and effective in clinical research. Compared to upfront surgery, patients receiving neoadjuvant targeted immunotherapy have a statistically significant increase in postoperative nononcologic mortality, but the difference is relatively small and may decrease with an increase in sample size. Numerous studies have shown that targeted immunotherapy is safe and well-tolerated [41, 42], and our two study groups did not have a significant difference in the occurrence of other major postoperative complications, indicating the safety of neoadjuvant targeted immunotherapy. Additionally, even though there is a slight increase in the risk of postoperative nononcologic mortality with neoadjuvant targeted immunotherapy, this risk is accidental and can be improved. With accumulated experience, we can reduce nononcologic mortality through improved targeted immunotherapy regimens, better preoperative evaluation, improved surgical skills, and enhanced postoperative supportive care regimens.
This study has potential limitations. Firstly, the inclusion criteria for the study might introduce bias, as the definition of vascular invasion includes both portal and hepatic veins, yet the subtype and extent of such invasions' influence on patient prognosis remain unexplored due to insufficient data. Secondly, the patient cohorts from four different medical centers in China, exhibited variation in indications for neoadjuvant targeted immunotherapy or upfront surgery, as well as inconsistencies in administered medications, contributing to increased heterogeneity. Thirdly, this is a retrospective study with small sample size, and prospective studies with larger sample size are needed to validate the effects of neoadjuvant targeted immunotherapy on short-term and long-term prognosis of HCC patients with macrovascular invasion. Lastly, considering the most of participant were of same gender (males) and of similar liver background disease (HBV). The results of this trial must have been affected following this deviation, especially that the number of all participants was relatively small.
Conclusion
The finding of this study suggested that preoperative targeted immunotherapy may be a relatively safe and beneficial treatment for certain HCC patients with macrovascular invasion. While facilitating the potential for curative resection, this approach also reduces the incidence of postoperative tumor recurrence. Nonetheless, such preoperative intervention might lead to chronic hepatic and biliary injury, resulting in heightened nononcologic mortality. As a result, the adoption of preoperative targeted immunotherapy followed by surgical resection for HCC with macrovascular invasion necessitates careful consideration regarding patient selection, timing, and regimens. Given that this study is a retrospective study with a small sample size, a prospective study of a larger sample size would provide more confidence in these conclusions.
Acknowledgements
Data analysis was assisted by Merck Sharp & Dohme company.
Funding
This work was supported by National Natural Science Foundation of China [No. 81972698]; CAMS Innovation Fund for Medical Sciences, CIFMS 2021-I2M-1-014; and National High Level Hospital Clinical Research Funding [No. 2022-PUMCH-C-047].
Informed consent and patient details
Informed consent was obtained from all enrolled patients, and approval was obtained from the ethics committees of the four medical centers including Peking Union Medical College Hospital, Beijing 302 Hospital, Jiangxi Cancer Hospital and Hebei Fourth Hospital.
Author contributions
Wu Xiang'an and Wan Yuxin: Data curation, Formal analysis, Writing - original draft, Visualization. Wang Sen and Chen Ye: Data curation, Writing - review & editing. Han Jiashu: Writing - original draft. Chao Wang, Meng Zhang, Hu Xiongwei and Song Biao: Data curation, Validation. Wan Xueshuai, Xu Haifeng, Zhao Haitao, Lu Xin, MaoYilei and Sang Xinting: Resources, Validation. Hong Zhixian and Wei Xiaoyong: Resources, Conceptualization, Supervision. Du Shunda: Funding acquisition, Conceptualization, Visualization, Supervision.
Declaration of generative AI in scientific writing
None used.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
2. Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N. et al. Recent progress in treatment of hepatocellular carcinoma. American journal of cancer research. 2020;10:2993-3036
3. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ. et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver international: official journal of the International Association for the Study of the Liver. 2015;35:2155-66
4. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M. et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720
5. EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. Journal of hepatology. 2018;69:182-236
6. Chan SL, Chong CC, Chan AW, Poon DM, Chok KS. Management of hepatocellular carcinoma with portal vein tumor thrombosis: Review and update at 2016. World J Gastroenterol. 2016;22:7289-300
7. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology. 2020; 28: 112-28.
8. Cheng S, Chen M, Cai J, Sun J, Guo R, Bi X. et al. Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus (2018 Edition). Liver Cancer. 2020;9:28-40
9. Cerrito L, Annicchiarico BE, Iezzi R, Gasbarrini A, Pompili M, Ponziani FR. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: Beyond the known frontiers. World J Gastroenterol. 2019;25:4360-82
10. Pinato DJ, Cortellini A, Sukumaran A, Cole T, Pai M, Habib N. et al. PRIME-HCC: phase Ib study of neoadjuvant ipilimumab and nivolumab prior to liver resection for hepatocellular carcinoma. BMC cancer. 2021;21:301
11. Li N, Feng S, Xue J, Wei XB, Shi J, Guo WX. et al. Hepatocellular carcinoma with main portal vein tumor thrombus: a comparative study comparing hepatectomy with or without neoadjuvant radiotherapy. HPB (Oxford). 2016;18:549-56
12. Kim BK, Kim DY, Byun HK, Choi HJ, Beom SH, Lee HW. et al. Efficacy and Safety of Liver-Directed Concurrent Chemoradiotherapy and Sequential Sorafenib for Advanced Hepatocellular Carcinoma: a Prospective Phase 2 Trial. International journal of radiation oncology biology physics. 2020;107:106-15
13. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X. et al. Neoadjuvant Three-Dimensional Conformal Radiotherapy for Resectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Randomized, Open-Label, Multicenter Controlled Study. Cancer medicine. 2019;37:2141-51
14. Zhao J, Wang J, Lu Y, Wu Y, Kuang D, Wang Y. et al. Neoadjuvant drug-eluting bead transarterial chemoembolization and tislelizumab therapy for resectable or borderline resectable hepatocellular carcinoma: A propensity score matching analysis. Eur J Surg Oncol. 2023;49:107106
15. Zeng ZM, Mo N, Zeng J, Ma FC, Jiang YF, Huang HS. et al. Advances in postoperative adjuvant therapy for primary liver cancer. World journal of gastrointestinal oncology. 2022;14:1604-21
16. Laface C, Fedele P, Maselli FM, Ambrogio F, Foti C, Molinari P. et al. Targeted Therapy for Hepatocellular Carcinoma: Old and New Opportunities. 2022; 14: 4028.
17. Kudo M, Ueshima K, Nakahira S, Nishida N, Ida H, Minami Y. et al. Adjuvant nivolumab for hepatocellular carcinoma (HCC) after surgical resection (SR) or radiofrequency ablation (RFA) (NIVOLVE): A phase 2 prospective multicenter single-arm trial and exploratory biomarker analysis. 2021; 39: 4070-.
18. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M. et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. 2020; 38: 2960-70.
19. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY. et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-905
20. Yang X, Xu H, Zuo B, Yang X, Bian J, Long J. et al. Downstaging and resection of hepatocellular carcinoma in patients with extrahepatic metastases after stereotactic therapy. Hepatobiliary surgery and nutrition. 2021;10:434-42
21. Sangro B, Chan SL, Kelley RK, Lau G, Kudo M, Sukeepaisarnjaroen W. et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol. 2024
22. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J. et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90
23. Huang Y, Zhang Z, Liao W, Hu K, Wang Z. Combination of Sorafenib, Camrelizumab, Transcatheter Arterial Chemoembolization, and Stereotactic Body Radiation Therapy as a Novel Downstaging Strategy in Advanced Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Case Series Study. Frontiers in oncology. 2021;11:650394
24. Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD. et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer. 2021;10:320-9
25. Ma YN, Jiang X, Song P, Tang W. Neoadjuvant therapies in resectable hepatocellular carcinoma: Exploring strategies to improve prognosis. Bioscience trends. 2024
26. Huang C, Zhu XD, Shen YH, Wu D, Ji Y, Ge NL. et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a retrospective analysis. 2021; 9: 19.
27. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-47
28. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-13
29. Pei Y, Li W, Wang Z, Liu J. Successful conversion therapy for unresectable hepatocellular carcinoma is getting closer: A systematic review and meta-analysis. Frontiers in oncology. 2022;12:978823
30. Zhang T, Merle P, Wang H, Zhao H, Kudo M. Combination therapy for advanced hepatocellular carcinoma: do we see the light at the end of the tunnel? Hepatobiliary surgery and nutrition. 2021;10:180-92
31. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M. et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-70
32. Zhang W, Hu B, Han J, Wang H, Wang Z, Ye H. et al. 174P A real-world study of PD-1 inhibitors combined with TKIs for HCC with major vascular invasion as the conversion therapy: A prospective, non-randomized, open-label cohort study. Annals of Oncology. 31: S1307.
33. Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ. et al. Lenvatinib Combined with Anti-PD-1 Antibodies Plus Transcatheter Arterial Chemoembolization for Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. J Hepatocell Carcinoma. 2021;8:1233-40
34. Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP, Pan ZY. et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249:195-202
35. Fan C, Kim A, Li S, Naidoo J, Cappelli LC, Brahmer JR. et al. Outcomes of immunotherapy-related hepatotoxicity from a multi-disciplinary toxicity team. J Cancer Res Clin Oncol. 2022
36. Imoto K, Kohjima M, Hioki T, Kurashige T, Kurokawa M, Tashiro S. et al. Clinical Features of Liver Injury Induced by Immune Checkpoint Inhibitors in Japanese Patients. Can J Gastroenterol Hepatol. 2019;2019:6391712
37. Doherty GJ, Duckworth AM, Davies SE, Mells GF, Brais R, Harden SV. et al. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO open. 2017;2:e000268
38. Barbier L, Fuks D, Pessaux P, Muscari F, Le Treut YP, Faivre S. et al. Safety of liver resection for hepatocellular carcinoma after sorafenib therapy: a multicenter case-matched study. Ann Surg Oncol. 2013;20:3603-9
39. Zhang HC, Wang LS, Miller E. Hepatobiliary and Pancreatic Adverse Events. Advances in experimental medicine and biology. 2021;1342:339-55
40. Zen Y, Yeh MM. Checkpoint inhibitor-induced liver injury: A novel form of liver disease emerging in the era of cancer immunotherapy. Seminars in diagnostic pathology. 2019;36:434-40
41. Zhang W, Zhang K, Liu C, Gao W, Si T, Zou Q. et al. Hepatic arterial infusion chemotherapy combined with anti-PD-1/PD-L1 immunotherapy and molecularly targeted agents for advanced hepatocellular carcinoma: a real world study. Frontiers in immunology. 2023;14:1127349
42. Laface C, Ranieri G, Maselli FM, Ambrogio F, Foti C, Ammendola M. et al. Immunotherapy and the Combination with Targeted Therapies for Advanced Hepatocellular Carcinoma. Cancers (Basel). 2023 15
Author contact
![]() Corresponding authors: Shunda Du, M.D. Department of Liver Surgery, Peking Union Medical College Hospital, PUMC and Chinese Academy of Medical Sciences, Dongcheng, Beijing 100730, China; E-mail: dushdcn; Tel: 86-18612671763. Zhixian Hong, M.D. Department of Hepatobiliary Surgery, the 302nd Hospital of Chinese PLA, Beijing, 100039, China; E-mail: zqyhzx2com; Tel: 86-13521037984. Xiaoyong Wei M.D. Department of Hepatobiliary Surgery, Jiangxi Provincial Cancer Hospital, Nanchang, Jiangxi, 330029, China; E-mail: 41906001com; Tel: 86-13576935622.
Corresponding authors: Shunda Du, M.D. Department of Liver Surgery, Peking Union Medical College Hospital, PUMC and Chinese Academy of Medical Sciences, Dongcheng, Beijing 100730, China; E-mail: dushdcn; Tel: 86-18612671763. Zhixian Hong, M.D. Department of Hepatobiliary Surgery, the 302nd Hospital of Chinese PLA, Beijing, 100039, China; E-mail: zqyhzx2com; Tel: 86-13521037984. Xiaoyong Wei M.D. Department of Hepatobiliary Surgery, Jiangxi Provincial Cancer Hospital, Nanchang, Jiangxi, 330029, China; E-mail: 41906001com; Tel: 86-13576935622.

 Global reach, higher impact
Global reach, higher impact