3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(10):3183-3198. doi:10.7150/jca.93789 This issue Cite
Research Paper
PSAT1 mediated EMT of colorectal cancer cells by regulating Pl3K/AKT signaling pathway
1. School of Pharmacy, Anhui University of Traditional Chinese Medicine, 230012 Hefei, Anhui, China.
2. The Key Laboratory of Hepatobiliary Pancreas, Southern District, Anhui Provincial Hospital, The First Affliated Hosnital of USTC, University of Science and Technology of China, 230022 Hefei, Anhui, China.
3. Department of Pathology, Anhui Provincial Hospital, The First Affliated Hosnital of USTC, University of Science and Technology of China, 230002 Hefei, Anhui, China.
# These authors contributed equally in the study.
Abstract
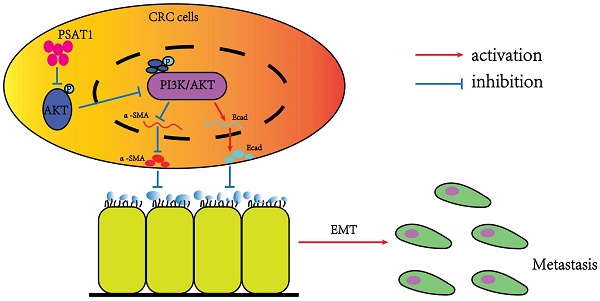
Background: The metastasis of colorectal cancer (CRC) is one of the significant barriers impeding its treated consequence and bring about high mortality, less surgical resection rate and poor prognosis of CRC patients. PSAT1 is an enzyme involved in serine biosynthesis. The studies showed that PSAT1 plays the part of a crucial character in the regulation of tumor metastasis. And Epithelial-Mesenchymal Transition (EMT) is a process of cell reprogramming in which epithelialcells obtain mesenchymal phenotypes. It is a crucial course in promoting cell metastasis and the progression of malignant tumors. The relationship between PSAT1 and EMT in colorectal cancer, as well as the underlying molecular mechanisms, remains enigmatic and warrants thorough exploration. These findings suggest that PSAT1 may serve as a promising therapeutic target for mitigating colorectal cancer metastasis and holds the potential to emerge as a valuable prognostic biomarker in forthcoming research endeavors.
Materials and Methods: Utilizing TCGA dataset in conjunction with clinical CRC specimens, our initial focus was directed towards an in-depth examination of PSAT1 expression within CRC, specifically exploring its potential correlation with the adverse prognostic outcomes experienced by patients. Furthermore, we conducted a comprehensive investigation into the regulatory influence exerted by PSAT1 on CRC through the utilization of siRNA knockdown techniques. In the realm of in vitro experimentation, we meticulously evaluated the impact of PSAT1 on various facets of CRC progression, including cell migration, invasion, proliferation, and colony formation. In order to elucidate the intricate effects in question, we adopted a multifaceted methodology that encompassed a range of assays and analyses. These included wound healing assays, transwell assays, utilization of the Cell Counting Kit-8 (CCK-8) assay, and colony formation assays. By employing this diverse array of investigative techniques, we were able to achieve a comprehensive comprehension of the multifaceted role that PSAT1 plays in the pathogenesis of colorectal cancer. This multifarious analysis greatly contributed to our in-depth understanding of the complex mechanisms at play in colorectal cancer pathogenesis. Using WB and PCR experiments, we found that PSAT1 has a role in regulating EMT development in CRC.In terms of mechanism, we found that PSAT1 affected EMT by Regulating Pl3K/AKT Signaling Pathway.
Results: Our investigation revealed a noteworthy down-regulation of PSAT1 expression in CRC specimens. Importantly, this down-regulation exhibited a significant positive correlation with the unfavorable prognosis of patients afflicted with CRC. Functionally, our study showcased that the siRNA-mediated knockdown of PSAT1 markedly enhanced various key aspects of CRC pathogenesis in an in vitro setting. Specifically, this included a substantial promotion of CRC cell migration, invasion, proliferation, and colony formation. Moreover, the silencing of PSAT1 also demonstrated a substantial promotion of the EMT process. Intriguingly, our research unveiled a hitherto unexplored mechanism underlying the regulatory role of PSAT1 in CRC and EMT. We have established, for the first time, that PSAT1 exerts its influence by modulating the activation of the PI3K/AKT Signaling Pathway. This mechanistic insight provides a valuable contribution to the understanding of the molecular underpinnings of CRC progression and EMT induction mediated by PSAT1.
Conclusions: In unison, our research findings shed light on the previously uncharted and significant role of the PSAT1/PI3K/AKT axis in the initiation of the EMT process in CRC. Furthermore, our discoveries introduce a novel biomarker with potential implications for the clinical diagnosis and treatment of CRC.
Keywords: PSAT1, Colorectal cancer, EMT, PI3K/AKT pathway, LY294002

 Global reach, higher impact
Global reach, higher impact