3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(12):2247-2255. doi:10.7150/jca.19461 This issue Cite
Research Paper
Develop and validation a nomogram to predict the recurrent probability in patients with major salivary gland cancer
1. Department of Hematology-Oncology, Chang Gung Memorial Hospital, Chiayi, Taiwan;
2. Department of Hematology-Oncology, Chang Gung Memorial Hospital, Kaohsiung, Taiwan;
3. Department of Hematology-Oncology, Chang Gung Memorial Hospital, Keelung, Taiwan;
4. Department of Hematology-Oncology, Chang Gung Memorial Hospital, Linkou, Taiwan;
5. Department of Surgery, Chang Gung Memorial Hospital, Linkou, Taiwan;
6. Graduate Institute of Clinical Medical Sciences, Chang Gung University College of Medicine, Taiwan.
Received 2017-2-2; Accepted 2017-5-18; Published 2017-7-20
Abstract
Objectives: Prediction of recurrent risk in patients with major salivary gland carcinoma (MSGC) after surgical treatment is an important but difficult task because of a broad spectrum of tumor histological subtypes and diverse clinical behaviors. This study aimed to develop and validate a nomogram to predict the recurrent probability in patients with MSGC.
Methods: A total of 231 consecutive patients with MSGC received curative-intend surgery between 2002 and 2014 from one medical center were selected as the training set. Clinicopathologic variables with the most significant values in the multivariate Cox regression were selected to build into a nomogram to estimate the recurrence probability. An independent validation set of 139 patients treated at the same period from 3 other hospitals were selected for external validation and calibration.
Results: The nomogram was developed on six significant predictive factors, including the smoking history, tumor grade, perineural invasion, lymphatic invasion, pathologic T- and N-classification, of tumor recurrence retained in the multivariate Cox model. The nomogram had a highly predictive performance, with a bootstrapped corrected concordance index of 0.82 for the training set and 0.78 for the validation set. The nomogram showed good calibration in predict 2-year and 5-year recurrence probability both in the training and validation set.
Conclusions: We developed and externally validated an accurate nomogram for prediction the tumor recurrence probability of patients with MSGC after surgical treatment. This nomogram may be used to assist clinician and patient in elaborating the recurrent risk and making decision for appropriate adjuvant treatment.
Keywords: major salivary gland cancer, recurrence, nomogram, validation, calibration
Introduction
Major salivary glands carcinoma (MSGC) is an uncommon malignancy accounting for less than 5% of all head and neck cancers [1]. The incidence rate of MSGC was 12/1,000,000 person-years and that was consistent in the United States during 1992 to 2006 [2]. MSGC is composed of a heterogeneous histological subtype with a wide variation of clinical course [3]. The standard treatment for localized MSGC is surgical resection; nevertheless, recurrence is observed in 23-43% of patients within 5 years after surgical treatment [4-11]. Because a wide variation of tumor behavior, the prediction of recurrent risk in patients with MSGC after surgical treatment is a difficult task for clinicians.
A number of parameters with predictive value have been identified in patients with MSGC, including age [4, 5,6,7], tumor size [4, 5, 6], histological subtype [12], tumor grade of histology [4, 6, 7, 12], resection margin [8, 9], local lymph nodes metastases [4, 6, 7], vascular invasion [7], lymphatic invasion [13] and perineural invasion [6,7]. However, the information regarding the effect of clinical variables on tumor relapse was inconsistent and varied widely because the rarity of disease, small numbers of patients and heterogeneous treatment modalities. Several scoring systems have been developed to predict the recurrent probability in patients with MSGC [5-7]. These scoring systems were limited because some were designed to use in part of major salivary glands [5, 6], had not yet been validated [7], or were not reproducible in different patient cohorts [11]. In addition, all these developed predicting models were developed based on population from the Western countries [5-7]. The clinical characteristics of MSGC in eastern countries differ from those in western countries, which included ethnical diversity [2], environmental exposures [14-16], surgical modalities and adjuvant treatment after operation. Such differences might cause inaccurate prediction of clinical outcome in eastern population using the same model. For instance, the incidence rate of mucoepidermoid and adenoid cystic carcinoma were similar among Whites and Asians whereas most other histologic subtypes had higher incidences rate among Whites based on a population study in the United States between 1992 and 2006 [2]. Furthermore, the difference of demographic and socioeconomic factors may also influence survival outcome of patients with MSGC [17].
A reliable prognostic tool for predicting recurrent probability for MSGC would be useful for identifying high risk patients, planning appropriate adjuvant therapeutic strategies, and designing optimal timing of follow up. This study performed a multicenter study to develop and externally validate a nomogram that predicts tumor recurrent probability in Asian patients with MSGC after surgical resection.
Patients and methods
Patient selection
In total, 370 patients who underwent curative-intent surgery of localized MSGC between 2002 and 2014 at the four affiliated hospitals of Chang Gung Memorial Hospital (CGMH) in Taiwan were identified from the institutional cancer registry database. The diagnosis of MSGC was confirmed and reviewed by expert pathologists according to the 2005 World Health Organization (WHO) classification [3]. Patients who had partial incision of tumor, recurrent tumors, prior radiotherapy or chemotherapy, and metastatic tumors, were excluded. The adjuvant treatment strategies, including radiotherapy and/or chemotherapy, were determined in a multidisciplinary cancer team conference based on poor prognostic factors (positive resection margin, perineural invasion, facial nerve invasion, or lymph node metastases). First, we evaluated 231 consecutive patients in one medical center (CGMH Linkou branch) as training set. The data obtained were analyzed to create the predictive nomogram for recurrent probability. We then evaluated an independent cohort (validation set) including 139 consecutively patients in three other hospitals (CGMH Keelung branch, CGMH Chiayi branch, and CGMH Kaohsiung branch) to validate the predictive model.
Data collection and follow-up
Demographic and clinicopathological variables were collected retrospectively. A total of 21 clinical variables for each patient were obtained from the clinical record. The overall survival (OS), cancer-specific survival (CSS), and recurrence-free survival (RFS) were calculated from the date of surgery to the date of any cause of death or the date last known to be alive, to the date of death due to cancer, and to the date of recurrence based on clinical and/or radiologic examination, respectively. The dates of the primary cancer diagnosis, primary surgery, recurrence pattern, and death of each patient were obtained from the institutional cancer center registry or the National Register of Death Database in Taiwan. Patients were followed until death or the end of the study (June 30, 2015). The Institutional Review Board for all branches of the CGMH approved this study (104-6042B), in compliance with the Helsinki Declaration (1996).
Statistical Analysis
Basic demographic data were summarized as n (%) for categorical variables, and mean with range, standard error (SE), or 95% confidence interval (CI) for continuous variables. Differences between groups were evaluated with independent t tests or Wilcoxon rank sum test for continuous variables, and x2 tests for categorical variables.
Survival outcome was calculated according to the Kaplan-Meier method. Log-rank tests were used to determine significant differences between the survival curves. Cox regression was used for univariate analyses on continuous and categorical prognostic factors, and multivariate Cox regression was used to estimate the predictive factors and their weights. Akaike information criterion (AIC) was used to develop different multivariate models by systematically removing predictors that were less statistically significant (P > 0.10) starting from a full model containing all common predictive factors which were statistically significant in the univariate analysis.
The predictive performance of models was evaluated by considering measures of discrimination and calibration. The bootstrapping method (1000 repetitions) was used to obtain a relatively unbiased estimate of the models' performance. The concordance index (c-index) and calibration plot was used to determine and validate the performance of the model to distinguish between high-risk and low-risk patients.
All statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) and R version 2.9.1 (The R Foundation for Statistical Computing, Vanderbilt University, Nashville, TN) using the Hmisc and Design libraries. All statistical assessments with p<0.05 were considered significant.
Results
Basic demographic data and survival outcome of training set and validation set
The distribution of demographic and clinical variables in the training set and validation set are compared in Table 1. All variables were similar distributed between the two cohorts, except age, numbers of patients with positive surgical margin, positive lymphatic invasion, and administration of adjuvant chemotherapy. Around 70% of the MSGC originated from parotid gland, and the most common histological subtype was adenoid cystic carcinoma, followed by mucoepidermoid carcinoma, and acinic cell carcinoma in both cohorts.
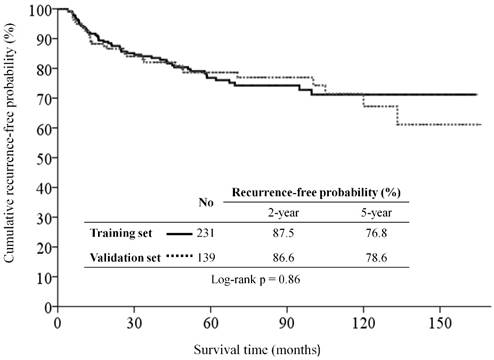
With a median follow-up period of 58.1 months (range, 1.4-169.5), the estimated 5-year OS, 5-year CSS, and 5-year RFS rates were 84.9%, 87.9%, and 76.8% in the training set, and were 80.4%, 85.9%, and 78.6% in the validation set, respectively. There were 51, including 14 (6.1%) local, 7 (3.0%) regional, and 30 (13.0%) distant metastases, of 231 patients in the training set, and 31, including 3 (2.2%) local, 5 (3.6%) regional, and 23 (16.5%) distant metastases, of 139 patients in the validation set had tumor recurrence at the end of study. The RFS curve was similar between the two groups (Figure 1).
Patient characteristics of the training set and validation set
| Variable | Category | Training set (n=231) | Validation set (n=139) | p value |
|---|---|---|---|---|
| Age | median (range) | 49 (7-91) | 52 (11-90) | 0.020 |
| Sex | male | 133 (57.6) | 66 (47.5) | 0.067 |
| Smoking history | yes | 79 (34.2) | 44 (31.7) | 0.35 |
| Drinking history | yes | 68 (29.4) | 35 (25.2) | 0.22 |
| Previous cancer history | yes | 6 (2.6) | 9 (6.5) | 0.24 |
| Comorbidity | yes | 66 (28.6) | 34 (24.5) | 0.40 |
| Tumor site | parotid | 161 (69.7) | 100 (71.9) | 0.21 |
| submandibular | 56 (24.2) | 36 (25.9) | ||
| sublingual | 14 (6.1) | 3 (2.2) | ||
| Histological type | adenoid cystic | 60 (26.0) | 35 (25.2) | 0.60 |
| mucoepidemoid | 51 (22.1) | 28 (20.1) | ||
| acinic cell | 45 (19.5) | 21 (15.1) | ||
| adenocarcinoma, nos | 21 (9.1) | 16 (11.5) | ||
| lymphoepidermoid | 20 (8.7) | 11 (7.9) | ||
| squamous cell | 10 (4.3) | 5 (3.6) | ||
| carcinoma ex | 15 (6.5) | 11 (7.9) | ||
| others | 9 (3.9) | 12 (8.6) | ||
| Tumor grade | I (well) | 85 (36.8) | 50 (36.0) | 0.68 |
| II (moderate) | 43 (18.6) | 31 (22.3) | ||
| III (poor) | 103 (44.6) | 58 (41.7) | ||
| pT-classification | T1 | 64 (27.7) | 41 (29.5) | 0.75 |
| T2 | 104 (45.0) | 57 (41.0) | ||
| T3 | 46 (19.9) | 31 (22.3) | ||
| T4a | 17 (7.3) | 10 (7.2) | ||
| pN-classification | No local lymph node | 188 (81.4) | 111 (79.9) | 0.62 |
| Local lymph nodes | 43 (18.6) | 28 (20.1) | ||
| 7th edition AJCC stage | 1 | 66 (26.8) | 40 (28.8) | 0.69 |
| 2 | 80 (38.1) | 47 (33.8) | ||
| 3 | 42 (18.2) | 31 (22.3) | ||
| 4a | 39 (16.9) | 21 (15.1) | ||
| Perineural invasion | yes | 69 (29.9) | 29 (20.9) | 0.07 |
| Vascular invasion | yes | 12 (5.2) | 13 (9.4) | 0.14 |
| Lymphatic invasion | yes | 12 (5.2) | 18 (12.9) | 0.01 |
| Surgical margin | involved | 153 (66.2) | 48 (34.5) | <0.001 |
| Postoperative radiotherapy | yes | 162 (70.1) | 91 (65.5) | 0.36 |
| Postoperative chemotherapy | yes | 7 (33.3) | 22 (15.8) | <0.001 |
Univariate and multivariate predictive factors of RFS in training set
In the univariate analysis from the training set, we identified 16 factors that were associated with recurrent probability (Supplementary table). Multivariate analysis identified pathologic T- and N-classification of the 7th edition American Joint Committee on Cancer (AJCC) staging system [18], perinueral invasion, tumor grade, and lymphatic invasion were the only independent predictive factors for tumor recurrence (Table 2). Moreover, patients who were ever or active smoking had a trend toward increasing risk of tumor recurrence than patients who were never smoking (p=0.10).
Kaplan-Meier recurrence-free survival curves for patients with major salivary gland carcinoma of the training set and validation set.
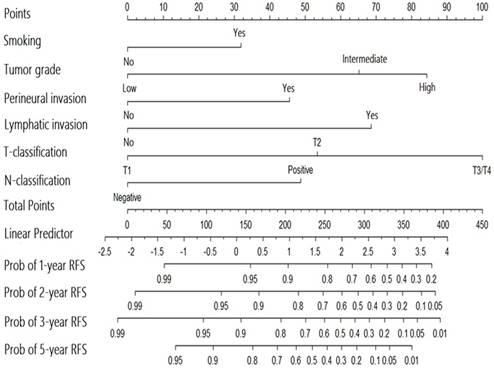
Nomogram for the prediction of recurrence in patients with major salivary gland cancer from the training set.
Variables statistically significantly associated with recurrence-free survival in the multivariate Cox regression analysis
| Variable | β (SE) | P value | HR (95% CI) |
|---|---|---|---|
| Smoking history | 0.478 (0.304) | 0.10 | 1.612 (0.889 to 2.924) |
| Tumor grade II | 0.977 (0.537) | 0.069 | 2.656 (0.927 to 7.613) |
| Tumor grade III | 1.263 (0.469) | 0.007 | 3.534 (1.411 to 8.856) |
| Perineural invasion | 0.684 (0.325) | 0.036 | 1.981 (1.047 to 3.748) |
| Lymphatic invasion | 1.027 (0.458) | 0.025 | 2.791 (1.139 to 6.843) |
| pT2 classification | 0.800 (0.558) | 0.152 | 2.225 (0.745 to 0.644) |
| pT3/T4 classification | 1.498 (0.570) | 0.009 | 4.471 (1.464 to 13.65) |
| Lymph node metastases | 0.730 (0.324) | 0.024 | 2.075 (1.100 to 3.915) |
Performance of the nomogram in training set
A nomogram for predicting the recurrence-free probability at 2- and 5-year was constructed with the six variables that had most significant values in multivariate analysis (Figure 2). The performance of the nomogram in training set was evaluated by the c-index and the calibration plot. The c-index of this model was 0.822 (95% CI, 0.771 to 0.873). In calibration plot, the performance of the ideal nomogram was plotted by the dotted line, in which the solid line showed that the actual relapse probability corresponded closely to present nomogram predicted recurrence-free probability at 2-years (Figure 3a) and 5-years (Figure 3b) after operation.
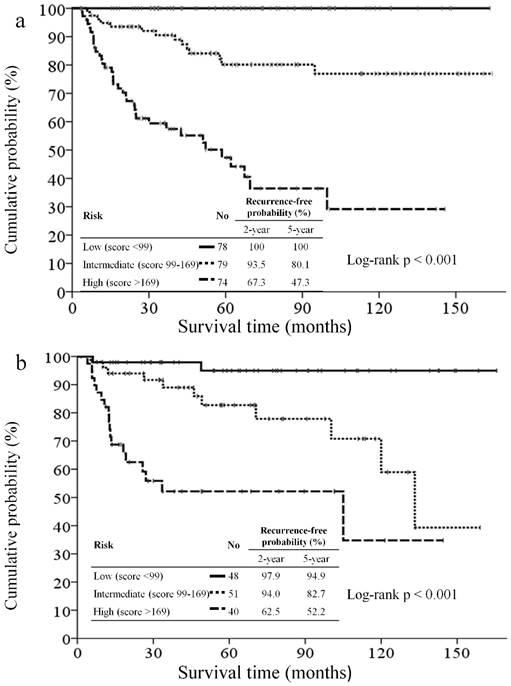
All patients were categorized tertiles according to the score generated by the nomogram. The RFS curve in patients from different groups is shown in Figure 4a. The 5-year RFS rate of patients in tertile 1 was 100% vs. in tertile 2, 80.1%, and in tertile 3, 47.3%. A significant statistical difference was identified within these three groups (Log-rank p <0.001).
Compare the performance of the AJCC staging system and nomogram in the training set
We compared the performance of the nomogram with that of the AJCC staging system for predicting recurrence-free probabilities (Table 3). The homogeneity likelihood ratio was 54.4 and 71.8 of the AJCC system and nomogram (p=0.004), respectively, indicated that present nomogram had a smaller difference within the model, and a better homogeneity than the AJCC system. The linear trend chi-square test was 80.5, and 96.0 of the AJCC system and nomogram, respectively. The c-index was 0.76 (95% CI, 0.70 to 0.83) and 0.82 (95% CI, 0.77 to 0.87) of the AJCC system and nomogram (p=0.023), respectively. Both tests indicated that present nomogram had better discrimination ability than the AJCC system. The AIC was 471.9 of the AJCC system and 464.5 of the nomogram. The smaller AIC value indicated better predictive stratification ability by using the nomogram than the AJCC system.
Assessing the prognostic performance of the AJCC stage and nomogram in training set and validation set
| Cohort | Model | Homogeneity | Monotonicity and discriminatory ability | Akaike information criterion (AIC)**** | |
|---|---|---|---|---|---|
| Likelihood ratio (LR) test* | Linear trend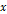 2 test** 2 test** | c-index*** (95% CI) | |||
| Training set | AJCC 7th stage | 54.4 | 80.5 | 0.76 (0.70 to 0.83) | 471.9 |
| Present nomogram | 71.8## | 96.0 | 0.82# (0.77 to 0.87) | 464.5 | |
| Validation set | AJCC 7th stage | 12.9 | 14.0 | 0.71 (0.62 to 0.80) | 269.6 |
| Present nomogram | 32.2# | 32.4 | 0.778# (0.70 to 0.86) | 260.2 | |
*Higher homogeneity likelihood ratio indicates a smaller difference within the staging system, it means better homogeneity
**Higher discriminatory ability linear trend indicates a higher linear trend between staging system, it means better discriminatory ability and gradient monotonicity
***A higher c-index means better discriminatory ability.
****Smaller AIC values indicate better optimistic prognostic stratification
# if p<0.05, ## if p<0.01
External validation of the nomogram in validation set
The nomogram was externally validated based on 139 patients from the validation set. The nomogram assigned a score to each patient in the validation set, and the c-index for the model was 0.78 (95% CI, 0.70 to 0.86). The calibration plot suggested that the actual relapse probability corresponded closely to nomogram-predicted relapse probability at 2-year (Figure 3c) and 5-year (Figure 3d) after operation. The RFS curve had significant in-group differences among patients in the validation cohort which categorized by the same score as in training set (Figure 4b). Again, a significant statistical difference was identified within these three groups (Log-rank p<0.001). The performance of the nomogram is better than that of the AJCC staging system in terms of the homogeneity likelihood ratio (32.2 vs. 12.9), the linear trend chi-square test (32.4 vs. 14.0), the c-index (0.78[95% CI, 0.70 to 0.86], vs. 0.71 [95% CI, 0.62 to 0.80]), and the AIC value (260.2 vs. 269.6) (Table 3).
Calibration plot of the nomogram predicted recurrence-free survival at 2-year, 5-year of the training set (3a and 3b, respectively), and 2-year, 5-year of the validation set (3c and 3d, respectively). The dotted line represents the ideal line where the actual probability of recurrence matches the predicted probability. The solid line represents the observed where the actual probability is slightly different from the predicted probability.
Kaplan-Meier recurrence-free survival curves for patients categorized tertiles according to the nomogram of the training set (4a) and validation set (4b).
Discussion
MSGC is an uncommon malignancy in Taiwan, as well as worldwide. Though some models had been established for prediction of its recurrence, the clinical use was limited for difference in tumor types, lack of validation in Asian population, no reproducible results and need of detailed pathological work [5-7]. The AJCC staging system was proposed for overall survival prediction [18]. The T and N-classification of AJCC system were not only important predictive factors for recurrence across all predictive models in previous studies [5-7] but we also found the AJCC staging system could be used for recurrence prediction. However, other important predictive clinical variables, including tumor grade [4,6,7,12], perineural invasion [6,7], and lymphatic invasion [13], were not taken into account in the AJCC staging system. We developed a nomogram using clinical variables in conjunction with the anatomic extent to predict the tumor recurrent probability of patients with MSGC based on 231 patients from one medical institute. The nomogram accurately predicted tumor recurrence-free probability, with a bootstrapped corrected c-index of 0.822, and 0.778 with an external independent validation cohort of 139 patients from three different institutes. Our study showed that this nomogram might be informative for the clinicians and patients with MSGC to estimate the recurrent risk after the surgical treatment.
The Memorial Sloan Kettering Cancer Center (MSKCC) recently proposed a prognostic nomogram, which constructed using the five most predictive cliniopathologic variables, including age, tumor grade vascular invasion, lymphatic invasion, and lymph node metastases, that predicted recurrent risk of major salivary gland carcinomas based on their cohort of 301 patients [7]. We previously externally validated the clinical utility of the MSKCC nomogram for predicting recurrent risk and our result showed a similar performance of the predictive accuracy between the MSKCC nomogram and the AJCC staging system [19]. Most importantly, the utility of MSKCC nomogram was limited especially as it tended to overestimate recurrent risk in high-risk group [19].
Tumor recurrence impacts a patient's quality of life, psychological burden and survival outcome. A more accurate predictive model of relapse provided clear information in counseling on adjuvant therapy. In the current study, the performance of the nomogram to predict tumor relapse probability is better than the AJCC staging system in terms of better homogeneity, and higher ability of discrimination and risk stratification of the model both in the training set and validation set. The superior performance of the present nomogram was because it integrated clinicopathologic variables, which were important for predicting the relapse risk, that are not accessible by the AJCC staging system. MSGC included a broad spectrum of tumor subtypes, which might present heterogeneous clinical course from an indolent behavior to a rapid lethal condition [3]. Therefore, overlook the impact of the clinicopathologic characteristics of the MSGC might limit the predictive value of clinical utility. Our analysis showed that incorporated clinicopathologic variables on the T- and N-classification increased the accuracy of the present nomogram than the AJCC staging system in Asian populations of patient with MSGC.
In addition to anatomic extent, our study identified that tumor grade, perineural invasion, lymphatic invasion, and smoking history were all important predictive factors of tumor relapse in Asian patients with MSGC. Tumor grade, perineural invasion, and lymphatic invasion are the most frequent reported prognostic factors of patients with MSGC [4,6,7,12,13]. Our analysis showed that addition of smoking history into our nomogram increased the risk stratification power of the nomogram (AIC value decreased from 464.9 to 464.5 and 260.6 to 260.2 after added smoking variable in the training cohort and validation cohort, respectively). Smoking was associated with increasing risk of developed benign salivary gland tumor [20], however, the relation of smoking and malignant salivary gland cancer still under debated [21], and seen probably influenced male only [16, 21]. Smoking induced oncogenic TP-53 mutation [22], which significantly associated higher relapse rate [24] and reduced survival [23], in head and neck squamous cell carcinoma. TP53 gene alternation also associated with a poor outcome in salivary gland cancer [25]. However, the mutation rate of TP53 in MSGC was lower than other type of cancer [26], indicated that the TP53 might not play a critical role of the tumorgienesis in MSGC. Some studies reported that smoking history is a poor prognostic factor of patients with MSGC in univariate analysis, however, the prognostic value of smoking was less diminished after adjusted for other clinicopathological characteristics [7]. Though the effect of smoking on survival outcome in patients with MSGC is inconclusive, our results suggest that smoking was a negative predictor and should be taken into account while predicting relapse risk in Asian patients with MSGC.
In Table 4, we summarized and compared known nomograms in prediction outcome for patients with MSGC from retrospective studies [7, 27, 28]. All the nomograms were developed based on combination of patient's clinical factor (age, sex, and smoking history) and pathologic characteristics of tumor (T-N-M classification, tumor grade, lymphatic invasion, perineural invasion, tumor dimension, and tumor site) with a similar performance in regard of concordance index. Ali S. et al developed two nomograms in prediction tumor recurrence and overall survival, respectively, based on 301 patient cohorts from the MSKCC in the United States [7, 27], however, the models were lack of external validation. Li Y. et al developed the nomogram in prediction of overall and cancer-specific survival based on 4218 patients from SEER database [28], the model was externally validated from an independent patient cohort in China. As the retrospective analysis of SEER database, the author incorporated treatment modalities including surgery and radiotherapy into the model, the model might have selection biased because some patients were medical unfit for those treatment. In addition, the use of antitumor therapy might be confounded by the clinician's preference, therefore, this model could not be routine use at the time of MSGC diagnosis. Most importantly, all three nomograms were developed based on patients in the United States, while ours was developed based on Asian population in Taiwan. We believed our nomogram might provide valuable clinical information to predict tumor recurrent probability in Asian patients with MSGC.
Age negatively impacts survival of patients with MSGC in several studies [27, 28]. Contrary, Ali S. et al reported age as a good prognostic factor [7]. Mixed results regarding the impact of age on survival were found in the literature. Our study showed no statistical significance between age and tumor recurrence. A well- designed, multicenter, prospective study may help to elucidate the relationship of age and tumor recurrence in patients with MSGC after surgical treatment.
Comparison of different nomogram of patients with major salivary gland cancer after cancer surgery
| Author, published year | Patient no. of the nomogram | Study site (Country) and period | Validation | Study outcome | Predictive factor within nomogram | Concordance index |
|---|---|---|---|---|---|---|
| Ali S7, 2013 | 301 | MSKCC (US), 1985-2009 | Interval validation | Tumor recurrence | age, tumor grade, vascular and perineural invasion, and nodal metastasis | 0.85 |
| Ali S27, 2014 | 301 | MSKCC (US), 1985-2009 | Internal validation | OS and CSS | age, clinical T4 stage, tumor grade, perineural invasion, and tumor dimension | 0.81 for OS, 0.86 for CSS |
| Li Y28, 2017 | 4,218 | SEER database (US), 2004-2013 | External validation | OS and CSS | age, sex, tumor site, tumor grade, surgery performed, radiation therapy and T-N-M classifications | 0.83 for OS, 0.81 for CSS |
| This study, 2017 | 231 | CGMH (Taiwan), 2002-2014 | External validation | Tumor recurrence | smoking history, tumor grade, perineural invasion, lymphatic invasion, pathologic T- and N-classification | 0.82 |
MSKCC, Memorial Sloan Kettering Cancer Center; US, United States; SEER, Surveillance, Epidemiology, and End Results program; OS, overall survival; CSS, cancer-specific survival; CGMH, Chang Gung Memorial Hospital.
The 5-year recurrent rate in the validation and training set of our study was 22% and 23%, which was better than western patients with MSGC by a range from 23-43% [4-11]. The difference might be possible contributed by, first, the advance of modern surgery and postoperative radiotherapy because all of our patients were treated between 2002 and 2014, whereas the majority of patients from the reports were treated before 2000. Second, a significant portion of our patients had been treated by postoperative chemotherapy, which was rarely provided for patients in western countries [5-7]. Because distant metastases were the most common site of recurrent tumor [8, 29], treatment with postoperative chemotherapy is a reasonable intent to reduce the recurrent probability for those with high risk group.
To our knowledge, this is the first study constructed a nomogram to predict recurrent risk in Asian patients with MSGC. Nomogram is designed based on statistical methodology to determine the likelihood of a certain event [30, 31]. Recent studies suggested that the use of the nomogram improved the accuracy of predicting value in oncologic practice. In current study, the risk of relapse event could be calculated according to the nomogram scoring based on clinicopathologic variables of each patient, therefore, the present nomogram could assign numerous predictions for the chances of relapse probability at 1-, 2-, 3- and 5-year. However, there are several limitations of this study. First, a selection bias might exist as ours was a retrospective study in nature. Second, we did not evaluate the influence of the adjuvant treatment as a predictive factor. The effectiveness of the adjuvant treatment may potentially affect survival, as such there was selection bias regarding which patients were offered the adjuvant treatment. Third, the present nomogram included smoking history of the patient, however, we were unable to evaluate the influence of daily smoking amount and exposure duration relevant to the relapse risk of MSGC. Four, the smoking history did not have significant statistic difference for recurrence probability in the multivariate analysis, the impact of smoking history on recurrent risk in MSGC need further correlation. Finally, our analysis included only patients from Taiwan with a relatively similar life style, in addition, the healthcare system and clinical practice in Taiwan may differ from other countries. A prospective, multi-country study would be helpful to validate the present nomogram before it can be used in clinics worldwide.
In conclusion, we had developed and externally validated an accurate nomogram for prediction the tumor recurrent probability of patients with MSGC after surgical treatment. This nomogram may be used to assist clinician and patient in elaborating the recurrent risk and in making decision for appropriate adjuvant treatment.
Acknowledgements
The authors would like to thank all members in the Chang Gung Memorial Hospital Cancer Center for their help in data collection. We would also like to extend our gratitude to Ms. Shu-Fang Huang and Ms. Vengi Ho from the Center for Clinical Research in Chang Gung Memorial Hospital for her invaluable contribution in biostatistics and figure illustration.
Competing Interests
This research was not funded by any public, commercial, or nonprofit agency. No competing financial interests exist.
References
1. Horner MJ, Ries LAG, Krapcho M. et al. editors. SEER Cancer Statistics Review, 1975-2006. Bethesda, MD: National Cancer Institute. 2009
2. Boukheris H, Curtis RE, Land CE, Dores GM. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992-2006: a population-based study in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2899-906
3. Barnes LB, Eveson JW, Reichart P, Sidransky D. Pathology and genetics of head and neck tumors. Lyon: IARC Press. 2005
4. Bell RB, Dierks EJ, Homer L, Potter BE. Management and outcome of patients with malignant salivary gland tumors. J Oral Maxillofac Surg. 2005;63:917-28
5. Vander Poorten VL, Balm AJ, Hilgers FJ. et al. The development of a prognostic score for patients with parotid carcinoma. Cancer. 1999;85:2057-67
6. Carrillo JF, Vazquez R, Ramirez-Ortega MC, Cano A, Ochoa-Carrillo FJ, Onate-Ocana LF. Multivariate prediction of the probability of recurrence in patients with carcinoma of the parotid gland. Cancer. 2007;109:2043-51
7. Ali S, Palmer FL, Yu C, DiLorenzo M. et al. A predictive nomogram for recurrence of carcinoma of the major salivary glands. JAMA Otolaryngol Head Neck Surg. 2013;139:698-705
8. Teo PM, Chan AT, Lee WY, Leung SF, Chan ES, Mok CO. Failure patterns and factors affecting prognosis of salivary gland carcinoma: retrospective study. Hong Kong Med J. 2000;6(1):29-36
9. Pohar S, Gay H, Rosenbaum P. et al. Malignant parotid tumors: presentation, clinical/pathologic prognostic factors, and treatment outcomes. Int J Radiat Oncol Biol Phys. 2005;61:112-28
10. Vander Poorten VL, Hart AA, van der Laan BF. et al. Prognostic index for patients with parotid carcinoma: external validation using the nationwide 1985-1994 Dutch Head and Neck Oncology Cooperative Group database. Cancer. 2003;97(6):1453-63
11. Takahama A Jr, Sanabria A, Benevides GM, de Almeida OP, Kowalski LP. Comparison of two prognostic scores for patients with parotid carcinoma. Head Neck. 2009;31(9):1188-95
12. Guntinas-Lichius O, Wendt TG, Buentzel J. et al. Incidence, treatment, and outcome of parotid carcinoma, 1996-2011: a population-based study in Thuringia, Germany. J Cancer Res Clin Oncol. 2015;141(9):1679-88
13. Wang YL, Li DS, Gan HL. et al. Predictive index for lymph node management of major salivary gland cancer. Laryngoscope. 2012;122(7):1497-506
14. Spitz MR, Fueger JJ, Goepfert H, Newell GR. Salivary gland cancer. A case-control investigation of risk factors. Arch Otolaryngol Head Neck Surg. 1990;116(10):1163-6
15. Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8(4):414-9
16. Swanson GM, Burns PB. Cancers of the salivary gland: workplace risks among women and men. Ann Epidemiol. 1997;7(6):369-74
17. Olarte LS, Megwalu UC. The Impact of demographic and socioeconomic factors on major salivary gland cancer survival. Otolaryngol Head Neck Surg. 2014;150(6):991-8
18. UICC TNM. Classification of Malignant Tumours; 7th edition. New York: Wiley & Liss. 2009
19. Chou WC, Chang KP, Lu CH. et al. Complementary role of the MSKCC nomogram to the AJCC system for the prediction of relapse of major salivary gland carcinoma after surgery. Head Neck. 2017;39(5):860-7
20. Freedman LS, Oberman B, Sadetzki S. Using time-dependent covariate analysis to elucidate the relation of smoking history to Warthin's tumor risk. Am J Epidemiol. 2009;170(9):1178-85
21. Sadetzki S, Oberman B, Mandelzweig L. et al. Smoking and risk of parotid gland tumors: a nationwide case-control study. Cancer. 2008;112(9):1974-82
22. Soussi T, Kato S, Levy PP, Ishioka C. Reassessment of the TP53 mutation database in human disease by data mining with a library of TP53 missense mutations. Hum Mutat. 2005;25:6-17
23. Mafune A, Hama T, Suda T. et al. Homozygous deletions of UGT2B17 modifies effects of smoking on TP53-mutations and relapse of head and neck carcinoma. BMC Cancer. 2015;15:205
24. Poeta ML, Manola J, Goldwasser MA. et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357(25):2552-61
25. Kiyoshima T, Shima K, Kobayashi I. et al. Expression of p53 tumor suppressor gene in adenoid cystic and mucoepidermoid carcinomas of the salivary glands. Oral Oncol. 2001;37(3):315-22
26. Soini Y, Kamel D, Nuorva K, Lane DP, Vähäkangas K, Pääkkö P. Low p53 protein expression in salivary gland tumours compared with lung carcinomas. Virchows Arch A Pathol Anat Histopathol. 1992;421(5):415-20
27. Ali S, Palmer FL, Yu C. et al. Postoperative nomograms predictive of survival after surgical management of malignant tumors of the major salivary glands. Ann Surg Oncol. 2014;21(2):637-42
28. Li Y, Ju J, Liu X. et al. Nomograms for predicting long-term overall survival and cancer-specific survival in patients with major salivary gland cancer: a population-based study. Oncotarget. 2017 Jan 30. doi: 10.18632/oncotarget.14905
29. Roh JL, Choi SH, Lee SW, Cho KJ, Nam SY, Kim SY. Carcinomas arising in the submandibular gland: high propensity for systemic failure. J Surg Oncol. 2008;97(6):533-7
30. Tang LQ, Li CF, Li J. et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. 2015:108 (1)
31. Dingemans SA, de Rooij PD, van der Vuurst de Vries RM. et al. Validation of six nomograms for predicting non-sentinel lymph node metastases in a Dutch breast cancer population. Ann Surg Oncol. 2016;23(2):477-81
Author contact
![]() Corresponding author: Wen-Chi Chou, M.D., Department of Hematology-Oncology, Chang Gung Memorial Hospital, Linkou, 5 Fu-Hsing Street, Kwei-Shan Shiang, Taoyuan 333, Taiwan. Tel: 886-3281200 Ext: 2517; Fax: 886-3-3285818; E-mail: wenchi3992com.tw
Corresponding author: Wen-Chi Chou, M.D., Department of Hematology-Oncology, Chang Gung Memorial Hospital, Linkou, 5 Fu-Hsing Street, Kwei-Shan Shiang, Taoyuan 333, Taiwan. Tel: 886-3281200 Ext: 2517; Fax: 886-3-3285818; E-mail: wenchi3992com.tw

 Global reach, higher impact
Global reach, higher impact