3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2010; 1:6-13. doi:10.7150/jca.1.6 This volume Cite
Research Paper
Breast cancer heterogeneity: mechanisms, proofs, and implications
1. Institute of Medicine, Chung Shan Medical University, Taiwan
2. Department of Obstetrics and Gynecology, Changhua Christian Hospital, Taiwan
3. Armed Forces Institute of Pathology and American Registry of Pathology, Washington DC, USA
4. Jilin University, China
Published 2010-6-1
Abstract
Human breast cancer represents a group of highly heterogeneous lesions consisting of about 20 morphologically distinct subtypes with substantially different molecular and/or biochemical signatures, clinical courses, and prognoses. This study analyzed the possible correlation between the morphological presentations of breast cancer and two hypothesized models of carcinogenesis, in order to identify the intrinsic mechanism(s) and clinical implications of breast cancer heterogeneity.
Keywords: breast cancer heterogeneity, mechanism, hypothesized model, clinical implication
1. Breast cancer heterogeneity
Human breast cancer represents a group of highly heterogeneous lesions consisting of about 20 morphologically distinct subtypes [1-2] (Table 1; Fig 1).
Morphological classification of human breast cancer
| # | Morphological classification | # | Morphological classification |
|---|---|---|---|
| 1 | Inflammatory | 11 | Clear cell |
| 2 | Pregnancy-associated | 12 | Medullary |
| 3 | Comedo | 13 | Secretory |
| 4 | Micropapillary | 14 | Signet ring cell |
| 5 | Papillary | 15 | Mucinous |
| 6 | Cribriform | 16 | Tubular |
| 7 | Solid | 17 | Lobular neoplasia |
| 8 | Clinging | 18 | Mixed cell types |
| 9 | Spindle cell | 19 | Apocrine |
| 10 | Neuroendocrine | 20 | Malignant myoepithelioma |
Among these subtypes, inflammatory and pregnancy-associated breast cancers have the most aggressive clinical course, in which many tumors had undergone extensive invasion or metastasis at the diagnosis [3-6]. In sharp contrast, small tubular and mucinous cancers have the most indolent clinical course, in which most pre-invasive cancers do not progress during patients' lifetime [1,2].
Breast cancer also has a highly variable profile of molecular and immunohistochemical signatures, and could be roughly divided into 5-categories based on their expression of estrogen receptor (ER), progesterone receptor (ER), human epidermal growth factor receptor-1 and 2 (HER-1 and 2), and cytokeratins 5/6 (CK 5/6) [7-9] (Table 2).
Molecular and immunohistochemical classification of human breast cancer
| Category | Signature | Treatment |
|---|---|---|
| Luminal A | ER(+) and/or PR(+); HER-2(-) | Endocrine RX; Tamoxifen |
| Luminal B | ER(+) and/or PR(+); HER-2(+) | Tratuzumab; Tamoxifen |
| Basal-like | ER(-); PR(-); HER-2(-);CK5/6(+); HER-1(+) | Neoadjuvant RX |
| HER-2(+)/ER(-) | HER-2(+); ER(-); PR(-) | Neoadjuvant RX |
| Normal breast-like | All markers(-) |
Breast tumor morphological heterogeneity. Sections from 4-different human breast tumors were stained with H&E. Note that although all are ductal carcinoma in situ, each has its unique morphological features.
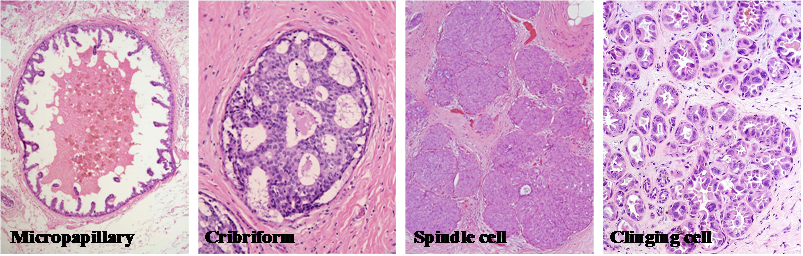
2. Mechanisms of heterogeneity
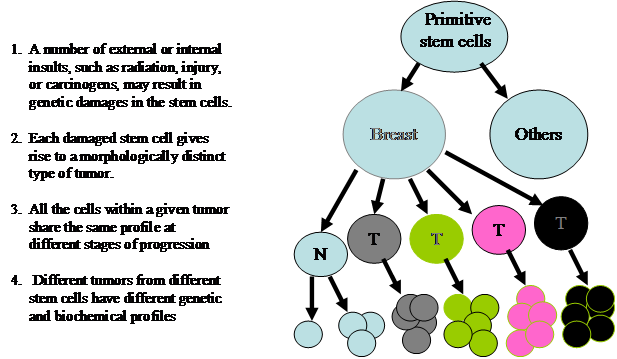
The mechanism(s) accounting for breast cancer heterogeneity remains elusive. However, two previously introduced theories, cancer stem cells and clonal evolution, appear to have provided some reasonable explanations. The theory of cancer stem cell was originated by Cohnheim in 1875 [10]. The main concept and major steps are depicted in Fig 2.
The theory of clonal evolution was introduced by Nowell in 1976 [11]. The main concept and major steps are depicted in Fig 3.
The theory of cancer stem cells for breast cancer heterogeneity
The theory of clonal evolution of breast cancer heterogeneity
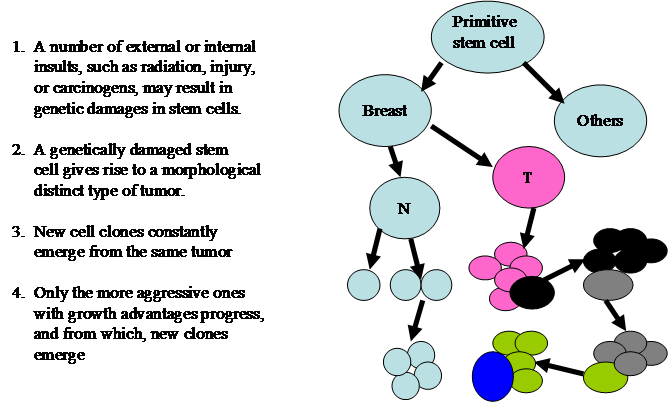
These theories share a number of similarities, including: (1) both believe that cancers are originated from cancer stem cells;(2) both believe that specific genetic or biochemical abnormalities are needed for carcinogenesis, and (3) both believe that the tumor microenvironment substantially influence the processes of carcinogenesis and tumor progression. However, these theories differ fundamentally in the main concept. The cancer stem cell theory believes that different tumors result from different stem cells, and that all cells within a given tumor could progress to a higher degree of malignancy. In sharp contrast, the clonal evolution theory believes that different tumors are originated from evolution of a single stem cell and that only the more aggressive clone progresses.
3. Proofs for each theory
The cancer stem cell theory is supported by several lines of evidence, including:
A. Different lobules have different morphological and immunohistochemical profiles
In H&E stained sections, all or nearly all lobules are clearly segregated by inter-lobular stromal tissues with a distinct boundary. All epithelial cells within a given lobule are morphologically and immunohistochemically similar, but they often differ substantially from cells in adjacent lobules. As shown in Fig 4, all cells within a large lobule in the center of the section are devoid of cytokeratin -19 (CK-19), whereas all or nearly all cells within adjacent lobules are CK-19 positive.
B. Malignancy-associated alterations in isolated lobules
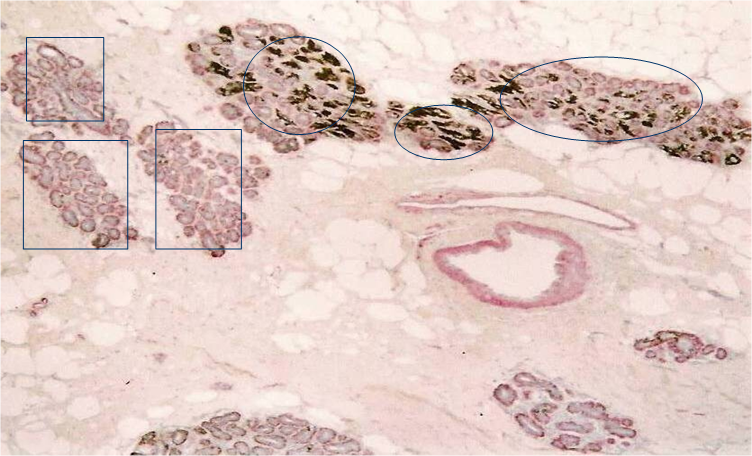
Our previous studies detected elevated cytoplasmic expression of HER-2 in some normal appearing lobules [12]. As shown in Fig 5, these lobules (circles) are segregated from other lobules with a distinct boundary. Most cells within these lobules show strong cytoplasm expression of HER-2, whereas all or nearly all cells in adjacent lobules (squares) are devoid of HER-2 expression.
Together, these findings suggest that different lobules are likely to arise from different stem cells, which constitutes the molecular base of heterogeneity for tumors subsequently derived from these lobules. This speculation is in total agreement with our previous findings in surgically operated rat submandibular glands with an integrated immunohistichemical and autoradiographical approach, which revealed the formation of morphologically and immunohistochemically unique new lobules within the residual gland by stem cells [13,14] (Fig 6).
Morphologically and immunohistochemically different lobules. A human breast tissue section was double immunostained for smooth muscle actin (red) and CK-19 (brown). Circle identifies a lobule consisting of all CK-19 negative cells. Arrows identify CK-19 positive cells in adjacent lobules.
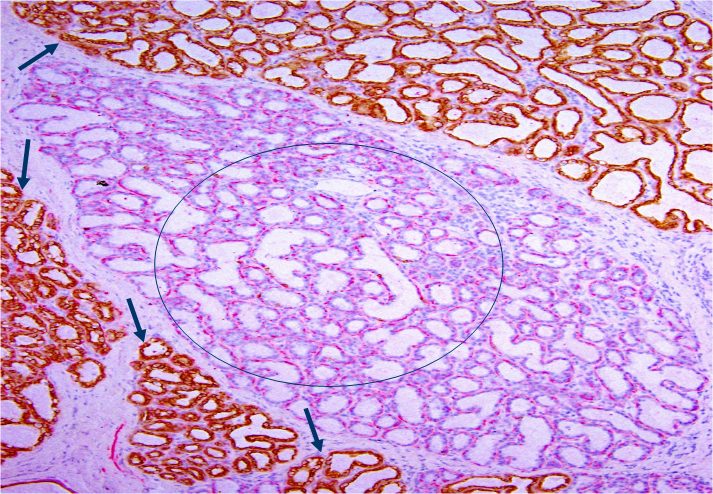
Aberrant HER-2 expression in isolated normal appearing lobules. A human breast tissue section was double immunostained for smooth muscle actin (red) and HER-2 (black).
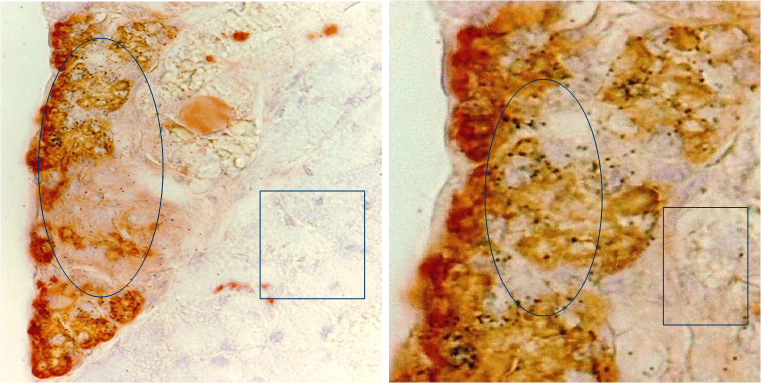
Formation of unique new lobules within residual submandibular glands (SMG) by stem cells after surgical operation. Rat SMG removed 4-weeks after partial removal of the gland and TdR injection was used for immunohistochemistry & autoradiography. Circles identify newly formed lobules. Squares identify pre-existing lobules within the residual gland.
The clonal evolution theory is also supported by several lines of evidence, including:
A. ER-negative cell clusters “budding” from ER- positive ductal tumor cores
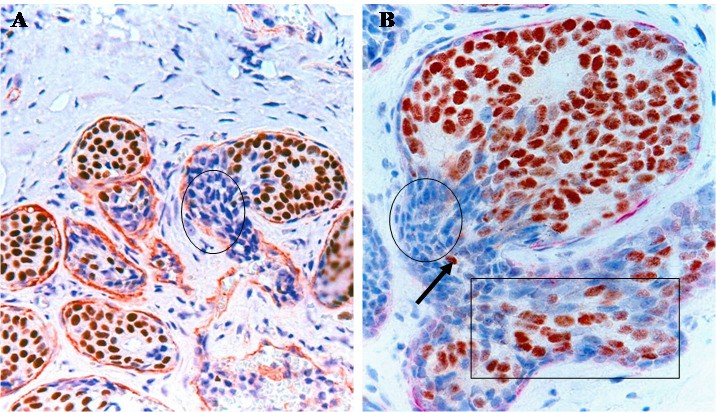
With a double immunohistochemical method, our previous studies revealed that a subset of ER-positive pre-invasive breast tumors harbored focal disruptions in their myoepithelial cell layers. Over 86% of these disruptions were overlaid by ER-negative cell clusters, but adjacent cells within the tumor core were ER-positive [15-18] (Fig 7). The size of ER-negative cell clusters appeared to increase with tumor progression. The tumor cells at the tip of these clusters gradually regained ER expression, but the cells near the tumor core were consistently devoid of ER expression (Fig 7b).
ER(-) cell “budding” from ER(+) tumors. Human breast tissue sections were double immunostained for SMA (red) and ER (brown). Circles identify ER(-) cells near the tumor cores. Squares identify ER(+) cells at the tip of an ER(-) cell cluster. Arrow identifies a ER(+) cell between the base and the tip of a ER(-) cell cluster.
B. “Budding” cell clusters and cells within tumor cores have a different molecular profile
Molecular analyses with microdissected “budding” cells and adjacent cells within the tumor core detected a markedly different rate of LOH and expression of invasion-related genes [17] (Fig 8).
4. Scientific and clinical implications
Together, these findings suggest that both the cancer stem cell and clonal evolution could contribute to breast cancer heterogeneity, in which, the cancer stem cell is more likely to contribute to lobular cancer heterogeneity, while the clonal evolution is more likely to contribute to ductal cancer heterogeneity.
If further validated, these theories and findings could have significant scientific and clinical implications. Scientifically, in order to identify the trigger factor(s) shared by all or most subtypes of breast cancers for their invasion and metastasis, a novel target highly representative to all or most subtypes is apparently needed to be elucidated, so that further studies could be carried out in comparable tissue samples. Clinically, breast cancers derived from clonal evolution are very likely to be more problematic for early detection and intervention for two main reasons:
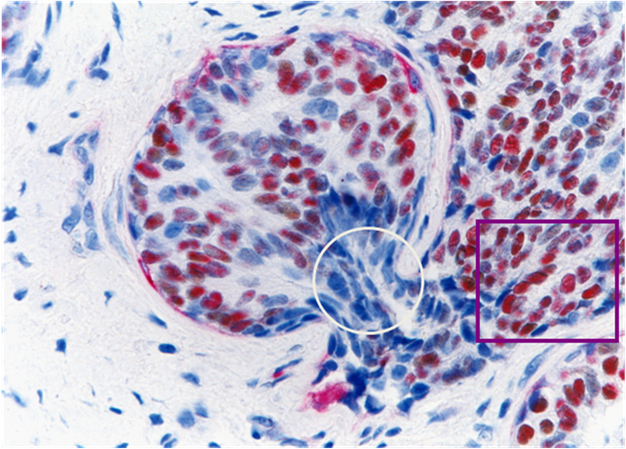
A. Tumor invasion or metastasis may occur at normal or hyperplastic stages
Our recent studies in nearly 1,000 cases of breast cancer have consistently shown that a subset of normal or hyperplastic breast duct clusters show malignancy-associated changes, including focal disruptions in the surrounding myoepithelial cell layer and basement membrane, expression of p53 and HER-2, morphological signs of stromal and vascular invasion [19,20] (Fig 9). These duct clusters may progress directly into invasive or metastatic lesions.
Differential expression of invasion-related genes between “budding” cells (circle) and cells within the tumor core (yellow square). Black squares identify differentially expressed genes between these two cell types of the same tumor.
Normal appearing breast ducts with malignancy-associated changes. A section of human breast tumor tissues was double immunostained with a specific marker for myoepithelial cells (red) and ER (brown). Circle identifies budding ER(-) cells from a ER(+) normal appearing duct. Square identifies ER(-) cells within a small dilated vein.
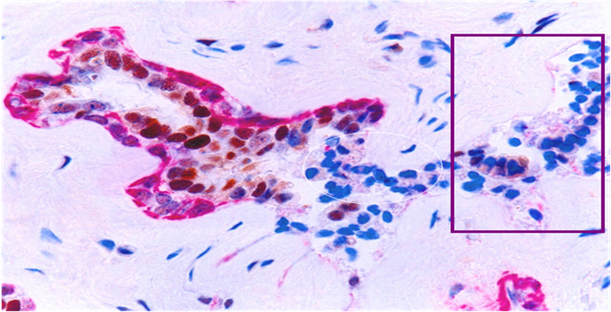
B. ER-negative cell clusters may represent “seeds” for drug resistant and recurrent tumors
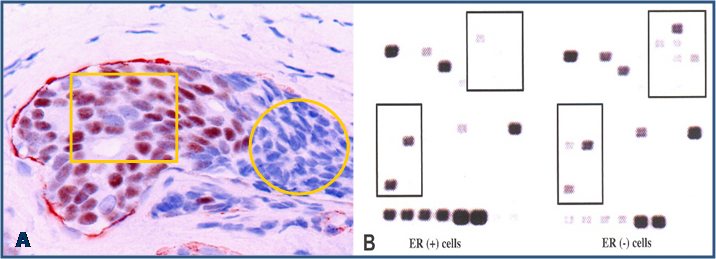
Our recent studies have consistently shown that ER-negative cells overlying focally disrupted myoepithelial cell layers are generally arranged as tongue- or finger-like projections invading the adjacent stromal tissue or vascular structures [15-18]. The size of these ER-negative cell clusters increased with tumor progression, and an increasing number of ER-positive cells were seen at the tips of these projections. In contrast, the ER-negative cell clusters overlying focally disrupted myoepithelial cell layers near the tumor core were consistently devoid of ER expression (Fig 10). These findings suggest these ER-negative cell clusters are likely to represent a population of a more aggressive cell clone that is under clonal evolution. Since these “budding” cells near the tumor core are consistently devoid of ER expression, they are unlikely to respond to tamoxifen and other hormonal-based therapies, representing “seeds” for drug resistant and recurrent breast tumors.
Changeable ER expression in “budding” cells overlying focally disrupted myoepithelial cell layers. Section was dobble immunostained with specific markers to the myoepithelial cell layer (red) and ER (brown). Circle identifies ER-negative cell cluster overlying focally disrupted myoepithelial cell layer. Square identifies ER-negative cell cluster derived ER-positive cells within the stromal tissue.
Acknowledgements
This study was supported in part by grant 2006CB910505 from the Ministry of Chinese Science and Technology Department, grant 30801176 from The National Natural Science Foundation of China, grants DAMD17-01-1-0129 and DAMD17-01-1-0130 from Congressionally Directed Medical Research Programs, grant BCTR0706983 from The Susan G. Komen Breast Cancer Foundation, and grant 2008-02 from US Military Cancer Institute and Henry M. Jackson Foundation.
The opinions and assertions contained herein represent the personal views of the authors and are not to be construed as official or as representing the views of the Department of the Army or the Department of Defense.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Rossen PP. Rosen's breast pathology. Philadelphia, PA: Lippncott Williams & Wilkins. 2001
2. Tavassoli FA. Pathology of the Breast. New York: Elsevier. 1992
3. Mathelin C, Annane K, Treisser A, Chenard MP, Tomaseeto C, Bellocq JP, Rio MC. Pregnancy and post-partum breast cancer: a prospective study. Anticancer Res. 2008;28(4C):2447-2452
4. Rodriguez AO, Chew H, Cress R, Xing G, McEivy S, Danielsen B, Smith L. Evidence of poorer survival in pregnancy-associated breast cancer. Obstet Gynecol. 2008;112(1):71-78
5. Mego M, DeGiorgi U, Hsu L, Ueno NT, Valero V, Jackson S. et al. Circulating tumor cells in metastatic inflammatory breast cancer. Ann Oncol. 2009;20(11):1824-1828
6. Man YG, Schwartz A, Levine PH, Teal C, Berg PE. BP1, a putative signature marker for inflammatory breast cancer and tumor aggressiveness. Cancer Biomark. 2009;5(1):9-17
7. Cianfrocca M, Gradishar W. New molecular classification of breast cancer. CA Cancer J Clin. 2009;59(5):303-313
8. Tang P, Skinner KA, Hicks DG. Molecular classification of breast carcinomas by immunohistochemical analysis: are we ready? Diagn Mol Pathol. 2009;18(3):125-32
9. Weigelt B, Mackay A, A'hern R, Natrajan R, Tan DS, Dowsett M. et al. Breast cancer molecular profiling with single sample predictors: a retrospective analysis. Lancet Oncol. 2010;11(4):339-349
10. Cohnheim J. Congenitales, quergestreiftes Muskelsarcom der Niere. Virchows Archiv fur Pathologische and Physiologic and fur Klinische Medizin. 1875;65:64-69
11. Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23-28
12. Zhang XC, Hashemi SS, Yousefi M, Gao CL, Sheng J, Mason J, Man YG. Atypical expression of c-erbB2 in cell clusters overlying focally disrupted breast myoepithelial cell layers: a potential sign for increasing cell motility and invasion. Int J Biol Sci. 2008;4:259-269
13. Man YG, Ball WD, Culp AJ, Hand AR, Moreira JE. Persistence of a perinatal cellular phenotype in the ducts of adult glands. J Histochem Cytochem. 1995;43(12):1203-1215
14. Man YG, Ball WD, Marchetti L, Hand AR. Contributions of intercalated duct cells to normal parenchyma of submandibular glands of adult rats. Anat Rec. 2001;263(2):202-14
15. Man YG, Tai L, Barner R, Vang R, Saenger JS, Shekitka KM. et al. Cell clusters overlying focally disrupted mammary myoepithelial cell layers and adjacent cells within the same duct display different immunohistochemical and genetic features: implications for tumor progression and invasion. Breast Cancer Res. 2003;5:R231-241
16. Yousefi M, Mattu R, Gao C, Man YG. Mammary ducts with and without focal myoepithelial cell layer disruptions show a different frequency of white blood cell infiltration and growth pattern: Implications for tumor progression and invasion. AIMM. 2005;13:30-37
17. Man YG, Zhang Y, Shen T, Vinh TN, Zeng X, Tauler J. et al. cDNA expression profiling identifies elevated expressions of tumor progression and invasion related genes in cell clusters of in situ breast tumors. Breast Cancer Res Treat. 2005;89:199-208
18. Man YG, Zhao CQ, Wang J. Breast tumor cell clusters and their budding derivatives show different immunohistochemical profiles during stromal invasion: implications for hormonal and drug therapies. Cancer Therapy. 2006;4:193-204
19. Man YG, Shen T, Weisz J, Berg PE, Schwartz AM, Mulshine JL, Sang QXA, Nieburgs HE. A subset of in situ breast tumor cell clusters lacks expression of proliferation and progression related markers but shows signs of stromal and vascular invasion. Cancer Detect Prev. 2005;29:323-331
20. Man YG, Nieburgs HE. A subset of cell clusters with malignant features in morphologically normal and hyperplastic breast tissues. Cancer Detect Prev. 2006;30(3):239-247
Author contact
![]() Corresponding author: Yan-gao Man, MD., PhD., Director of Gynecologic and Breast Research Laboratory, Department of Gynecologic and Breast Pathology, Armed Forces Institute of Pathology and American Registry of Pathology. Tel: 202-782-1612; Fax: 202-782-3939; E-mail: manosd.mil
Corresponding author: Yan-gao Man, MD., PhD., Director of Gynecologic and Breast Research Laboratory, Department of Gynecologic and Breast Pathology, Armed Forces Institute of Pathology and American Registry of Pathology. Tel: 202-782-1612; Fax: 202-782-3939; E-mail: manosd.mil

 Global reach, higher impact
Global reach, higher impact