Impact Factor
ISSN: 1837-9664
J Cancer 2010; 1:23-26. doi:10.7150/jca.1.23 This volume Cite
Research Paper
Age and tumor size predicts lymph node involvement in Hürthle Cell Carcinoma
1. Department of Surgery, University of Arizona, Tucson, AZ 85724-5131, USA;
2. Department of Surgery, University of California, San Francisco, CA, USA;
3. Surgery Branch, National Cancer Institute, Bethesda, MD, USA
Published 2010-6-2
Abstract
Introduction: Hürthle cell carcinoma (HCC) is a rare tumor that tends to metastasize to the lymph nodes. Some studies have correlated size of Hürthle cell tumors with the risk of malignancy. Whether the size of HCC correlates with the risk of lymph node (LN) metastases, to our knowledge has not been addressed.
Methods: A retrospective analysis was performed on all patients diagnosed with HCC on final pathology between 1997 and 2008. The tumor size and lymph node status was obtained for each patient. The data were analyzed utilizing Student's t-test and the Fisher's exact test to calculate the two-tailed p-value.
Results: Out of 39 patients diagnosed with HCC 3(8%) had LN metastases; 1 had ipsilateral central LN metastasis and 2 had ipsilateral central and lateral LN metastasis. LN dissection was performed in patients with known metastasis (2 were evident on preoperative ultrasound and 1 intraoperatively). Patients with LN metastasis were older than those without (mean age: 86.7 and 56.4 years, respectively), had larger tumors (mean size: 6 and 4 cm) and were commonly male (2 of 3). No tumor < 5cm presented with lymph node involvement (3/15 with >5cm cancer had node metastasis, 0/24 with <5cm cancer had node metastasis).
Conclusions: Similar to what has been found in patients with papillary thyroid cancer, older male patients with Hürthle cell carcinomas greater than 5cm are more likely to have lymph node metastasis. Our data suggest that these patients may benefit from a prophylactic ipsilateral central neck dissection at the time of their initial operation.
Keywords: Hurthle cell carcinoma, Lymph node metastasis, Age, Tumor size, Prediction
Introduction
Hürthle cell carcinoma (HCC) is a distinct and rare subtype of well-differentiated thyroid cancers. The aggressive nature of HCC stems from the higher propensity for multifocality, lymph node (LN) metastasis and distant metastasis compared to the other types of well-differentiated thyroid cancer. The limitation, however, is the difficulty in obtaining an accurate diagnosis preoperatively. Unlike the more common papillary thyroid cancer, HCC cannot be diagnosed on fine-needle aspiration (FNA). Rather, FNA typically yields the diagnosis of Hürthle cell neoplasm. The definition of a neoplasm differs in the literature, with some recommending the presence of greater than 50% Hürthle cells 1, 2, while others use greater than 75% 3, 4 as the cutoff. Regardless of the percent cellularity, the presence of capsular and/or vascular invasion is required to make the diagnosis of HCC on histopathology.
Attempts have been made to identify clinical parameters that can accurately predict the malignant potential of Hürthle cell neoplasms.2 However, tumor size has been shown to be predictive of malignancy in several studies.1, 5-7 Our group previously demonstrated that the risk of malignancy in a Hürthle cell neoplasm was 55% for tumors > 4 cm and only 13% for tumors ≤ 4 cm.5 This finding is corroborated in other studies that found that between 44%8 and 65%1 of neoplasms > 4 cm were malignant. Determining the malignant potential of Hürthle cell neoplasm is important because it can change the initial surgical approach from a lobectomy to a total thyroidectomy. Similarly, determining the risk of LN metastasis can also help in operative planning. The aim of this study was to determine if tumor size correlated with LN metastasis in patients diagnosed with HCC.
Materials and Methods
We retrospectively analyzed the records of all patients who underwent a thyroid operation at the University of California, San Francisco from February 1997 to September 2008. Patients who were diagnosed with Hürthle cell carcinoma on final pathology were used for analysis. Patient demographics, preoperative imaging results, intraoperative findings, and postoperative outcome data were collected. The institutional review board at the University of California, San Francisco, approved the study.
A fine needle aspiration (FNA) of suspicious thyroid nodules and/or lymph nodes was performed for diagnosis. An ultrasound (US) of the neck was performed to evaluate the thyroid gland and lymph node basins. The type of surgical procedure was dictated by clinical parameters: A thyroid lobectomy was performed on patients with a thyroid neoplasm and no contralateral thyroid nodules or suspicious cervical lymphadenopathy, with a completion thyroid lobectomy if final pathology revealed HCC; a total thyroidectomy was performed on patients with a thyroid neoplasm and contralateral thyroid nodules or suspicious cervical lymphadenopathy; a total thyroidectomy and cervical lymph node dissection (central and/or lateral) was performed when suspicious lymphadenopathy was found preoperatively on ultrasound, or during the operation.
Results
During the study period, 39 patients were diagnosed with Hürthle cell carcinoma. The majority of patients with HCC were women and the mean age at diagnosis of all patients was 58.7 years (Table 1). The preoperative diagnosis included 31 (79%) patients with Hürthle cell neoplasm, 3 (7%) with thyroid goiter, 2 (5%) with HCC, 2 (5%) with follicular cell neoplasm and 1 (2%) with Graves' disease. Four patients had risk factors for thyroid cancer; 1 had a family history of thyroid cancer, and 3 patients had a history of radiation exposure.
Characteristics of patients with Hürthle cell carcinoma
| Characteristics | Values | |
|---|---|---|
| Total Patients | 39 | |
| Age (years), mean ± SD | 58.7 ± 15.9 | |
| Gender | ||
| Female, n (%) | 23 (59) | |
| Male, n (%) | 16 (41) | |
| Race | ||
| Caucasian, n (%) | 31 (79) | |
| Asian, n (%) | 4 (10) | |
| African American, n (%) | 1 (2) | |
| Latino, n (%) | 1 (2) | |
| Other, n (%) | 2 (5) | |
| Pathologic findings | ||
| Multifocal | 7 (18) | |
| Capsular invasion | 17 (44) | |
| Vascular invasion | 23 (59) | |
| Lymphatic invasion | 5 (13) | |
| Lymph node metastases | 3(8) | |
At the initial operation, 22 (56%) patients underwent a thyroid lobectomy, 14 (36%) total thyroidectomy, and 3 (8%) total thyroidectomy and lymph node dissection. Patients who underwent an initial thyroid lobectomy underwent a subsequent operation for a completion thyroidectomy. Of the 3 patients who received a lymph node dissection, 1 had ipsilateral central LN metastases identified intraoperatively and underwent a central LN dissection, and the other 2 had lateral LN metastasis identified on preoperative ultrasound and underwent an ipsilateral central and lateral LN dissection.
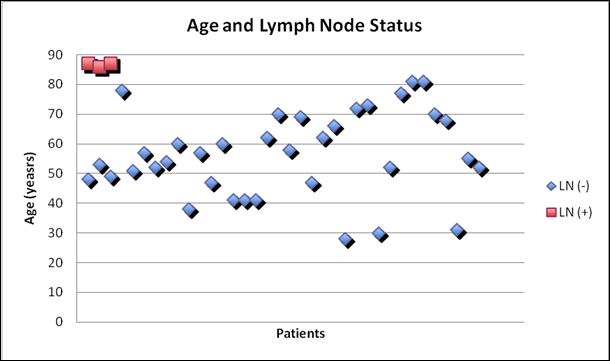
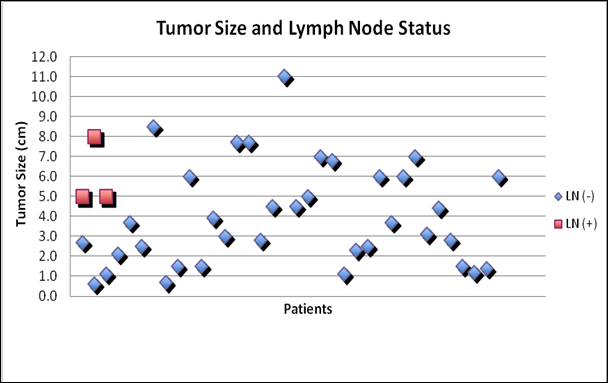
Though there was no gender difference between the two groups, patients with LN metastasis were older than those without (Figure 1: mean age 86.7 and 56.4 years, respectively). Similarly, patients with LN metastasis had larger tumors than those without (Figure 2: mean size 6 and 4 cm, respectively). Tumor size was predictive of lymph node metastasis as no patient with tumor < 5cm presented with lymph node involvement (3/15 with >5cm cancer had node metastasis, 0/24 with <5cm cancer had node metastasis).
Several histologic differences were evident between patients with and without lymph node metastasis. All patients with lymph node metastasis had widely invasive tumors with capsular invasion, vascular invasion and multifocal disease. Of the 36 tumors without LN metastasis, 9 (25%) were moderately-to-widely invasive, 14 (39%) had capsular invasion, 20 (56%) had vascular invasion, and 4 (11%) were multifocal. Only extent of invasion and multifocality were associated with LN metastasis.
Relationship between patient age and lymph node status in patients with Hürthle cell carcinoma
Relationship between tumor size and lymph node status in patients with Hürthle cell carcinoma
Discussion
Our group previously showed that the overall rate of malignancy in FNA proven Hürthle cell neoplasms was 21% and that 55% of these tumors larger than 4cm were HCC. No single clinical factor can accurately predict the malignant potential of Hürthle cell neoplasms. However, identifying such clinical factors can guide operative decision-making. For example, it has been suggested that a total thyroidectomy, rather than a lobectomy, be considered for older patients with tumors larger than 4cm.8
In this series, we sought to determine if the size of the HCC tumor could predict the presence of LN metastasis. In our patient cohort, 8% of patients with HCC were found to have LN metastasis. This is similar to other reported rates of 3% to 13%.3, 9 Others, however, have report higher rates of 18% to 25%.10, 11 Despite the associated risk of LN metastasis in patients with HCC, no studies have shown that HCC size predicts LN involvement.
This study demonstrates that HCC tumor size predicts LN metastasis. Patients who presented with LN metastasis had larger tumors than those who presented without LN metastasis. Interestingly, no patients with cancers < 5cm presented with LN metastasis. On the other hand, 20% of patients with cancers > 5cm were found to have LN metastasis.
This study also demonstrates that all patients with LN metastasis had widely invasive and multifocal tumors. This finding has been corroborated in another study that demonstrated that all seven patients with LN metastasis had widely invasive HCC.9 The presence of capsular invasion or vascular invasion, however, did not predict LN involvement. Though HCC commonly affects women more often than men, gender was not an associated risk factor for developing LN metastasis.
Older age is considered a risk factor for malignancy in patients with Hürthle cell neoplasms.8 Similarly, we found that advanced age was a risk for the presence of LN metastasis in patients with HCC. Specifically, all of our patients who presented with LN metastasis were octogenarians (Figure 1). This finding suggests that older patients with large HCC are at risk for developing lymph node metastasis.
In summary, as size has been shown to predict the malignant potential of Hürthle cell neoplasms and this study demonstrate that size and age are also predictive of LN metastasis in patients with HCC. Our data suggest that consideration should be given to performing a prophylactic ipsilateral central neck dissection at the time of their initial operation in older patients with large tumors.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Chen H, Nicol TL, Zeiger MA. et al. Hürthle cell neoplasms of the thyroid: are there factors predictive of malignancy? Ann Surg. 1998;227:542-6
2. McHenry CR, Thomas SR, Slusarczyk SJ, Khiyami A. Follicular or Hürthle cell neoplasm of the thyroid: can clinical factors be used to predict carcinoma and determine extent of thyroidectomy? Surgery. 1999;126:798-802
3. Kushchayeva Y, Duh QY, Kebebew E, Clark OH. Prognostic indications for Hürthle cell cancer. World J Surg. 2004;28:1266-70
4. Giorgadze T, Rossi ED, Fadda G. et al. Does the fine-needle aspiration diagnosis of "Hürthle-cell neoplasm/follicular neoplasm with oncocytic features" denote increased risk of malignancy? Diagn Cytopathol. 2004;31:307-12
5. Sippel RS, Elaraj DM, Khanafshar E. et al. Tumor size predicts malignant potential in Hürthle cell neoplasms of the thyroid. World J Surg. 2008;32:702-7
6. Pisanu A, Sias L, Uccheddu A. Factors predicting malignancy of Hürthle cell tumors of the thyroid: influence on surgical treatment. World J Surg. 2004;28:761-5
7. Lopez-Penabad L, Chiu AC, Hoff AO. et al. Prognostic factors in patients with Hürthle cell neoplasms of the thyroid. Cancer. 2003;97:1186-94
8. Zhang YW, Greenblatt DY, Repplinger D. et al. Older age and larger tumor size predict malignancy in hürthle cell neoplasms of the thyroid. Ann Surg Oncol. 2008;15:2842-6
9. Stojadinovic A, Hoos A, Ghossein RA. et al. Hürthle cell carcinoma: a 60-year experience. Ann Surg Oncol. 2002;9:197-203
10. Mills SC, Haq M, Smellie WJ, Harmer C. Hürthle cell carcinoma of the thyroid: Retrospective review of 62 patients treated at the Royal Marsden Hospital between 1946 and 2003. Eur J Surg Oncol. 2009;35:230-4
11. Soh EY, Clark OH. Surgical considerations and approach to thyroid cancer. Endocrinol Metab Clin North Am. 1996;25:115-39
Author contact
![]() Corresponding author: Marlon A. Guerrero, MD, University of Arizona, Department of Surgery, 1501 N. Campbell Ave, #4327D, Tucson, AZ 85724-5131. mguerreroarizona.edu
Corresponding author: Marlon A. Guerrero, MD, University of Arizona, Department of Surgery, 1501 N. Campbell Ave, #4327D, Tucson, AZ 85724-5131. mguerreroarizona.edu

 Global reach, higher impact
Global reach, higher impact