Impact Factor
ISSN: 1837-9664
J Cancer 2011; 2:67-75. doi:10.7150/jca.2.67 This volume Cite
Research Paper
miR-21 Expression in Pregnancy-Associated Breast Cancer: A Possible Marker of Poor Prognosis
1. Laboratory of Pathology, National Institutes of Health, National Cancer Institute, Bethesda, MD, USA;
2. Department of Pathology and Cytopathology, Universidad Autonoma Nuevo Leon, Monterrey, México.
Received 2011-1-30; Accepted 2011-2-7; Published 2011-2-7
Abstract
Aims: microRNAs (miRNAs) are a class of small noncoding RNAs that can act as key modulators in tumorigenesis-related genes. Specifically, it has been suggested that miR-21 overexpression plays a role in the development and progression of breast cancer. So far, the role of miRNAs in pregnancy-associated breast cancer (PABC) has not been investigated.
Methods and Results: We evaluated miR-21 expression by quantitative RT-PCR in 35 patients, 25 with PABC and 10 control breast cancer cases not pregnancy-associated with similar clinicopathological features. We then analyzed protein expression for PTEN, BCL2 and PDCD4 as miR-21 target genes by IHC, and finally correlated the results with patients' clinicopathological features.
Significant overexpression of miR-21 in PABC tumors compared to normal adjacent tissue was found. Overexpression of miR-21 was frequently found in high grade tumors with loss of hormone receptor expression and was significantly associated with positive lymph nodes (p=0.025). In PABC patients, PTEN, BCL2 and PDCD4 target protein expression was decreased in 80%, 76% and 40% respectively.
Conclusion: Our study supports the involvement of miR-21 in breast cancer progression and metastasis formation in PABC implying a role of this miRNA as a marker for poor prognosis in PABC patients.
Keywords: Pregnancy-associated breast cancer, breast cancer, microRNA, miR-21, PTEN.
Introduction
Pregnancy-associated breast cancer (PABC) is defined as cancer of the mammary gland diagnosed during pregnancy, up to 1 year after delivery or at any time while the patient is lactating 1. Although it is a relatively infrequent condition with an incidence rate of approximately 1-3 cases per 10,000 pregnancies, frequently delayed diagnosis leads patients to present with large tumors and increased number of positive lymph nodes, both elements associated with poor survival 2, 3 .
Recent studies have implicated microRNAs (miRNAs) in different types of cancer, acting as key modulators of tumorigenesis. miRNAs are small fragments of non-coding RNA that are post-transcriptionally involved in the regulation of gene expression. Since the first miRNA lin 4 was identified in Caenorhabditis elegans as being involved in development and differentiation processes, more than 1,000 miRNAs have been identified in the human genome 4. Approximately 3% of genes in the human genome encode for miRNAs, and up to 30% of human protein-coding genes may be regulated by them 5. Furthermore, it was previously demonstrated that in breast cancer cells there is a causal link between specific miRNAs and alteration of proliferation, migration and apoptosis, thus contributing to cancer pathogenesis 6, 7 .
miRNA profiling studies in different solid tumors have shown a cancer type-specific deregulation of tumor suppressor genes 8, 9. In particular, miR-21 has been found to be frequently overexpressed in different solid tumors, including breast cancer 10, 11. It was demonstrated that miR-21 may affect tumor progression through down-regulation of different targeted tumor suppressor genes such as phosphatase and tensin homolog deleted on chromosome 10 (PTEN), programmed cell death 4 (PDCD4), B-cell lymphoma protein (BCL2), tropomyosin 1 (TPM 1) among others 12-14. The potential importance of miR-21 expression as a marker of poor prognosis in breast cancer has been suggested 15, however its role in PABC patients has not been investigated.
In this study, we analyzed the expression levels of miR-21 in a series of PABC cases to evaluate its role in the transcriptional regulation of its target genes (PTEN, PDCD4 and BCL2) and therefore its impact on tumor onset and progression. We then correlated the results with clinicopathological data to assess its potential use as prognostic marker in PABC patients.
Materials and Methods
Patients
A total of 35 patients, 25 with PABC and 10 with similar clinicopathological features of breast cancer but not pregnancy-associated, were obtained from the surgical pathology archives of the Laboratory of Pathology, National Cancer Institute, Bethesda, MD, USA after IRB approval. Hematoxylin and eosin (H&E) stained slides were reviewed to confirm the diagnosis and to evaluate their respective histopathological features. Medical records were reviewed for relevant clinical data, and hormone receptor expression data (estrogen receptor (ER), progesterone receptor (PR), and Her-2/neu status) in the surgical pathology report were added to our database.
RNA extraction
Five micron formalin-fixed paraffin-embedded (FFPE) tissue sections were deparaffinized, rehydrated in graded ethanol and manually needle microdissected under a microscope to obtain separate amounts of tumor and matched normal breast tissue. RNA was extracted using the RecoverAllTM Total Nucleic Acid Isolation Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's recommendations. A total of 60 μl of elution solution containing low molecular weight RNA (<200 bases) was obtained in which concentration and quality of the RNA isolated was determined by measuring its absorbance using the NanoDropTM 1000 spectrophotometer (Thermo Scientific Inc.). RNA final concentration was normalized in all samples.
miR-21 quantification by real-time PCR
Real-time PCR analysis of miRNA was performed as previously described 16. A two-step protocol requiring reverse transcription with miRNA-specific primer, followed by real-time PCR with a TaqMan® probe was used. MicroRNA's quantitative assay for miR-21 (assay ID: 000397, Applied Biosystems) was used to evaluate mature miRNA expression, while RNU6B (assay ID: 001093) was used as endogenous control.
Each RT reaction contained 10 ng of purified low molecular weight RNA in a final volume of 20 µl. One μl of RT product was used in duplicate for each quantitative real time-PCR reaction. Expression data were acquired and analyzed in 96-well-plate format using an ABI Prism 7000 Sequence Detection System (Applied Biosystems). Relative amount of miRNA was calculated by comparative threshold cycle (CT) method. For normalization of RT-PCR results, RNU6B was used as an endogenous control. Changes in expression levels in tumor samples are shown as relative (fold change) to their normal corresponding tissue.
Immunohistochemistry
Protein expression of PTEN, PDCD4 and BCL2 was evaluated by immunohistochemistry using PTEN C-term rabbit monoclonal antibody Clone: Y184 (Epitomics, Burlingame, CA, USA); BCL2 mouse monoclonal antibody Clone: 124 (Imgenex, San Diego, CA, USA) and PDCD4 rabbit monoclonal antibody Clone: ab51495 (Abcam, Cambridge, MA, USA).
Paraffin-embedded sections (5 μm) were deparaffinized and rehydrated in graded alcohol. Tissue sections were microwaved in 10 mM sodium citrate pH 6.0 for 15 minutes and then allowed to cool down. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide in phosphate buffered saline (PBS) for 10 minutes. Sections were then incubated for 1 hour at room temperature with the BCL2 antibody or overnight at 4°C for PDCD4 and PTEN antibodies. For negative controls, sections were incubated in parallel with their respective matched isotype non-immune IgG. After rinsing, the sections were incubated with the appropriate secondary antibodies for 30 minutes (EnVision+TM Dual Link Kit, Dakocytomation, Carpinteria, CA). The staining was visualized with 3,3'-diaminobenzidine as chromogen and slides were counterstained with hematoxylin, dehydrated and finally mounted. Human thyroid gland carcinoma was used as a positive control for PTEN, whereas normal colon tissue was used for the PDCD4 staining. For the BCL2 antibody, a lymph node section was used as a positive control.
Level of expression was scored semi-quantitatively based on intensity of staining and the proportion of tumor cells in the selected section. Intensity was recorded as 0 (no staining), 1 (weak staining), 2 (moderate staining) or 3 (strong staining), and the percentage of stained cytoplasmic/nuclear area was recorded. All cases with moderate or strong staining in >10 percent of the cells were considered positive 17.
Statistical Analysis
Results are expressed as the mean values and standard error of the mean. Statistical analyses for RT-PCR results were performed on ∆CT data. SPSS version 13.0 (SPSS, Chicago IL) software was used for statistical analysis of data with the unpaired 2-tailed Student t-test. P values less than 0.05 were considered statistically significant. Survival analysis was performed for overall survival (OS) time, and survival curves were constructed according to the Kaplan-Meier product-limit method and compared by the log-rank test.
Results
Clinicopathological data
There were no significant differences in clinicopathogical features between the PABC and control groups, as summarized in Table 1.
Clinicopathological characteristics of patients with pregnancy-associated breast cancer (PABC) and the control group.
| Variable | PABC N (%) | CONTROL N (%) | p value |
|---|---|---|---|
| Age in years (Mean) | 37 | 38 | 0.355 |
| Tumor histology | 1.00 | ||
| Poorly differentiated | 17 (68) | 7 (72) | |
| Moderately differentiated | 8 (32) | 3 (28) | |
| Lymph node metastasis | 1.000 | ||
| Positive | 16 (64) | 6 (60) | |
| Negative | 9 (36) | 4 (40) | |
| Hormone receptors status | 0.361 | ||
| ER-positive | 3 (19) | 3 (30) | |
| ER-negative | 13 (81) | 5 (50) | |
| PR-positive | 6 (38) | 3 (30) | 1.000 |
| PR-negative | 10 (62) | 5 (50) | |
| Her-2/neu-positive | 1 (06) | 3 (30) | 0.090 |
| Her-2/neu-negative | 15 (94) | 5 (50) |
The mean age at diagnosis of patients in the PABC group was 37 vs. 38 years in the control group of patients with not pregnancy-associated breast cancer. Age distribution was similar in both groups; more than 70% of patients were older than 35 years. All tumors were infiltrating ductal carcinomas; 32% were moderately differentiated and 68% were poorly differentiated tumors, with similar percentage in the control group (28% and 72% respectively). Lymph node metastasis was present in 64% vs. 60% in the control group. In the PABC cases compared with the control group, 19% vs. 30% of patients were ER-positive, 62% vs. 50% were PR-negative, and 94% vs. 50% were HER-2/neu-negative.
miR-21 expression
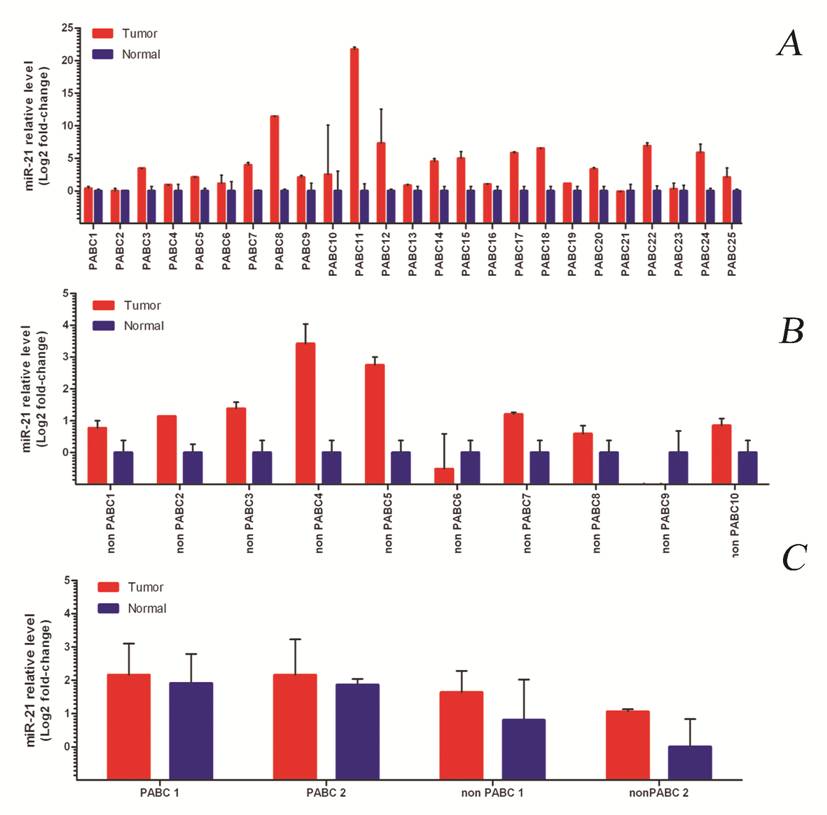
We consistently found overexpression of miR-21 in the PABC group, and in 80% of the tumor vs. matched normal adjacent tissue in the control group (Figure 1A-B).
When the overall level of miR-21 expression was compared within the two groups, tumors from PABC cases presented a much higher level of expression compared to their normal counterparts (average 11.1 vs. 1.8 fold). We also found higher expression of miR-21 in the normal breast tissue of the PABC group in comparison with normal breast in the non-PABC group (Figure 1C). However, the overall expression of miR-21 in the normal breast tissue of PABC cases was still lower than in the PABC tumors.
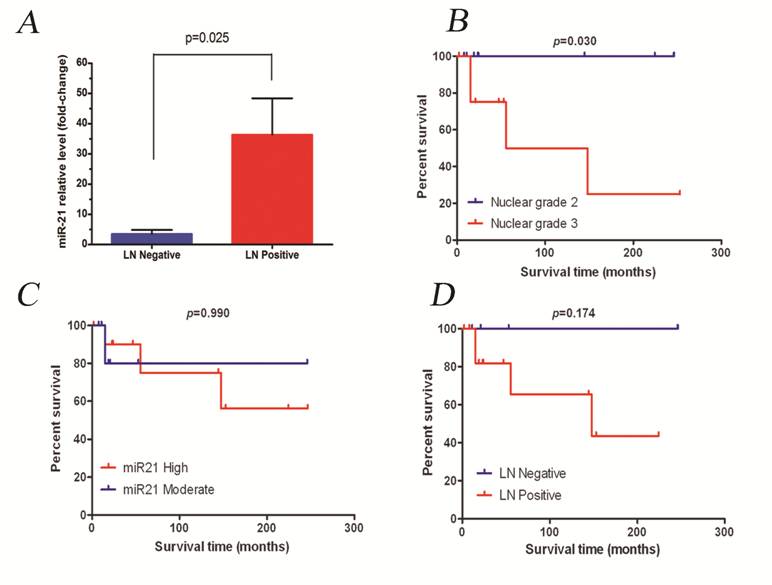
The median fold-change for all PABC cases was 5-fold. This value was used then to divide patients into two groups: miR-21 moderate expressers (n=11, 31%) and miR-21 high expressers (n=13, 37%). Since all cases showed overexpression of miR-21 compared to normal tissue, no low-expressers category was included. Tumors with a high overexpression level of miR-21 correlated positively with the presence of metastatic lymph nodes (p=0.025, Figure 2A). The other clinicopathological features of the PABC cases did not significantly correlate with miR-21 expression levels (Table 2).
Targeted gene expression
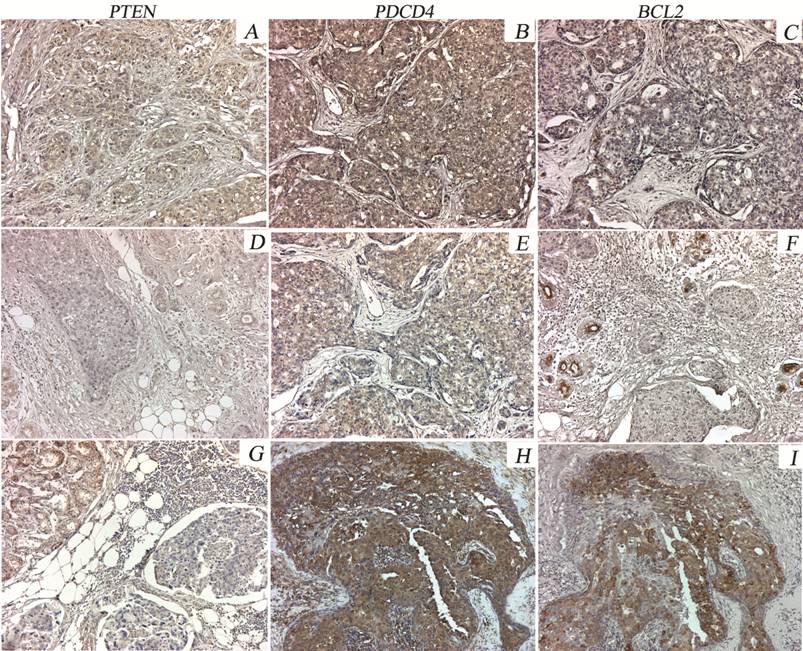
Protein levels of PTEN, PDCD4 and BCL2 were lower in tumors compared with adjacent normal tissue in both groups. PTEN protein expression was negative in 80% of PABC cases vs. 70% of the tumors in the control group. PDCD4 expression was negative in 40% of the PABC cases vs. 20% in the control group. For the BCL2 gene, 76% of PABC tumors were negative vs. 40% of tumors in the control group (Table 3 and Figure 3). PTEN negative expression was significantly correlated with increased miR-21 expression (p=0.044).
miR-21 expression in normal and tumor samples of PABC cases (1A) and the control group (1B). C) Representative cases of PABC and non-PABC showing different levels of miR-21 expression. Note the increased levels of miR-21 expression in normal breast tissue of the PABC group vs. the control group.
Survival analysis of the PABC group
Figure 2B-D summarizes survival analysis. Median follow-up for the PABC group was 35.5 months. No statistically significant difference was observed in survival time between the PABC and non-PABC groups; however, within cases that overexpressed miR-21, a worse prognosis was seen in the group that presented with a higher expression level (p=0.990). According to tumor histological features and patient clinical data, the presence of lymph nodes was the only feature that was significantly associated with worse patient survival (p=0.174 and p=0.030, respectively).
miR-21 expression levels (moderate/high) of tumor vs. normal sample and correlation with the clinicopathological features of PABC patients.
| Variable | miR-21 expression* | p value¶ | |
|---|---|---|---|
| Moderate N (%) | High N (%) | ||
| Age (years) | 1.000 | ||
| < 35 | 3 (15) | 2 (10) | |
| ≥ 35 | 8 (40) | 7 (35) | |
| Histologic grade† | 1.000 | ||
| Grade 1 | (0) | (0) | |
| Grade 2 | 4 (16) | 4 (16) | |
| Grade 3 | 8 (32) | 9 (36) | |
| Lymph node metastasis | 0.068 | ||
| Positive | 6 (26) | 10 (44) | |
| Negative | 6 (26) | 1 (04) | |
| ER status | |||
| Negative | 7 (44) | 6 (38) | 1.000 |
| Positive | 1 (06) | 2 (12) | |
| PR status | |||
| Negative | 5 (31) | 5 (31) | 1.000 |
| Positive | 3 (19) | 3 (19) | |
| Her-2/ neu status | |||
| Negative | 8 (50) | 8 (50) | - |
| Positive | (0) | (0) | |
PTEN, BCL2 and PDCD4 protein expression analyzed by immunohistochemistry.
| Variable | PABC N (%) | CONTROL N (%) | p value |
|---|---|---|---|
| PTEN | |||
| Positive | 5 (20) | 3 (30) | 0.661 |
| Negative | 20 (80) | 7 (70) | |
| BCL2 | |||
| Positive | 6 (24) | 4 (40) | 0.420 |
| Negative | 19 (76) | 6 (60) | |
| PDCD4 | |||
| Positive | 15 (60) | 8 (80) | 0.230 |
| Negative | 10 (40) | 2 (20) |
A) miR-21 expression in PABC samples with metastatic lymph nodes. B-D) Impact of some clinicopathological variables on overall survival for PABC patients.
Immunostaining patterns of PTEN, PDCD4 and BCL2 proteins in representative PABC cases. PTEN is moderately expressed in the normal breast tissue and is weak or negative in most of the tumor cells (A, D, G). Heterogeneous PDCD4 expression was observed in PABC tumors, with either weak or moderate staining in different cases (B, E, H). Moderate BCL2 expression in the normal breast tissue adjacent to the tumor was found. Variable staining was seen in the tumor areas (negative to moderate intensity) (C, F, I).x20.
Discussion
Pregnancy-associated breast cancer is usually associated with advanced disease status and poor prognosis, although a causal effect of the hormonal milieu has not been demonstrated 18. Delayed diagnosis due to pregnancy or breastfeeding has been suggested as one of the reasons for advanced breast cancer in these patients. Recently, in a registry study performed in a large Swedish population with breast cancer, the 5-year survival rate for PABC was only 52%, significantly less than the 80% survival rate for age-matched patients with a not-recent pregnancy 19. Low overall survival in PABC has been associated with advanced disease and loss of hormone receptor expression 20. Positive regional lymph nodes are found more often in PABC patients than in non-PABC patients (59%-83% vs. 38%-54%). In our study, PABC patients presented similar clinicopathological features as in previously published reports, most breast cancer samples were poorly differentiated tumors, presenting at the time of diagnosis 2 cm or more in size and a high rate of positive lymph nodes (64% of the PABC cases). A high frequency of negative hormone receptor tumors was also found (ER, PR and Her-2/neu).
Over the past decades, the published literature has demonstrated that gene expression profiling may be a useful tool to define the signature of breast cancer and predict the prognosis or response to treatment. However, similar investigations need to be done to evaluate miRNAs that may interfere with such gene expression signature.
A cancer-specific miRNA profile has been investigated before for breast cancer. Altered expression of some genes related with breast cancer tumorigenesis and metastasis has been shown to be linked to specific miRNAs that may potentially act as tumor suppressor or oncogenic miRNAs. Iorio et al. 21 demonstrated the existence of a breast cancer-specific signature with genome-wide miRNA expression profiling that may help to predict prognosis and therapeutic response.
Overexpression of miR-21 has been consistently reported to be up-regulated in many solid tumors, including breast cancer, when tumor expression is compared with matched normal tissue. Si et al. first established a breast cancer cell line with miR-21 knockdown (MCF-7) and used it in a xenograft model; resulting tumors were reduced in size, with low proliferation rate and increased apoptosis in comparison with the negative control 22.
miR-21 has been demonstrated to play a role in regulating cell apoptosis through modulating target genes as BCL2 and PDCD4 22. Special attention has been focused on the anti-apoptotic response of breast cancer patients with chemotherapy resistance, where miR-21 has been found not only to reduce BCL2 protein expression but to also activate a whole pathway that is related to inhibitors of apoptosis proteins and multidrug-resistant proteins 23.
PTEN has been also suggested to be a miR-21 target gene. Expression of miR-21 was found negatively correlated with PTEN expression; high expression of miR-21 was frequently found in the more aggressive breast cancer cases 24.
Our results are in agreement with previous publications 15, 24, in that most of the tumors overexpressed miR-21. Forty-five percent of the tumor samples presented with a high level of miR-21 expression (more than the median value). This group of cases was significantly correlated with positive lymph node metastasis (p=0.024), and with poor prognosis.
miR-21 has been linked with numerous target genes (PTEN, TPM1, PDCD4, BCL2 and recently the maspin genes), all of them associated with increased invasion and metastatic potential in breast cancer 25.
In our study, when we validated some of the targeted genes by immunohistochemistry, a statistically significant inverse correlation was found only between PTEN and miR-21 overexpression. We observed loss of PTEN protein expression in almost 80% of the tumors in both groups (PABC vs. control). This rate is higher than reported in previous publications that have frequently shown loss of PTEN protein expression in 38- 48% of advanced breast cancer 26, 27. The difference in results may be due to the different features of our study group, in which most of the PABC cases presented with poorly differentiated tumors in patients with advanced disease, positive lymph node metastasis and negative hormone receptors. Koninki et al. found frequent loss of PTEN expression in breast tumors that are Her-2 receptor-negative, suggesting that low expression of PTEN is one of the co-existent molecular mechanisms that lead to resistance to trastuzumab 28, 29.
The PDCD4 protein has been known as a tumor suppressor involved not only in apoptosis but also in cell transformation and tumor progression. Recently, an inverse correlation between miR-21 and PDCD4 expression has been proposed 30. In our study we found that 40% of the tumors in PABC were PDCD4 negatively expressed, results that are in agreement with previous reports using invasive breast cancer cases.
The BCL2 protein has been proposed as a prognostic marker in breast cancer and it seems to be associated with ER status, increased dedifferentiation, and deregulated proliferation 31. In our study, 76% percent of the PABC samples showed loss of BCL2 protein expression, some of them correlated with negative hormone receptor status (of the BCL2 negative tumors, 89% were also ER-negative, 66% PR- negative and 75% Her-2/neu-negative), supporting the idea that loss of hormone receptors is associated with loss of BCL2 expression.
Taken together, the results suggest that overexpression of miR-21 is involved in many cellular processes that are altered in cancer, such as dedifferentiation, proliferation and apoptosis, playing an important role in pathogenesis of breast cancer through different specific targeted genes. Our study supports the association between PTEN and miR-21 in PABC.
Interestingly, we found increase overall expression level of miR-21 in normal breast tissue of PABC in comparison with the control group. It is well known that during pregnancy there is an elevated rate of proliferation and dedifferentiation of the mammary epithelial and myoepithelial cells, stem cells beside the pathological alterations of the microenvironment. A potential link between tumor and developmental biology of the mammary glands during pregnancy has been suggested especially in the myoepithelial cells of PABC, which exhibit unique alterations in p63 expression that is suggested represent a biologically more aggressive cell population, which could potentially progress to invasive lesions 32.
This observation suggests that differential miRNA expression may occur as a part of the mammary gland histological and physiological reorganization during pregnancy, lactation or involution that may modify gene expression and contribute to tumor development or progression. Further studies are needed to determine how the miRNA profiles change in the mammary gland during pregnancy.
In conclusion, our results demonstrate that miR-21 is overexpressed in PABC. Changes in the expression of target genes such as PTEN, PDCD4, BCL2 support the concept that miR-21 may play an important oncogenic role not only in breast cancer but also in breast tissue during pregnancy. Expression of miR-21 may be a possible indicator of poor prognosis in breast cancer for this group of patients.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, NCI.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Petrek JA. Breast cancer and pregnancy. Journal of the National Cancer Institute. 1994(16):113-121
2. Ulery M, Carter L, McFarlin BL, Giurgescu C. Pregnancy-associated breast cancer: significance of early detection. Journal of midwifery & women's health. 2009;54(5):357-363
3. Pentheroudakis G, Pavlidis N. Cancer and pregnancy: poena magna, not anymore. Eur J Cancer. 2006;42(2):126-140
4. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843-854
5. Sassen S, Miska EA, Caldas C. MicroRNA: implications for cancer. Virchows Arch. 2008;452(1):1-10
6. Miska EA. How microRNAs control cell division, differentiation and death. Current opinion in genetics & development. 2005;15(5):563-568
7. Gaur A, Jewell DA, Liang Y. et al. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer research. 2007;67(6):2456-2468
8. Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nature reviews. 2006;6(4):259-269
9. Lu J, Getz G, Miska EA. et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834-838
10. Selcuklu SD, Yakicier MC, Erson AE. An investigation of microRNAs mapping to breast cancer related genomic gain and loss regions. Cancer genetics and cytogenetics. 2009;189(1):15-23
11. Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochemical Society transactions. 2009;37(Pt 4):918-925
12. Qi L, Bart J, Tan LP. et al. Expression of miR-21 and its targets (PTEN, PDCD4, TM1) in flat epithelial atypia of the breast in relation to ductal carcinoma in situ and invasive carcinoma. BMC cancer. 2009;9:163
13. Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647-658
14. Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. The Journal of biological chemistry. 2008;283(2):1026-1033
15. Yan LX, Huang XF, Shao Q. et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA (New York, NY). 2008;14(11):2348-2360
16. Chen Y, Gelfond JA, McManus LM, Shireman PK. Reproducibility of quantitative RT-PCR array in miRNA expression profiling and comparison with microarray analysis. BMC genomics. 2009;10:407
17. Callagy GM, Pharoah PD, Pinder SE. et al. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res. 2006;12(8):2468-2475
18. Kroman N, Mouridsen HT. Prognostic influence of pregnancy before, around, and after diagnosis of breast cancer. Breast (Edinburgh, Scotland). 2003;12(6):516-521
19. Bladstrom A, Anderson H, Olsson H. Worse survival in breast cancer among women with recent childbirth: results from a Swedish population-based register study. Clinical breast cancer. 2003;4(4):280-285
20. Halaska MJ, Pentheroudakis G, Strnad P. et al. Presentation, management and outcome of 32 patients with pregnancy-associated breast cancer: a matched controlled study. The breast journal. 2009;15(5):461-467
21. Iorio MV, Casalini P, Tagliabue E, Menard S, Croce CM. MicroRNA profiling as a tool to understand prognosis, therapy response and resistance in breast cancer. Eur J Cancer. 2008;44(18):2753-2759
22. Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26(19):2799-2803
23. Bourguignon LY, Xia W, Wong G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates beta-catenin signaling and NFkappaB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. The Journal of biological chemistry. 2009;284(5):2657-2671
24. Huang GL, Zhang XH, Guo GL. et al. Clinical significance of miR-21 expression in breast cancer: SYBR-Green I-based real-time RT-PCR study of invasive ductal carcinoma. Oncology reports. 2009;21(3):673-679
25. The world Health Organization Histological Typing of Breast Tumors--Second Edition. The World Organization. American journal of clinical pathology. 1982;78(6):806-816
26. Tsutsui S, Inoue H, Yasuda K. et al. Reduced expression of PTEN protein and its prognostic implications in invasive ductal carcinoma of the breast. Oncology. 2005;68(4-6):398-404
27. Chung MJ, Jung SH, Lee BJ, Kang MJ, Lee DG. Inactivation of the PTEN gene protein product is associated with the invasiveness and metastasis, but not angiogenesis, of breast cancer. Pathology international. 2004;54(1):10-15
28. Koninki K, Barok M, Tanner M. et al. Multiple molecular mechanisms underlying trastuzumab and lapatinib resistance in JIMT-1 breast cancer cells. Cancer letters. 2010;294(2):211-219
29. Zhang S, Yu D. PI(3)King Apart PTEN's Role in Cancer. Clin Cancer Res. 2010;16(17):4325-30
30. Lankat-Buttgereit B, Goke R. The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biology of the cell. 2009;101(6):309-317
31. Dawson SJ, Makretsov N, Blows FM. et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. British journal of cancer. 2010;103(5):668-675
32. Hsiao YH, Su YA, Tsai HD, Mason JT, Chou MC, Man YG. Increased invasiveness and aggressiveness in breast epithelia with cytoplasmic p63 expression. International journal of biological sciences. 2010;6(5):428-442
Author contact
![]() Corresponding author: Maria J Merino. Laboratory of Pathology, National Institutes of Health, National Cancer Institute, 10 Center Drive Bldg 10/Room 5B53, 9000 Rockville Pike, Bethesda, MD 20892, USA. Telephone number: 301-4963326; Fax number: 301-4801458; mjmerinonih.gov
Corresponding author: Maria J Merino. Laboratory of Pathology, National Institutes of Health, National Cancer Institute, 10 Center Drive Bldg 10/Room 5B53, 9000 Rockville Pike, Bethesda, MD 20892, USA. Telephone number: 301-4963326; Fax number: 301-4801458; mjmerinonih.gov

 Global reach, higher impact
Global reach, higher impact