Impact Factor
ISSN: 1837-9664
J Cancer 2011; 2:153-164. doi:10.7150/jca.2.153 This volume Cite
Research Paper
A Retrospective Review of the Frequency and Nature of Acute Hypersensitivity Reactions at a Medium-Sized Infusion Center: Comparison to Reported Values and Inconsistencies Found in Literature
1. Infusion Center, University of California, San Diego Moores Comprehensive Cancer Center
2. Department of Clinical Investigations, University of California, San Diego Moores Comprehensive Cancer Center
3. Department of Biostatistics, University of California, San Diego Moores Comprehensive Cancer Center
4. Department of Pharmacy, University of California, San Diego Moores Comprehensive Cancer Center
5. Department of Medicine, Division of Hematology and Oncology, University of California, San Diego School of Medicine and Medical Center
* These authors contributed equally to this work.
Received 2010-11-4; Accepted 2011-3-3; Published 2011-3-10
Abstract
Purpose: To evaluate acute hypersensitivity reactions at the UCSD Moores Cancer Center in San Diego, compare our findings to those reported previously in the literature, and examine the effectiveness of the objective grading scale as represented by the Common Terminology Criteria for Adverse Events (CTCAE).
Patients and Methods: Using the available pharmacy and electronic medical record data from 2006-2010, we examined our reported hypersensitivity reactions (HSRs) using the CTCAE v.3.0 and v.4.0. A thorough literature review was also performed to compare our findings with those previously reported.
Results: We found 222 cases of HSRs, of which 50% were due to immunotherapeutics. Most were grade 1 or 2 by any CTCAE criteria. The clinical presentation of HSRs varied between drug classes. Using different versions of grading schema led to inconsistencies in ~50% of all HSRs. Fifty-two percent of all cases not due to blood products were rechallenged on the same day. The reported literature HSR frequencies for each causative agent showed a striking variability, possibly indicating that previous studies used a wide variety of grading and reporting systems for adverse events.
Conclusion: HSRs are common in clinical practice, and most are mild or moderate. There are inconsistencies in reporting HSRs between studies. The existence of several grading schema and subjective definitions of hypersensitivity could be contributing to poor clinical generalizability. Along with an improved system of reporting HSRs to minimize underreporting, a standard system of objectively assessing HSRs is necessary for purposes of research and clinical practice.
Keywords: hypersensitivity reactions, immunotherapeutics, literature review
Introduction
Acute hypersensitivity reactions (HSRs) are a known source of great stress to patients, their families, nurses, other patients, and physicians1. In past assessments, 52% of a nursing staff has reported that infusion reactions are draining and frightening to them, and 42% of nurses feel that physicians do not adequately inform patients about the risk associated with an intravenous infusion2. Around 88% of outpatient and 62% of inpatient nurses consider infusion reactions frightening to other patients, with the potential to cause anxiety and confusion2,3. Since the opening of the Rebecca and John Moores UCSD Cancer Center (MCC) in San Diego, California in 1978, it was anecdotally believed that no patient had ever experienced a respiratory arrest-level HSR in the Infusion Center until May 2007, which prompted our clinical team to elucidate our adverse event profile, compare it to reports published previously, and review our practices regarding intravenous infusion of drugs with increased risk of hypersensitivity. The development of a variety of assessment tools, of novel therapies, and of evolving premedication schema in the past decade has made standardization of assessment challenging; this has resulted in substantial misrepresentation of HSR incidence and severity, both to patients and providers. To improve care for patients receiving these therapies, and to improve the safety and efficacy of outpatient administration for these therapies, we felt it necessary to evaluate the HSR environment; specifically, what should be addressed is the true frequency and incidence in the modern setting, the factors which providers take into account when assessing and treating HSRs, and the scientific soundness of certain treatment methods. Given the amount and variety of therapies administered at our center, it was felt to be an appropriate environment for such an initial evaluation.
Materials and Methods
The retrospective review was approved by the University of California, San Diego Institutional Review Board. HSRs taking place from June 2006 until January 2010 in the MCC were reported by clinical staff as part of the Infusion Center Standard Operating Procedure using the electronic Quality Variance Reporting (eQVR, Incident Reporting 2.0, University of California) system, a web-based event tracking system for collecting and analyzing data regarding patient care service quality. During this time the MCC administered over 30,850 infusions to about 4,000 patients. All HSRs reported by eQVR were reviewed twice, independently, by PAD and YM. Baseline data, including patient demographics, history of known allergies, premedications administered (and the adherence to this institution's existing standard premedication protocols), agent suspected of causing the HSR, signs and symptoms, reaction management, and the decision to same-day rechallenge were collected, using the PCIS (Siemens Invision Clinicals, Siemens Medical Solutions, Malvern, PA) and Hyperspace Clinical EMR (Epic Systems, Verona, WI) electronic medical record system. Since the eQVR links each HSR to a unique medical record number, the team verified that there were no duplicate HSR reports. Each HSR was graded retrospectively (not at time of event) using three separate grading systems - the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 Allergic Reaction/Hypersensitivity (AR), CTCAE v.3.0 Cytokine-Release Syndrome/Acute Infusion Reaction (CRS), and CTCAE v.4.0 Infusion-Related Reaction87. Reaction attribution to an agent was ascertained from practitioners' notes of the event in question. ADRs were analyzed for various characteristics using Microsoft Excel 2003 (Microsoft, Redmond, Washington). Total drug administration at the MCC was determined by utilizing three pharmacy drug database systems (PCIS, Siemens, Epic) used during the time period. A thorough literature review of package inserts, prospective and retrospective studies, and anecdotal case reports (dating from the time of the registrational clinical trials for each agent until 2010) was then performed in the interest of determining HSR frequency and incidence at other locations. This was done during 2010 by performing searches of the combinations of the terms “adverse effect,” “hypersensitivity,” “adverse reaction,” “adverse drug reaction” with the names of therapeutic agents in PubMed and MedLine, with 280 reports found. The selected 100 unique reports were in English with a primary focus on acute adverse effects of chemotherapeutic and biotherapeutic agent or experiences with acute hypersensitivity events in oncology outpatient settings. These reports were analyzed for the method of HSR assessment, HSR frequency and incidence, changes in administration practice, differences in perceived and actual HSR risk on the part of patients and providers, pharmacological HSR mechanisms, and, where applicable, impact on the institution.
Statistical Analysis
Cohen's weighted kappa was calculated to assess the consistency between CTCAE v.3.0 AR and CTCAE v.3.0 CRS, where large discrepancies between HSR grades are weighted more heavily than similar grades. The weighted kappa statistic was assessed using the Landis and Koch scale (1977), which translates the numerical score into categories of poor, slight, fair, moderate, substantial, or almost perfect. The average HSR grades were compared between subjects rechallenged and those that were not, overall and by drug category, using the Mantel-Haenszel chi-square test of a linear association.
Results
We found a total of 222 documented HSRs in our Infusion Center from June 2006 until January 2010. The median age of female and male patients was 47 and 69 years old, respectively; 59% of patients evaluated were women (Table 1). Patients were evaluated for average exposure to the causative agent at the time of the reaction, defined as the number of previous times that a patient received the therapy plus the one causing the HSR. Of medications, platinums were most likely to cause an HSR with extensive exposure. In 12 cases, HSRs took place on secondary exposure to a medication (i.e., re-treatment with the agent following disease recurrence for patients who had been treated with it after the initial diagnosis). For those, the average exposure to the agent was as follows: platinums, 2.3; immunotherapy, 1; iron products, 1.
Approximately 60% of patients had no known allergies and 18% were known to have a single allergen. There was no correlation between the number of known allergens and the likelihood of having a reaction to a particular agent or drug class.
Frequency and incidence of reactions
The total number of reactions by therapeutic agent, administrations of the HSR-causing agent, patients receiving the agent during the specified time period, rate of HSRs per administration ('administration frequency') and per patient ('incidence'), and values found in the literature are summarized in Table 2. When compared to individual agents, rituxumab caused the majority of HSRs (27%), followed by paclitaxel (10%). It should be noted that all iron agents (responsible for 5.9% of all HSRs) were classified together; because of the low HSR incidence to iron agents, they were not separated into low- and high-molecular weight preparations.
Patient demographics and prior allergy history.
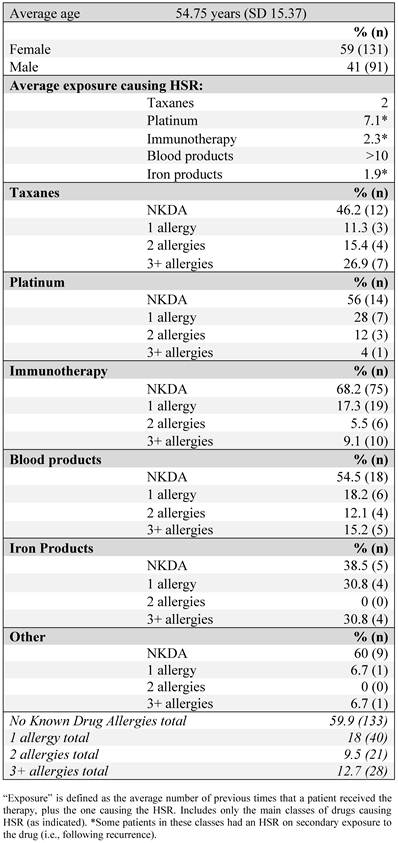
Total HSRs with drugs suspected of their causality, June 2006-January 2010.
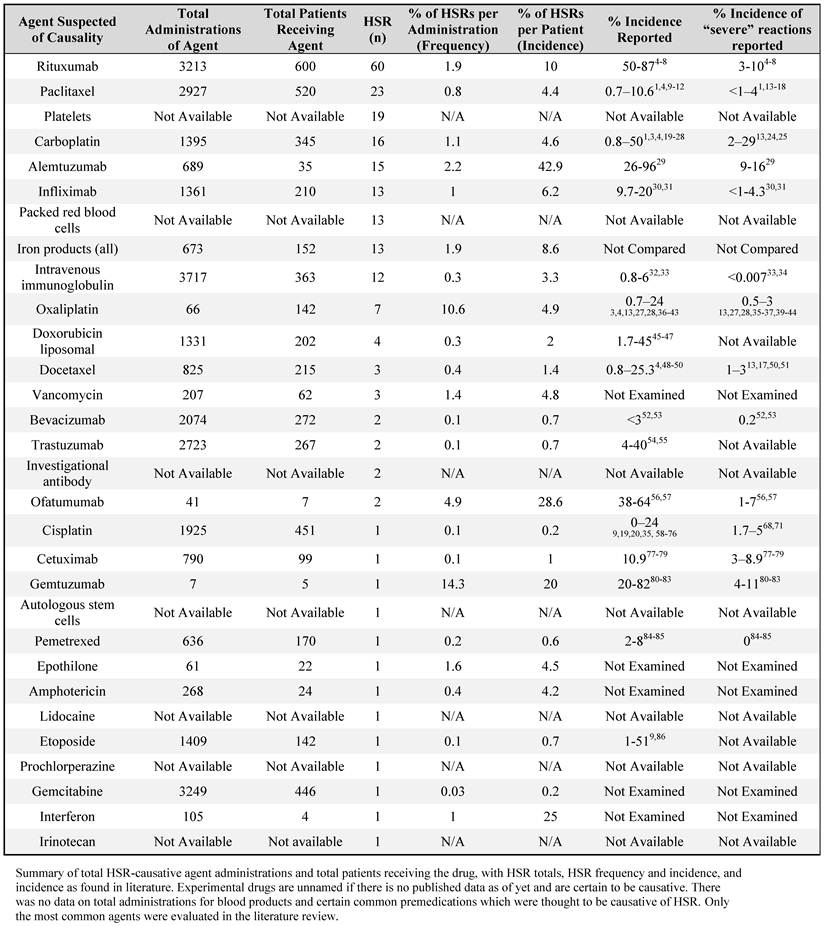
Total administration of certain agents could not be ascertained, either because they were too frequently used (as with reactions to premedications), experimental with yet-unpublished data, or otherwise unavailable to the study team (as with blood products). Values reported in the literature are noted in terms of general HSR incidence and incidence of severe HSR. Only the most common agents were evaluated in this literature review.
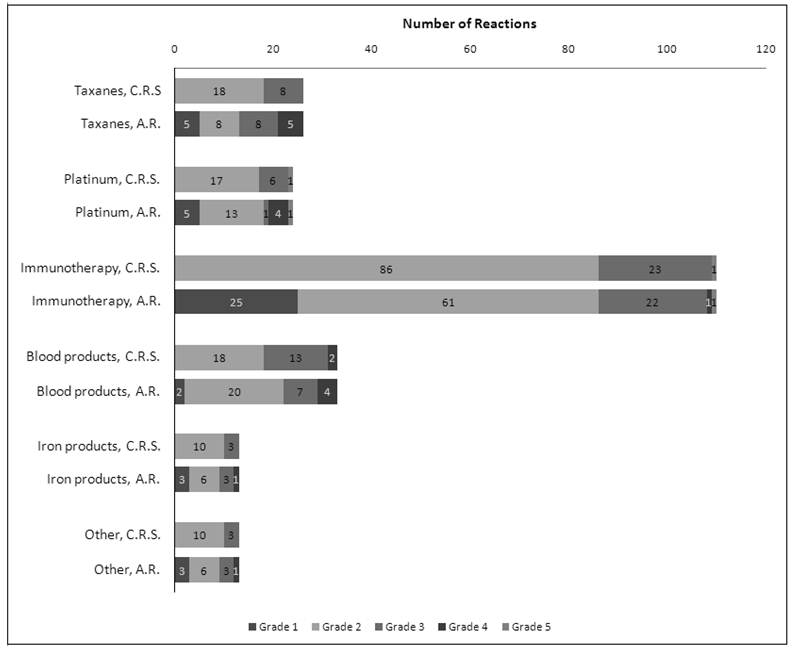
HSR-causative agents, as a percentage of the total number of HSRs, separated by drug class, June 2006-January 2010. The upper chart includes blood products. 'Other treatment' comprises all agents with a single HSR case. The lower chart is a breakdown of causative agents within the class of immunotherapeutic agents.
There were substantial differences between HSR administration frequency and incidence for several common HSR-causing agents; specifically, rituxumab had a 1.9% admininistration frequency and a 10% incidence, paclitaxel had a 0.8% administration frequency and a 4.4% incidence, and alemtuzumab had a 2.2% administration frequency and 42.9% incidence at our center.
The most commonly-administered drug at our center, of available data, was intravenous immunoglobulin (given 3,717 times), followed by gemcitabine (given 3,249 times) and rituxumab (given 3,213 times). However, rituxumab was given to the greatest number of patients (n=600), followed by paclitaxel (n=520) and cisplatin (n=451).
Figure 1 outlines the breakdown of causative agents into specific drug classes. Immunotherapeutics, as a class, accounted for half of the HSRs, with rituxumab accounting for 54% of the cases within the immunotherapeutic class. When blood products, accounting for 33 cases, are excluded, HSRs to immunotherapeutics account for 58% of all HSRs, taxanes 14%, platinum agents 13%, and iron products 7%. An investigational monoclonal antibody (still in clinical trials, with safety data unpublished) was felt to be responsible for 2 of the HSRs.
Reaction Severity
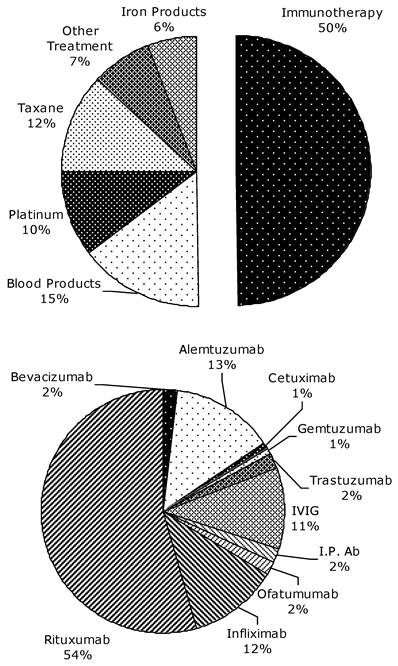
All sign, symptom and rechallenge data reported is provided on a per-HSR case, rather than per-patient, basis. Since only 8 patients experienced more than one HSR, the rates per HSR case should be similar to the rates per patient. The profile of the most commonly reported signs and symptoms per drug class is shown in Figure 2. The distribution of signs and symptoms reported at below 20% was more varied. Patients experiencing HSRs to taxanes reported the following signs or symptoms in more than 50% of cases: thoracic symptoms (chest pain, tightness, and pressure - 61.5%), respiratory symptoms (dyspnea, wheezing, and desaturation - 53.8%), and dermatological symptoms (46.1%). For platinum agents, these were respiratory symptoms (68%) and dermatological symptoms (64%). In regard to immunotherapeutic agents, blood products, and iron products, the most common findings were chills and rigors (46.4%), dermatological symptoms (36.4%), and both dermatological and respiratory symptoms (38.5%), respectively. The treatment methods for HSRs to each drug class were very similar, utilizing mainly diphenhydramine (>60% of all cases), and intravenous hydrocortisone and oxygen (≥20% of cases each). There was no deviation from this instution's standard premedication protocol in the case of any HSR.
HSRs graded with the 3 different criteria of the CTCAE demonstrated an evident difference depending on which criterion of the CTCAE v.3.0 (AR or CRS) was utilized (Figure 3). Using the CTCAE v.3.0 CRS and CTCAE v.4.0 Infusion-Related Reaction schema led to identical grade values. The HSR grades were consistent across all schemas in approximately 50% of cases, though this frequency was slightly lower among taxanes (34.6% consistency) and iron products (30.8% consistency). Overall, Cohen's weighted kappa was 0.487, a value representing “moderate agreement” by Landis and Koch's scale. Weighted kappa was lowest for iron products (0.255) and taxanes (0.287), and highest for blood products (0.562). Figure 4 illustrates both the relative totals of each grade as well as the differences in these totals as a result of different grading systems. The majority of HSRs were moderate-to-severe (CTCAE grade 2-3), with two HSRs resulting in death (one to carboplatin and one to gemtuzumab).
Rechallenge
There were 98 (52% of HSRs not due to blood products) attempts to rechallenge patients on the same day following HSR. Nearly all (n=92) were successful. Only 3 HSRs due to blood products were followed by rechallenge, and all were successful. Immunotherapeutics were rechallenged most often (69% of all rechallenged agents), with generally good success (only 5 cases could not be rechallenged successfully). Regardless of the grading method used, the overall HSR grade distribution was significantly lower for those reactions that were rechallenged than for those that were not (p-value<0.05). There was a larger difference in the HSR grade distributions between these groups when the CTCAE AR grading system was used. However, when restricted to immunotherapy reactions, the HSR grades distributions were essentially the same in these groups. Twenty-one cases were rechallenged following reactions that were grade 3-4 by both criteria.
Frequencies of the three most common signs and symptoms reported per drug class. Since each HSR may have presented with more than one symptom, the frequencies do not add to 100% in each class. Dermal symptoms include erythema, flushing, pruritis, and urticaria. Respiratory symptoms include dyspnea, wheezing, and desaturation. Thoracic symptoms include chest pain, tightness, and pressure (but do not include cardiac S&S). All reported S&S were those not present at baseline.
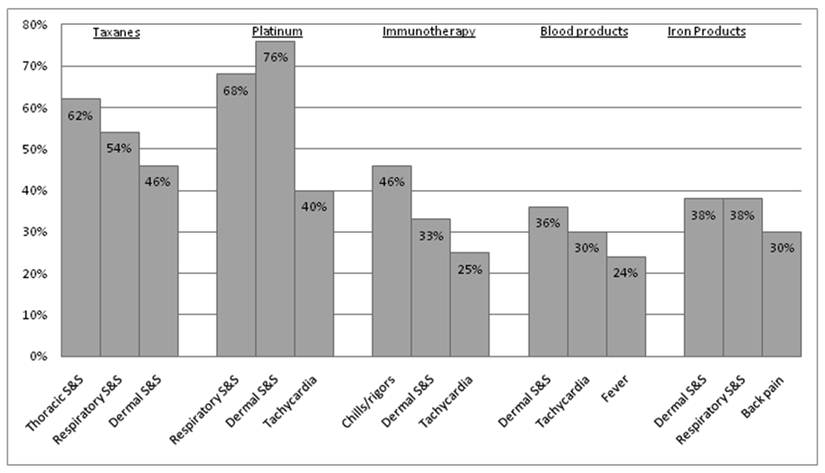
Breakdown of HSRs, June 2006-January 2010, as graded by CTCAE v3.0
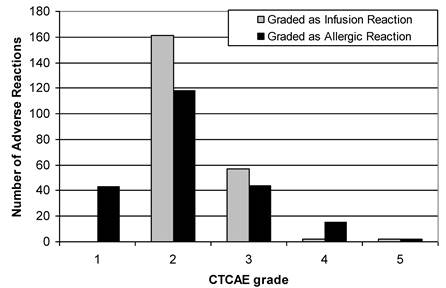
Differences in breakdown of HSRs of a particular grade when using CTCAE v.3. Cytokine Release Syndrome / Acute Infusion Reaction (C.R.S.) and CTCAE v.3 Allergic Reaction / Hypersensitivity (A.R.). There were no grade 1 reactions as per CTCAE v.3 Acute Hypersensitivity / Infusion Reaction.
Discussion
Severe HSRs are reported in ≤ 5% of all chemotherapy infusions, with platinum compounds and taxanes accounting for the greatest risk, but milder HSRs are certainly no rarity in any infusion center13. HSR risk is quoted by physicians when presenting treatment options to their patient and is utilized in appropriate infusion center staffing, so there is no question that the study of HSRs is one which will remain relevant.
Our review of the literature, while covering only major agents, revealed that there are enormous disparities in HSR risk not only between our data and published reports, but also among the reports themselves. There are substantial confounding factors which must be remembered when quoting reaction risk, including inconsistencies in the CTCAE v.3.0, the most commonly-used HSR assessment tool in oncology today88. The terminology currently used to describe an HSR is by no means standard.
The commonplace term 'allergic reaction' fell under criticism as scientifically inaccurate as early as 197973. An allergy - that is, a type I, IgE-mediated immune response - is facilitated by a sensitization period of repeat exposure to the allergen. HSRs to carboplatin and oxaliplatin support this feature; the incidence of HSRs per patient population increases with the number of doses given and in cases of documented occupational exposure to platinum salts. However, a longer platinum-free interval between courses of carboplatin has been correlated to an increased incidence of HSR1,3,19,20,22-28,37,40,89. The IgE-mediated mechanism is thus not wholly accurate, and has led researchers to question the validity of the reaction as an allergic one, to consider the possibility of a non-immunological histamine release, and to even view the nature of a HSR as idiosyncratic19,23.
Similarly, there is a lack of consensus regarding mechanisms of HSRs to monoclonal antibodies. Frequent initial-exposure reactions to cetuximab, alemtuzumab, and rituxumab counter IgE-mediated hypersensitivity5,7,29; infliximab, however, is known to cause reactions after multiple rounds of therapy90,91.
There is a great deal of conviction, however, that taxane HSRs are non-IgE-mediated. These HSRs are most frequent at first or second exposure, are severe only during these administrations, nearly all patients rechallenged after the first administration are able to tolerate subsequent cycles, and they are dose- and rate-dependent12,17,49,92. Nonetheless, the majority of studies reviewed here used the CTCAE AR grading criteria.
Today's premedication protocols do not always reflect those environments in which trials were initially conducted. Many early cisplatin trials did not utilize glucocorticoids and antihistamines, as is commonly done today9,58,59,61,62,64,68,69,73, which may account for the decreased incidence in more recent studies. There are, additionally, documented decreases of over 50% in HSR incidence (general and severe) in trials where premedication for docetaxel and paclitaxel was standard11,14-16,48,49,51,93-98. Citation errors remain; as Weiss and colleagues have noted, citation of older publications with a different premedication protocol as references in modern reports has led to significant discrepancies.
In clinical trials, investigators continue to employ a wide variety of grading scales. The CTCAE itself has undergone several revisions (v.4 released May 2009), but as late as 2003, teams have used early versions of the CTCAE for assessing severity of HSRs81,82. The World Health Organization, Eastern Cooperative Oncology Group, and Radiation Therapy Oncology Group sometimes use their own scales for grading allergic reactions. Furthermore, the majority of trials reviewed here used a subjective variety of terms to define a reaction; the definitions of each of these terms, where provided, infrequently corresponded to those in the CTCAE. Some studies graded each sign and symptom of a HSR separately using the CTCAE, rather than as general condition. At least one team has proposed a completely new, 3-grade, system for anaphylaxis whereas another has suggested elimination of the anaphylaxis category altogether99-102.
The CTCAE v.3.0 itself poses another problem. In using AR and CRS scales, the same hypersensitivity reaction can be graded as moderate (grade 2), severe (grade 3), or life-threatening (grade 4); this was evident at our center. Moreover, the CTCAE v.3.0 indicates parenteral rescue medications in grade ≥3 allergic reactions, whereas these are given at the first, mildest, sign of a HSR in most infusion centers88, including ours. Many inconsistencies have been removed in the CTCAE v.4.0, which provides nearly identical gradations of allergic reactions and infusion-related reactions, and features a new category, anaphylaxis, which is consistent with the other relevant categories.
Some reports provide a risk of HSR per number of infusions and others per number of patients, which adds an additional layer of inconsistency. This is not irrelevant; as our data indicates, the difference in reported percentages can be hundredfold (Table 2 subselects for those reports which provided incidence figures in the same manner as do we). Often, the calculation method is not specified in published reports, establishing a potential for misquoting. Our review showed at least three published articles citing previous research erroneously.
The importance of consistent grading is not trivial; reported differences in percentages can lead to misconceptions about HSRs and their management, misinformation given to patients as they prepare to initiate treatment, and inappropriate staffing in infusion centers. Only with a variety of prospective evaluations of therapies using a standardized grading scheme will we understand the true reaction frequencies, an exact profile, and an evidence-based method for the decision to rechallenge.
While the most obvious use of a standard grading system is in data reporting for clinical research, the grade for a HSR can have bearing on the physician's clinical judgment. Another past literature review indicated that patients who had a mild-to-moderate (grade 1-2) reaction on first exposure are likely to tolerate rechallenge with a drug; this is contraindicated in patients having a grade 3-4 reaction13. However, some reports asserted that mild-to-moderate reactions to monoclonal antibodies only require a decrease of rate, rather than cessation altogether103. Immediate re-treatment, particularly on the same day with the same preparation, is especially important for outpatient treatment centers and their patients. If performed properly, it can result in the minimizing of treatment time and costs without adversely affecting patient safety12.
This topic should also continue to be explored for educational purposes. At our institution, the full-time staffing of the Infusion Center with dedicated Physician Assistants experienced in the medical management of patients experiencing HSRs has resulted in high rechallenge success rates and comprehensive management of HSRs. However, we found that the likelihood of the decision to rechallenge taxanes (a drug class with an unclear rechallenge indication) increased over time, with no similar trend in average grade of the reaction; coincidentally, the timeline of this study paralleled the initiation of a mid-level practitioner in the Infusion Center. This suggests that the deciding practitioner's knowledge of HSR management increases with time and is the key factor in making such a decision.
Finally, the study of HSR mechanism remains significant. Although IgE-mediated and non-IgE-mediated reactions can be similar in clinical presentation, they are vastly disparate in mode of development, and this may have bearing on the pharmacological interaction of rescue medication90. Whether the mechanism of a reaction is relevant to clinical management remains under debate19. Specifically, one report pointed out that treatment of a docetaxel HSR with antihistamines may be detrimental, as doing so inhibits cytochrome P450, which is responsible for docetaxel elimination49. Since many sites, including ours, have a standing protocol for HSRs which includes antihistamines, an intravenous steroid, and possibly epinephrine, it is worthwhile to extend research in this area13,103.
In this study, HSR frequency and incidence was based solely on eQVR reporting, and there is a strong suspicion of underreporting or erroneous reporting of HSR frequency by eQVR; thus, there may be an underestimation of true reaction frequency and incidence (particularly among milder HSRs). In addition, this study was a retrospective analysis of a single-center experience at an academic medical center; thus, extrapolation of the findings to other institutions must be done with care. We have implemented a comprehensive, prospective, multi-year study of HSRs at the MCC Infusion Center which bypasses use of the eQVR system; however, continued study of HSRs at other institutions is necessary to validate the conclusions.
Conclusion
The findings presented here indicate an inadequacy in the systematic reporting of acute hypersensitivity reactions to non-oral medications. HSR incidence tends to vary widely between reports, and thus the incidence and characteristics at MCC tend to reflect some, while strongly conflicting with others. A variety of CTCAE criteria and interpretations, difference in reporting rates as administration frequency versus incidence, an evolution of premedication, and citation errors all contribute to this issue. Since a multitude of clinical decisions is based upon the conclusions of reported literature, it is necessary to devise or formally adopt a system used universally for reporting HSRs. Finally, modern word processing software should be utilized to minimize referencing errors. The findings must be validated in larger, multi-center settings with special emphasis on preventing underreporting or erroneous reporting.
Acknowledgements
The team would like to thank the pharmacy and nursing staff at the UCSD Moores Cancer Center for their assistance with this study and for their continued dedication to patient care.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Markman M, Kennedy A, Webster K. et al. Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol. 2000;18:102-5
2. Colwell HH, Mathias SD, Ngo NH. et al. The impact of infusion reactions on oncology patients and clinicians in the inpatient and outpatient practice settings: oncology nurses' perspectives. J Infus Nurs. 2007;30:153-60
3. Bonosky K, Miller R. Hypersensitivity reactions to oxaliplatin: what nurses need to know. Clin J Oncol Nurs. 2005;9:325-30
4. Escalante CP, Oh JH, Baum DD. et al. Immediate adverse reactions to chemotherapy: Experience of a large ambulatory treatment center. ASCO Annual Meeting. J Clin Oncol. 2006:8558
5. Genentech Inc. Rituxan (rituximab) [package insert]. South San Francisco, California: Genentech Inc. 2008
6. Plosker GL, Figgitt DP. Rituximab: a review of its use in Non-Hodgkin's Lymphoma and Chronic Lymphocytic Leukaemia. Drugs. 2003;63:803
7. Onrust SV, Lamb HM, Barman Balfour JA. Rituximab. Drugs. 1999;58:79-88
8. Sehn LH, Donaldson J. et al. Rapid infusion rituximab in combination with steroid-containing chemotherapy can be given safely and substantially reduces resource utilization. Blood. 2004;104:1407
9. Weiss RB. Hypersensitivity reactions. Semin Oncol. 1992;19:458-77
10. Feldweg AM, Lee CW, Matulonis UA. et al. Rapid desensitization for hypersensitivity reactions to paclitaxel and docetaxel: a new standard protocol used in 77 successful treatments. Gynecol Oncol. 2005;96:824-9
11. Weiss RB, Donehower RC, Wiernik PH. et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263-8
12. Olson JK, Sood AK, Sorosky JI. et al. Taxol hypersensitivity: rapid retreatment is safe and cost effective. Gynecol Oncol. 1998;68:25-8
13. Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601-9
14. Zanotti KM, Markman M. Prevention and management of antineoplastic-induced hypersensitivity reactions. Drug Saf. 2001;24:767-79
15. Myers JS. Paclitaxel-related hypersensitivity reactions and monitoring recommendations. Clin J Oncol Nurs. 2002;6:177-8
16. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332:1004-14
17. Shepherd GM. Hypersensitivity reactions to chemotherapeutic drugs. Clin Rev Allergy Immunol. 2003;24:253-62
18. Teva Pharmaceuticals. Paclitaxel injection [package insert]. Irvine, California: Teva Pharmaceuticals. 2008
19. Saunders MP, Denton CP, O'Brien ME. et al. Hypersensitivity reactions to cisplatin and carboplatin--a report on six cases. Ann Oncol. 1992;3:574-6
20. Weidmann B, Mulleneisen N, Bojko P. et al. Hypersensitivity reactions to carboplatin. Report of two patients, review of the literature, and discussion of diagnostic procedures and management. Cancer. 1994;73:2218-22
21. Teva Parenteral Medicines. Carboplatin injection [package insert]. Irvine, California: Teva Parenteral Medicines. 2007
22. Hendrick AM, Simmons D, Cantwell BM. Allergic reactions to carboplatin. Ann Oncol. 1992;3:239-40
23. Morgan JS, Adams M, Mason MD. Hypersensitivity reactions to carboplatin given to patients with relapsed ovarian carcinoma. Eur J Cancer. 1994;30A:1205-6
24. Polyzos A, Tsavaris N, Kosmas C. et al. Hypersensitivity reactions to carboplatin administration are common but not always severe: a 10-year experience. Oncology. 2001;61:129-33
25. Schwartz JR, Bandera C, Bradley A. et al. Does the platinum-free interval predict the incidence or severity of hypersensitivity reactions to carboplatin? The experience from Women and Infants' Hospital. Gynecol Oncol. 2007;105:81-3
26. Tonkin KS, Rubin P, Levin L. Carboplatin hypersensitivity: case reports and review of the literature. Eur J Cancer. 1993;29A:1356-7
27. Brandi G, Pantaleo MA, Galli C. et al. Hypersensitivity reactions related to oxaliplatin (OHP). Br J Cancer. 2003;89:477-81
28. Gowda A, Goel R, Berdzik J. et al. Hypersensitivity Reactions to oxaliplatin: incidence and management. Oncology. 2004;18:1671-5
29. Bayer Healthcare Pharmaceuticals Inc. Campath (alemtuzumab) [package insert]. Wayne, New Jersey: Bayer Healthcare Pharmaceuticals Inc. 2008
30. Centocor Ortho Biotech Inc. Infliximab (Remicade for IV injection) [package insert]. Malvern, Pennsylvania: Centocor Ortho Biotech Inc. 2009
31. Schaible TF. Long-term safety of infliximab. Can J Gastroenterol. 2000;14(Suppl C):29C-32C
32. Misbah SA, Chapel HM. Adverse effects of intravenous immunoglobulin. Drug Saf. 1993;9:254-62
33. Gurcan HM, Ahmed AR. Frequency of adverse events associated with intravenous immunoglobulin therapy in pemphigus or pemphigoid. Ann Pharmacother. 2007;41:1604-10
34. Brennan VM, Salome-Bentley NJ, Chapel HM. Prospective audit of adverse reactions occurring in 459 primary antibody-deficient patients receiving intravenous immunoglobulin. Clin Exp Immunol. 2003;133:247-51
35. Elligers KT, Davies M, Sanchis D. et al. Rechallenge with cisplatin in a patient with pancreatic cancer who developed a hypersensitivity reaction to oxaliplatin. Is skin test useful in this setting? J Pancreas. 2008;9:197-202
36. de Lemos ML. Acute reactions to chemotherapy agents. J Oncol Pharm Pract. 2006;12:127-9
37. Gammon D, Bhargava P, McCormick MJ. Hypersensitivity reactions to oxaliplatin and the application of a desensitization protocol. Oncologist. 2004;9:546-9
38. Meyer L, Zuberbier T, Worm M. et al. Hypersensitivity reactions to oxaliplatin: cross-reactivity to carboplatin and the introduction of a desensitization schedule. J Clin Oncol. 2002;20:1146-7
39. Saif MW. Hypersensitivity reactions associated with oxaliplatin. Expert Opin Drug Saf. 2006;5:687-94
40. Siu SW, Chan RT, Au GK. Hypersensitivity reactions to oxaliplatin: experience in a single institute. Ann Oncol. 2006;17:259-61
41. Thomas RR, Quinn MG, Schuler B. et al. Hypersensitivity and idiosyncratic reactions to oxaliplatin. Cancer. 2003;97:2301-7
42. Syrigou EI, Karapanagiotou EM, Alamara CV. et al. Hypersensitivity reactions to oxaliplatin: a retrospective study and the development of a desensitization protocol. Clin Colorectal Cancer. 2009;8:106-9
43. Andre T, Boni C, Mounedji-Boudiaf L. et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-51
44. Teva Parenteral Medicines. Oxaliplatin for injection [package insert]. Irvine, California: Teva Parenteral Medicines. 2009
45. Gabizon AA. Pegylated Liposomal Doxorubicin:Metamorphosis of an old drug into a new form of chemotherapy. Cancer Inv. 2001;19:424-36
46. Chanan-Khan A, Szebeni J, Saway S. et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil®): possible role in hypersensitivity reactions. Ann Oncol. 2003;14:1430-37
47. Skubitz KM. Phase II Trial of pegylated-liposomal doxorubicin (Doxil) in sarcoma. Cancer Inv. 2003;21:167-76
48. Lee C, Gianos M, Klaustermeyer WB. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann Allergy Asthma Immunol. 2009;102:179-87
49. Ardavanis A, Tryfonopoulos D, Yiotis I. et al. Non-allergic nature of docetaxel-induced acute hypersensitivity reactions. Anticancer Drugs. 2004;15:581-5
50. Sanofi-Aventis US. Taxotere (Docetaxel injection concentrate) [package insert]. Bridgewater, New Jersey: Sanofi-Aventis US. 2008
51. Bernstein BJ. Docetaxel as an alternative to paclitaxel after acute hypersensitivity reactions. Ann Pharmacother. 2000;34:1332-5
52. Genentech Inc. Avastin (bevacizumab) [package insert]. South San Francisco, CA: Genentech Inc. 2007
53. Reidy DL, Chung KY, Timoney JP. et al. Bevacizumab 5 mg/kg can be infused safely over 10 minutes. J Clin Oncol. 2007;25:2691-5
54. Genentech Inc. Herceptin (trastuzumab) [package insert]. South San Francisco, California: Genentech Inc. 2003
55. Beeram M, Burris HA, Modi S. et al. A phase I study of trastuzumab-DM1, a first-in-class HER2 antibody-drug conjugate (ADC), given every 3 weeks to patients with HER2+ metastatic breast cancer. Presented at the American Society of Clinical Oncology Annual Meeting, Chicago, Illinois, May 29-June 3. 2008
56. Wierda WG, Kipps TJ, Mayer J. et al. Ofatumumab As Single-Agent CD20 Immunotherapy in Fludarabine-Refractory Chronic Lymphocytic Leukemia. J Clin Oncol. 2010;28:1749-55
57. Castillo J, Milani C, Mendez-Allwood J. Ofatumumab, a second-generation anti-CD20 monoclonal antibody, for the treatment of lymphoproliferative and autoimmune disorders. Expert Opin Investig Drugs. 2009;18:491-500
58. Anderson T, Javadpour N, Schilsky R. et al. Chemotherapy for testicular cancer: current status of the National Cancer Institute Combined Modality Trial. Cancer Treat Rep. 1979;63:1687-92
59. Baum ES, Gaynon P, Greenberg L. et al. Phase II study of cis-dichlorodiammineplatinum(II) in childhood osteosarcoma: Children's Cancer Study Group Report. Cancer Treat Rep. 1979;63:1621-7
60. Blumenreich MS, Needles B, Yagoda A. et al. Intravesical cisplatin for superficial bladder tumors. Cancer. 1982;50:863-5
61. Cheng E, Cvitkovic E, Wittes RE. et al. Germ cell tumors (II): VAB II in metastatic testicular cancer. Cancer. 1978;42:2162-8
62. Gralla RJ, Casper ES, Kelsen DP. et al. Cisplatin and vindesine combination chemotherapy for advanced carcinoma of the lung: A randomized trial investigating two dosage schedules. Ann Intern Med. 1981;95:414-20
63. Koren C, Yerushalmi R, Katz A. et al. Hypersensitivity reaction to cisplatin during chemoradiation therapy for gynecologic malignancy. Am J Clin Oncol. 2002;25:625-6
64. Merrin CE. Treatment of genitourinary tumours with cis-dichlorodiammineplatinum(II): experience in 250 patients. Cancer Treat Rep. 1979;63:1579-84
65. O'Brien ME, Souberbielle BE. Allergic reactions to cytotoxic drugs-an update. Ann Oncol. 1992;3:605-10
66. Rozencweig M, von Hoff DD, Slavik M. et al. Cis-diamminedichloroplatinum (II). A new anticancer drug. Ann Intern Med. 1977;86:803-12
67. Shlebak AA, Clark PI, Green JA. Hypersensitivity and cross-reactivity to cisplatin and analogues. Cancer Chemother Pharmacol. 1995;35:349-51
68. Thigpen T, Shingleton H, Homesley H. et al. cis-Dichlorodiammineplatinum(II) in the treatment of gynecologic malignancies: phase II trials by the Gynecologic Oncology Group. Cancer Treat Rep. 1979;63:1549-55
69. Von Hoff DD, Schilsky R, Reichert CM. et al. Toxic effects of cis-dichlorodiammineplatinum(II) in man. Cancer Treat Rep. 1979;63:1527-31
70. Weiss RB. Hypersensitivity reaction to cancer chemotherapy. Semin Oncol. 1982;9:5-13
71. Weiss RB, Bruno S. Hypersensitivity reactions to cancer chemotherapeutic agents. Ann Intern Med. 1981;94:66-72
72. Planner RS, Weerasiri T, Timmins D. et al. Hypersensitivity reactions to carboplatin. J Natl Cancer Inst. 1991;83:1763-4
73. Wiesenfeld M, Reinders E, Corder M. et al. Successful re-treatment with cis-dichlorodiammineplatinum(II) after apparent allergic reactions. Cancer Treat Rep. 1979;63:219-21
74. Gottlieb JA, Drewinko B. Review of the current clinical status of platinum coordination complexes in cancer chemotherapy. Cancer Chemother Rep. 1975;59:621-8
75. Khan A, Hill JM, Grater W. et al. Atopic hypersensitivity to cisdichlorodiammine platinum (II) and other platinum complexes. Cancer Res. 1975;35:2766-70
76. Zanotti KM, Rybicki LA, Kennedy AW. et al. Carboplatin Skin Testing: A Skin-Testing Protocol for Predicting Hypersensitivity to Carboplatin Chemotherapy. J Clin Oncol. 2001;19:3126-9
77. Cunningham D, Humblet Y, Siena S. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal carcinoma. N Engl J Med. 2004;351:337-45
78. Waqar SN, Tan BR, Zubal B. et al. Race and albuterol premedication are risk factors for hypersensitivity reactions to cetuximab (abstract 20503). J Clin Oncol. 2008;26:a20503
79. ImClone Systems Inc and Bristol-Myers Squibb Company. Erbitux (cetuximab) [package insert]. Branchburg and Princeton, New Jersey: ImClone Systems Inc and Bristol-Myers Squibb Company. 2004
80. Wyeth Pharmaceuticals. Mylotarg (gemtuzumab) [package insert]. Philadelphia, Pennsylvania: Wyeth Pharmaceuticals. 2008
81. Giles FJ, Cortes JE, Halliburton JA. et al. Intravenous corticosteroids to reduce gemtuzumab ozogamicin infusion reactions. Ann Pharmacotherap. 2003;37:1182-5
82. Sievers EL, Larson RA, Stadtmauer EA. et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244-54
83. Leopold LH, Berger MS, Feingold J. Acute and long-term toxicities associated with gemtuzumab ozogamicin (Mylotarg) therapy of acute myeloid leukemia. Clin Lymphoma. 2002;2(Suppl 1):S29-34
84. Eli Lilly and Company. Alimta (pemetrexed) [package insert]. Indianapolis, Indiana: Eli Lilly and Company. 2005
85. Hanna N, Shepherd FA, Fossella FV. et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with nonsmall cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589-97
86. Hudson MM, Weinstein HJ, Donaldson SS. et al. Acute hypersensitivity reactions to etoposide in a VEPA regimen for Hodgkin's disease. J Clin Oncol. 1993;11:1080-4
87. The Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, versions 3.0 and 4.0. http://ctep.cancer.gov/forms
88. Cmelak AJ, Lordick F, Borner M. et al. Management of infusion reactions in clinical trials and beyond - the US and EU perspectives. Oncology. 2009;23(Suppl 1):18
89. Freedman SO, Krupey J. Respiratory allergy caused by platinum salts. J Allergy. 1968;42:233-237
90. Chung CH, O'Neil BH. Infusion reactions to monoclonal antibodies for solid tumors: immunologic mechanisms and risk factors. Oncology. 2009;23:14-7
91. Baert F, Noman M, Vermeire S. et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601-8
92. Peereboom DM, Donehower RC, Eisenhauer EA. et al. Successful retreatment with Taxol after major hypersensitivity reactions. J Clin Oncol. 1993;11:885-90
93. Schrijvers D, Wanders J, Dirix L. et al. Coping with toxicities of docetaxel (Taxotere). Ann Oncol. 1993;4:610-1
94. Tankanow RM. Docetaxel: a taxoid for the treatment of metastatic breast cancer. Am J Health Syst Pharm. 1998;55:1777-91
95. Verweij J, Catimel G, Sulkes A. et al. Phase II studies of docetaxel in the treatment of various solid tumours. Eur J Cancer. 1995;31A(Suppl 4):S21-S24
96. Wanders J, Schrijvers D, Bruntsch U. The EORTC-ECTG experience with acute hypersensitivity reactions in Taxotere studies. Proc Am Soc Clin Oncol. 1993;12:73
97. Verwey J, Clavel M, Chevalier B. Paclitaxel (Taxol) and Docetaxel (Taxotere): not simply two of a kind. Ann Oncol. 1994;5:495-505
98. Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD. et al. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol. 1994;12:2654-66
99. Brown AF, McKinnon D, Chu K. Emergency Department anaphylaxis: a review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108:861-866
100. Gleich GJ, Leiferman KM. Anaphylaxis: Implications of monoclonal antibody use in oncology. Oncology. 2009;23:7-13
101. Bailie GR, Clark JA, Lane CE. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant. 2005;20:1443-9
102. Sanders RP, Geiger TL, Heddle N. et al. A revised classification scheme for acute transfusion reactions. Transfusion. 2007;47:621-8
103. Chung CH. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist. 2008;13:725-32
Author contact
![]() Corresponding author: Yuri Matusov, Clinical Trials Office, University of California, San Diego Moores Cancer Center, 3855 Health Sciences Drive, MC 0698, La Jolla, California 92093-0698. Tel: (858) 246-0357; Fax: (858) 822-5380; Email: matusovedu
Corresponding author: Yuri Matusov, Clinical Trials Office, University of California, San Diego Moores Cancer Center, 3855 Health Sciences Drive, MC 0698, La Jolla, California 92093-0698. Tel: (858) 246-0357; Fax: (858) 822-5380; Email: matusovedu

 Global reach, higher impact
Global reach, higher impact