Impact Factor
ISSN: 1837-9664
J Cancer 2011; 2:228-231. doi:10.7150/jca.2.228 This volume Cite
Case Report
Unusual Ovarian Stromal Tumor with Radiation Changes Mimicking Carcinoma
Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, USA.
Received 2011-3-23; Accepted 2011-4-26; Published 2011-4-27
Abstract
Radiation-related changes including fibrosis, nuclear enlargement, hyperchromasia and cytoplasmic vacuolization may alter the appearance of normal ovarian tissue and ovarian tumors. We describe radiation-related changes in ovarian stromal neoplasm with mixed features of sclerosing stromal tumor and fibrothecoma. The right ovarian mass was discovered in a 38 year-old woman with past history of invasive squamous cell carcinoma of the cervix treated with cone biopsy and brachytherapy. The low power architecture of cellular pseudolobules and small sheets of tumor cells with scattered hyaline plaques was consisted with the pattern of combined sclerosing stromal tumor and fibrothecoma. However, the presence of severe cytologic atypia, as well as clear cell and signet ring differentiation and arrangements of tumor cells in single files and nests, raised a possibility of primary or metastatic carcinoma. The tumor cells were positive for calretinin, vimentin, inhibin, and WT1 and negative for AE1/3, cytokeratin 7 and 20, CD99, estrogen and progesterone receptors, mammaglobin, chromogranin, and S100 protein. Based on the results of immunostains and a subsequently provided history of radiation, a diagnosis of sex cord stromal tumor with mixed fibrothecoma and sclerosing stromal differentiation was made. Radiation-related atypia and fibrosis in sex cord stromal tumor may create a pattern mimicking carcinoma and therefore, in the presence of unusual histology, the use of immunohistochemistry is recommended.
Keywords: ovary, immunohistochemistry, sclerosing stromal tumor, fibrothecoma, radiation-related changes
Introduction
The ovary is one of the most radiosensitive organs and its function is affected even with low doses of radiation. Radiation treatment changes the appearance of normal ovarian tissue and ovarian tumors and causes ovarian failure (1-3). Typical changes in the normal ovary include destruction of primordial follicles, stromal fibrosis, and sclerosis of blood vessels. Radiation changes in benign and malignant ovarian tumors may create a histologic pattern that is difficult to interpret. To our knowledge, there are no previous reports of radiation-related changes in ovarian sex cord-stromal tumors. We present an unusual stromal neoplasm with features of sclerosing stromal tumor and fibrothecoma (1, 4, 5) in a patient previously treated with radiation for carcinoma of the cervix. The differential diagnosis and the use of immunohistochemical stains are discussed.
Case Report
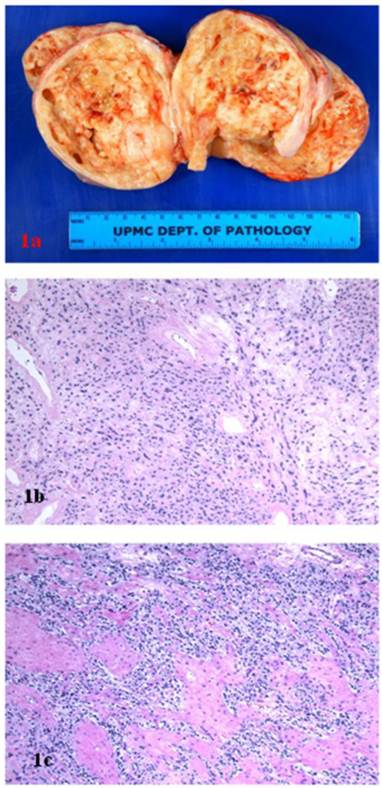
A 38 year-old woman gravida 1 and para 0 presented with a pelvic mass discovered during the work-up following treatment for cervical cancer. Her past medical history included Stage IIB invasive squamous cell carcinoma of the cervix, treated with cone biopsy and brachytherapy. On an ultrasound, a large heterogeneous mass on the right side of the uterus was identified. A positron-emission tomography/computed tomography scan showed a heterogeneously enhancing tumor arising from the right adnexa/broad ligament, with borderline FDG activity consistent with benign characteristics. Magnetic resonance imaging revealed a 10.1 x 10.1 x 10.2 cm heterogeneously enhancing right solid ovarian mass. With preoperative diagnosis of ovarian tumor, the patient underwent bilateral salpingo-oophorectomy. The right tubo-ovarian complex weighed 356 grams and included ovary replaced by 11.5 X 9.5 x 7.0 cm solid mass and an unremarkable fallopian tube. The surface of ovarian tumor was tan-pink and smooth, with focal hemorrhagic adhesions. The cut sections revealed solid tan-white and nodular, fleshy tissue, with multiple cystic spaces filled with clear serous fluid (Fig 1A). The opposite ovary and fallopian tube were grossly unremarkable.
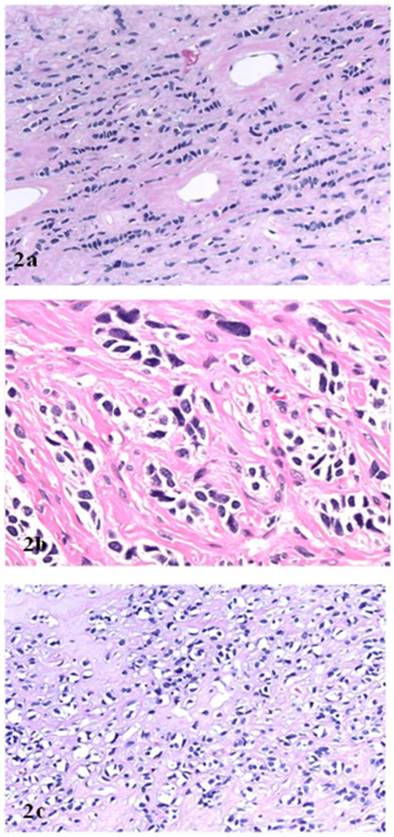
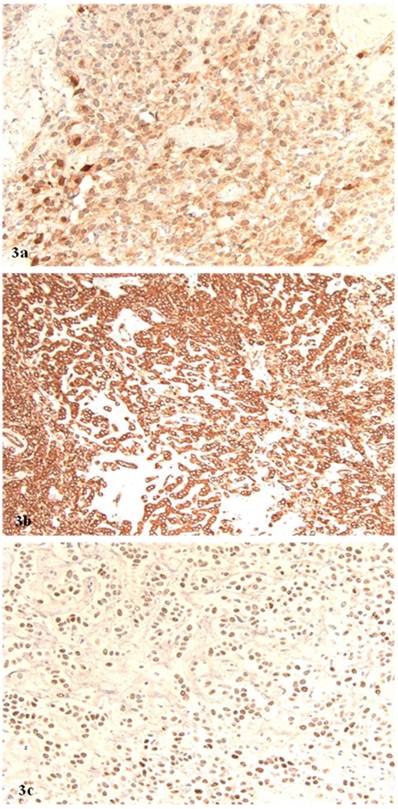
Microscopically, the right ovarian tumor showed two different patterns. One consisted of rounded cellular pseudolobules embeded in the fibrous stroma (Fig 1B), the other of solid sheets or separate small nests of tumor cells with scattered hyaline plaques (Fig 1C). The pseudolobules contained mostly small cells with scant cytoplasm and hyperchromatic nuclei, often arranged in cords mimicking single files of metastatic lobular carcinoma (Fig 2A). The tumor cells forming solid sheets were larger with hyperchromatic nuclei and clear, vacuolated cytoplasm (Fig 2B). In some areas, cytoplasmic vacuoles pushed nuclei to the periphery creating signet-ring pattern (Fig 2C). The mitotic activity was 0 per 10 high power fields. Numerous thick-walled blood vessels and areas of cystic degeneration and edema were present throughout the tumor. No definite diagnosis was made on an intraoperative consultation. Although the low power architecture favored stromal tumor, the high power features especially cytologic atypia, clear cell and signet-ring differentiation were suggestive of primary or metastatic carcinoma. The results of the immunostains included: positive nuclear and cytoplasmic staining for calretinin (Fig 3A), positive membranous and cytoplasmic staining for vimentin, positive nuclear staining for WT (Fig 3C) and focally positive cytoplasmic staining for inhibin 1. The immunostains for CD99, epithelial markers (AE1/AE3, CK 7 and CK 20), estrogen receptor (ER), progesterone receptor (PR), mammaglobin, chromogranin, and S100 protein were negative. Based on the history of radiation treatment and immunohistochemical profile, a diagnosis of ovarian stromal tumor with features of fibrothecoma and sclerosing stromal tumor and superimposed radiation effect was made. The opposite ovary showed changes consistent with status post radiation treatment including stromal fibrosis, prominent thick walled blood vessels and foci of foamy histiocytes, and hemosiderin-laden macrophages.
A. Ovarian stromal tumor, gross photograph. B. Sclerosing stromal tumor component (hematoxillin and eosin, original magnification 20 x). C. Fibrothecoma component (hematoxillin and eosin, original magnification 20 x).
A. “Single file” pattern (hematoxillin and eosin, original magnification 40 x). B. “Clear cell” differentiation (hematoxillin and eosin, original magnification 40 x). C. “Signet ring cell” differentiation (hematoxillin and eosin, original magnification 40 x).
A. Positive nuclear and cytoplasmic staining for calretinin (original magnification, 40 x). B. Positive cytoplasmic staining for vimentin (original magnification 40 x). C. Positive nuclear staining for WT1 (original magnification 40 x).
Discussion
Radiation treatment damages DNA of tumor cells and surrounding normal tissues. The damage is either a direct result of radiation or is due to exposure to free radicals produced by the interaction of radiation and water within the cell. Typical morphologic changes in tissues subjected to radiation affect both, the epithelium and the stroma (1-3). The nuclei of epithelial cells become enlarged, pleomorphic and hyperchromatic and show irregular chromatin clumping. The cytoplasm develops granular, vacuolated, or clear appearance due to the dilatation and damage of cytoplasmic organelles associated with the destruction of lysosomes. The stromal changes include enlargements of fibroblasts and prominent hyalinization and thickening of blood vessels. The radiosensitivity of individual cells depends on their mitotic index. Therefore, the neoplastic cells are more radiosensitive than cells of normal tissues. As a result, radiation-related changes alter the appearance of normal tissues as well as benign and malignant tumors and therefore may create diagnostic difficulties. Since the surgery remains the primary treatment for ovarian carcinoma, there are only a few reports describing the effects of radiation and chemotherapy treatment on histology of ovarian tumors (2,3,6,7). McCluggage et al. reported morphologic alterations in ovarian serous and endometrioid carcinomas initially treated with chemotherapy (6). As in radiation treatment, the changes affected nuclei and cytoplasm of tumor cells and the surrounding stroma. There was nuclear enlargement, irregularity, hyperchromasia, smudging and irregular chromatin clumping. The cytoplasm was either vacuolated, foamy or deeply eosinophilic. There was also prominent stromal fibrosis associated with inflammation, fat necrosis, foamy histiocytes, cholesterol clefts and hemosiderin laden macrophages. It was noted that morphologic alterations made tumor grading and typing very difficult. A similar observation was published recently by Chew et al., who described histologic changes in ovarian serous carcinoma treated with chemotherapy (7). As a result of the treatment, part of the tumor developed “clear cell differentiation.” The distinction from a true clear cell carcinoma was based on the results of immunostains.
Our case illustrates how radiation treatment may change the appearance of benign ovarian stromal tumor and create a diagnostic dilemma, especially during the intraoperative examination. The cytoplasmic vacuolization, nuclear enlargement and hyperchromatism resulting from the radiation may suggest a clear cell differentiation. The prominent stromal fibrosis dividing tumor cells into cords and small nests may create a pattern mimicking single file arrangements of metastatic lobular carcinoma. Finally, the signet-ring differentiation may raise a possibility Krukenberg tumor.
Both, our report and the previous studies indicate that iatrogenic changes should always be taken into consideration when an ovarian tumor shows confusing and unusual features. Radiation and chemotherapy can alter the original histology and make the distinction between different histologic types of ovarian tumors very difficult. As it is evident in our case, sex cord stromal tumors after radiation treatment may develop cellular alterations suggestive of a carcinoma. In conclusion, it is important to recognize radiation and chemotherapy changes, to obtain past clinical history, and resort to immunohistochemical stains when necessary.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Kurman RJ. Blaustein's Pathology of the Female Genital Tract; 5th edition. New York: Springer-Verlag. 2001
2. Cohen LE. Cancer treatment and the ovary: the effects of chemotherapy and radiation. Ann N Y Acad Sci. 2008;1135:123-5
3. Lo Presti A, Ruvolo G, Gancitano RA, Cittadini E. Ovarian function following radiation and chemotherapy for cancer. Eur J Obstet Gynecol Reprod Biol. 2004;113( Suppl 1):S33-40
4. Scully RE, Young RH, Clement PB. Atlas of Tumor Pathology. Tumors of the Ovary and Maldeveloped Gonads, Fallopian Tube, and Broad Ligament; 3rd edition. Washington, DC: AFIP. 1998
5. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of the Breast and Female Genital Organs. Lyon: IARC Press. 2003
6. McCluggage WG, Lyness RW, Atkinson RJ. et al. Morphologic effects of chemotherapy on ovarian carcinoma. J Clin Pathol. 2002;55:27-31
7. Chew I, Soslow RA, Park KJ. Morphologic changes in ovarian carcinoma after neoadjuvant chemotherapy: Report of a case showing extensive clear cell changes mimicking clear cell carcinoma. Int J Gynecol Pathol. 2009;28:442-6
Author contact
![]() Corresponding author: Mirka W. Jones, M.D., Department of Pathology Magee-Womens Hospital of UPMC, 300 Halket Street Pittsburgh, Pennsylvania 15213. E-mail: mjones2edu, fax (412) 641-1675, tel. (412) 641-1909.
Corresponding author: Mirka W. Jones, M.D., Department of Pathology Magee-Womens Hospital of UPMC, 300 Halket Street Pittsburgh, Pennsylvania 15213. E-mail: mjones2edu, fax (412) 641-1675, tel. (412) 641-1909.

 Global reach, higher impact
Global reach, higher impact