Impact Factor
ISSN: 1837-9664
J Cancer 2011; 2:309-316. doi:10.7150/jca.2.309 This volume Cite
Review
Novel Monoclonal Antibodies for Cancer Treatment: The Trifunctional Antibody Catumaxomab (Removab®)
Fresenius Biotech GmbH, Munich, Germany
Received 2011-4-29; Accepted 2011-5-24; Published 2011-5-25
Abstract
The trifunctional antibody (trAb) catumaxomab is characterized by a unique ability to bind three different cell types: tumor cells; T-cells; and accessory cells. It binds to epithelial cell adhesion molecule (EpCAM) on tumor cells, the CD3 antigen on T-cells, and to type I, IIa, and III Fcγ receptors (FcγRs) on accessory cells (e.g. natural killer cells, dendritic cells, and macrophages). Catumaxomab exerts its anti-tumor effects via T-cell-mediated lysis, antibody-dependent, cell-mediated cytotoxicity, and phagocytosis via activation of FcγR-positive accessory cells. Catumaxomab represents a self-supporting system, as no additional immune cell activation is required for tumor eradication. The efficacy and safety of catumaxomab have been demonstrated in a pivotal phase II/III study in malignant ascites (MA) and supporting phase I/II studies. It is administered as four intraperitoneal (i.p.) infusions of 10, 20, 50, and 150 µg on days 0, 3, 7, and 10, respectively. Catumaxomab was approved for the i.p. treatment of MA in patients with EpCAM-positive carcinomas where standard therapy is not available or no longer feasible in the European Union in April 2009. It is the first trAb and the first drug in the world approved specifically for the treatment of MA. Catumaxomab was awarded the Galen of Pergamon Prize, which recognizes pharmacological research for developing new and innovative drugs and diagnostics, in the specialist care category in 2010. The use of catumaxomab in other indications and additional routes of administration are currently being investigated to further exploit its therapeutic potential in EpCAM-positive carcinomas.
Keywords: catumaxomab, epithelial cell adhesion molecule (EpCAM), anti-EpCAM × anti-CD3, trifunctional antibody, targeted cancer immunotherapy, malignant ascites
Introduction
The development of monoclonal antibodies (mAbs), which act via antibody-dependent cell-mediated cytotoxicity (ADCC), represented a significant advance in cancer immunotherapy.1 Bispecific antibodies (bsAbs), which bind to tumor cells and T-cells, and act via T-cell-mediated lysis, are currently in clinical development.2,3 The trifunctional antibody (trAb) catumaxomab (Removab®, Fresenius Biotech GmbH, Munich, Germany) is characterized by a unique ability to bind three different cell types: tumor cells, T-cells, and accessory cells.4-6 It was approved in the European Union (EU) in April 2009 for the intraperitoneal (i.p.) treatment of malignant ascites (MA) in patients with epithelial cell adhesion molecule (EpCAM)-positive carcinomas where standard therapy is not available or no longer feasible. Catumaxomab is the first trAb and the first drug in the world approved specifically for the treatment of MA.
Catumaxomab
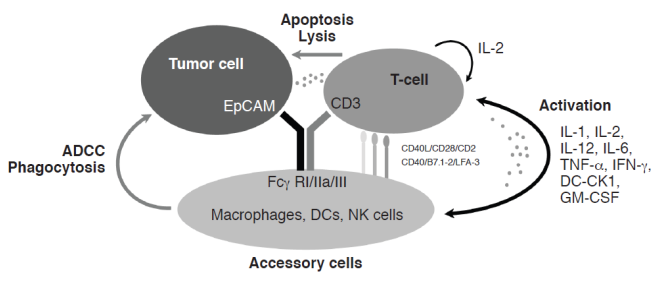
Catumaxomab has two different antigen-binding specificities: one for EpCAM on tumor cells and one for the CD3 antigen on T-cells. In addition, catumaxomab binds, via its intact Fc region, to type I, IIa, and III Fcγ receptors (FcγRs) on accessory cells, e.g. natural killer (NK) cells, dendritic cells (DCs), and macrophages. Catumaxomab exerts its anti-tumor effects via T-cell-mediated lysis,7 ADCC, and phagocytosis via activation of FcγR-positive accessory cells (Figure 1).5,6 Its anti-tumor activity is assisted by the induction of T-cell-secreted cytokines, such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α.8 An important aspect of catumaxomab's mode of action is that no additional activation of immune cells is required for effective tumor eradication, so it is a self-supporting system.
Malignant Ascites
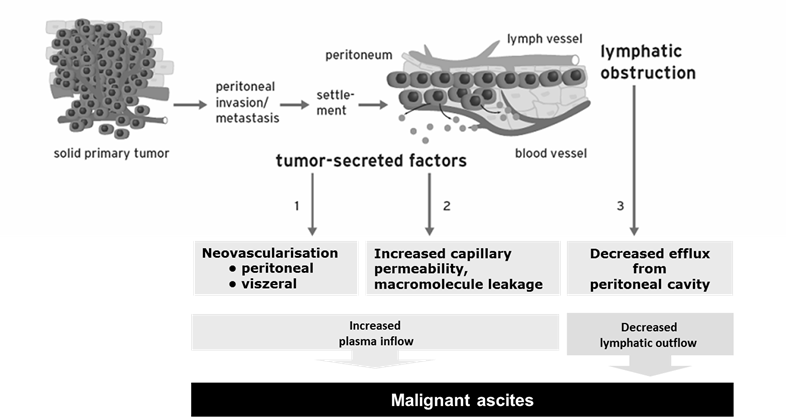
MA is an increased accumulation of protein-containing fluid within the peritoneal cavity that is caused by i.p. spread of cancer. It is associated with advanced ovarian cancer, gastrointestinal malignancies, and other carcinomas, and leads to abdominal pain and swelling, dyspnea, nausea, vomiting, malnutrition, and anorexia.9,10 Patients with MA have a poor quality of life,9,11 and a poor prognosis, with median overall survival (OS) of approximately 1-6 months.9,12,13 The causes of MA are independent of the origin of the primary tumor (Figure 2).14-17 Tumor-secreted factors lead to tumor neovascularization and increased capillary permeability, resulting in increased plasma inflow into the peritoneal cavity. Tumor cells obstruct lymphatic drainage, leading to decreased fluid efflux from the peritoneal cavity.
Rationale for use of Catumaxomab in the Treatment of MA
Prior to the approval of catumaxomab, no agents were specifically approved for the treatment of MA and treatment options, such as peritoneovenous shunts, paracentesis, and diuretics, are only palliative.11 There was thus a need for an effective treatment for MA. The rationale for the use of catumaxomab for the i.p. treatment of MA was four-fold: 1) epithelial tumors spreading into the peritoneal cavity play a major role in the development of MA; 2) epithelial tumors frequently express EpCAM;18-21 3) in the peritoneal cavity, EpCAM is a tumor-specific antigen; and 4) immune effector cells are present in MA.22,23 Targeting EpCAM by i.p. administration of catumaxomab leads to a depletion of epithelial tumor cells in the peritoneal cavity and a sustained reduction of MA production.
Catumaxomab mechanism of action. ADCC = antibody-dependent cell-mediated cytotoxicity, CK = cytokine, DC = dendritic cell, EpCAM = epithelial cell adhesion molecule, Fcγ R = Fcγ receptor, GM-CSF = granulocyte-macrophage colony-stimulating factor, IL = interleukin, IFN = interferon, LFA = lymphocyte function-associated antigen, NK = natural killer, TNF = tumor necrosis factor.
Pathophysiology of malignant ascites.
Clinical development of catumaxomab in malignant ascites.
| Study number | Indication (No. of treated patients) | Phase | Study design | Key results |
|---|---|---|---|---|
| STP-REM-0124 | Malignant ascites due to ovarian cancer (23) | I/II | Multicenter, multinational, open label, uncontrolled, sequential dose escalation | Recommended dose 10, 20, 50, 150 µg Efficacy: Reduction of ascites flow; no requirement for puncture in 22 patients at study end |
| IP-REM-PK-01-EU | Malignant ascites due to epithelial cancer (13) | II | Multicenter, open label, pharmacokinetic | i.p. catumaxomab measurable in plasma t1/2: geometric mean ~2 days High inter-subject variability |
| IP-REM-AC-0125 | Malignant ascites due to epithelial cancer (157) | II/III | Multicenter, multinational, two arm, randomized, open label | Statistically significant and clinically relevant superiority of catumaxomab plus paracentesis versus paracentesis alone |
Clinical Development of Catumaxomab in MA
The clinical development of catumaxomab in MA consisted of three key studies: an open-label phase I/II dose-finding study (STP-REM-01);24 a pharmacokinetic study (IP-REM-PK-01-EU); and a pivotal phase II/III study (IP-REM-AC-01)25 (Table 1). A phase II and two phase I studies in other indications (ovarian cancer [AGO-OVAR-2.10],26 peritoneal carcinomatosis [IP-REM-PC-01-DE],27 and intra-abdominal epithelial cancers [IP-REM-GC-01]),28 provided supporting efficacy and safety data. In total, 270 patients received catumaxomab in these studies.
STP-REM-01
This study investigated the maximum tolerated dose (MTD) of catumaxomab in 23 women with recurrent ascites due to treatment-refractory ovarian cancer.24 Patients received four or five 6-hour i.p. catumaxomab infusions of 5-200 μg on days 0, 3, 6, and 9 for the first four dose groups and a fifth infusion on day 13 for the fifth dose group. Catumaxomab produced a significant and sustained reduction in ascites flow rate, and 22 patients did not require paracentesis between the last infusion and end of the study (day 37). EpCAM-positive tumor cells in ascites were reduced by up to 5 logs and were eliminated to levels below the limit of detection. The MTD was determined to be 10, 20, 50, 200, and 200 μg.
Most adverse events were reversible and resolved without sequelae. Frequent adverse events were transient fever (83%), nausea (61%), and vomiting (57%), which were mostly grade 1/2. Although there was no clear relationship between catumaxomab dose and the severity of adverse events, which is similar to other cancer immunotherapies,29 a dose schedule of 10, 20, 50, and 150 μg that is well below the MTD was recommended for further studies.
IP-REM-PK-01-EU
This was an open-label, multicenter, pharmacokinetic study in 13 patients who received four i.p. catumaxomab infusions of 10, 20, 50, and 150 μg. In most patients, the catumaxomab concentration increased with the number of infusions and the doses applied. The highest concentrations of catumaxomab were found in ascitic fluid, the site of intended efficacy. Catumaxomab could be detected in plasma after the third and fourth i.p. infusions, demonstrating systemic availability. Inter-patient variability was high. The geometric mean maximum plasma drug concentration (Cmax) was approximately 0.5 ng/mL and the mean terminal plasma elimination half-life (t1/2) was approximately 2.5 days.30
IP-REM-AC-01
This pivotal phase II/III, multicenter study was a two-arm, randomized (2:1), open-label design that compared catumaxomab plus paracentesis with paracentesis alone (control) in 258 patients stratified by cancer type (ovarian or nonovarian; n=129: 85 catumaxomab/44 control in each group).25 Catumaxomab was administered as four 6-hour i.p. infusions of 10, 20, 50, and 150 µg on days 0, 3, 7, and 10, respectively. Puncture-free survival, defined as the time after day 0 (control group)/1 day after last infusion (catumaxomab group) to the first need for therapeutic paracentesis or death, whichever occurred first, was the primary endpoint. Secondary endpoints included time to next therapeutic paracentesis, ascites signs and symptoms, OS, and safety. The main inclusion criteria were resistance to chemotherapy or chemotherapy no longer feasible, at least one previous puncture within 5 weeks before screening, symptomatic ascites with a volume of >1 L, EpCAM-positive tumor cells in the ascites, and a Karnofsky Index ≥60.
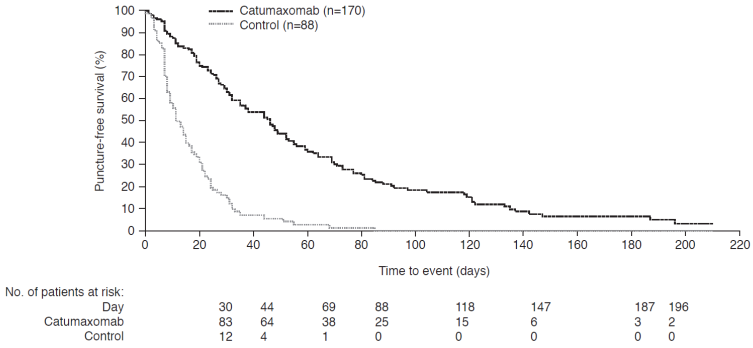
Catumaxomab significantly prolonged puncture-free survival versus paracentesis alone in the intent-to-treat (ITT) population, in the pooled (Figure 3), ovarian, and nonovarian cancer populations (all p<0.0001). The median difference was 35 (95% CI: 25-45), 41 (95% CI: 32-50), and 23 (95% CI: 8-38) days in the pooled, ovarian, and nonovarian cancer populations, respectively. The hazard ratio (HR) for the pooled population corresponded to a risk reduction of 75% for puncture or death. Catumaxomab also significantly prolonged the median time to next therapeutic paracentesis versus paracentesis alone: 77 versus 13, 71 versus 11, and 80 versus 15 days in the pooled, ovarian, and nonovarian cancer populations, respectively (all p<0.0001). This corresponds to a saving of about five punctures for catumaxomab, which is clinically relevant as there is continuous protein loss with each puncture that leads to cachexia and a potential risk of infection and bowel perforation.
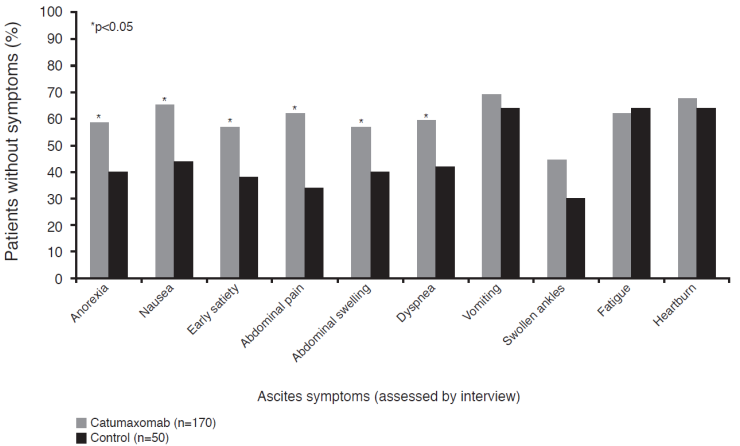
Ascites signs and symptoms were analyzed 8 days after the last catumaxomab infusion or after day 0 in the control group.25 Catumaxomab significantly (p<0.05) improved ascites-related symptoms in six of 10 categories (abdominal pain, nausea, abdominal swelling, dyspnea, anorexia, and early satiety) (Figure 4) and in all four sign categories (abdominal distension dull to percussion, shifting dullness, fluid thrill, and bulging flanks). There was a trend towards prolonged OS with catumaxomab, although the study was not powered or designed to detect statistically significant differences in OS. Median OS was 72 versus 68, 110 versus 81, and 52 versus 49 days in the pooled, ovarian, and nonovarian cancer populations, respectively. In the subgroup of patients with gastric cancer, there was a statistically significant difference between catumaxomab and paracentesis alone (71 versus 44 days, p=0.0313).25 A long-term survival analysis showed that the 6- and 12-month survival rates for catumaxomab plus paracentesis versus paracentesis alone in the ITT population were 27.5% versus 6.7% and 11.4% versus 3.4%, respectively.31
Catumaxomab had an acceptable safety profile: most adverse events were generally mild to moderate and fully reversible. The majority of patients (n=131; 83%) received all four i.p. infusions. The most common drug-related adverse events were cytokine-release-related symptoms (CRRSs), i.e. pyrexia, nausea, and vomiting, and abdominal pain. These symptoms are due to catumaxomab's mechanism of action and are well-known side effects of antibody therapy.32,33 Transient increases in liver parameters and white blood cell abnormalities occurred but were rarely considered to be clinically significant. There was no distinctive pattern of adverse events corresponding to specific infusions.
The results of this study demonstrated that catumaxomab, administered as a sequence of four i.p. infusions of 10, 20, 50, and 150 μg, had a positive risk-benefit profile. Catumaxomab plus paracentesis resulted in significant prolongation of puncture-free survival and puncture-free time, pronounced reduction of ascites-related symptoms, and improvement in OS. The safety profile of catumaxomab is defined by its mechanism of action and the i.p. route of administration. Adverse events are predictable, limited, reversible, and manageable.
Puncture-free survival in the pooled intent-to-treat population in the pivotal phase II/III study.25
Ascites-related symptoms in the pivotal phase II/III study.25
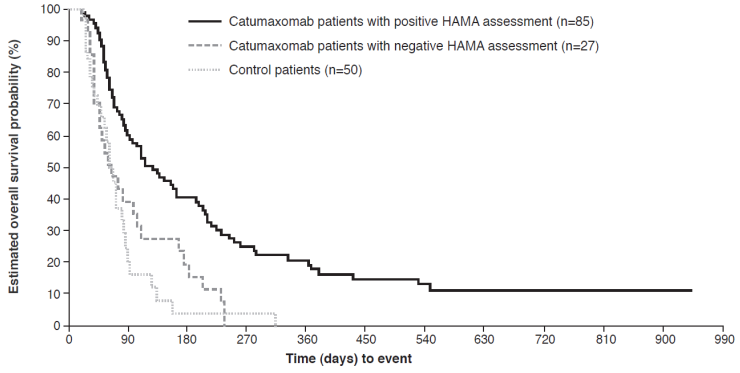
Overall survival in HAMA-positive versus HAMA-negative patients* in the pooled population in the pivotal phase II/III study.37 *Assessed 8 days after last catumaxomab infusion.
Immunological Response to Catumaxomab
Although the induction of human anti-murine antibodies (human anti-mouse antibodies [HAMAs] and human anti-rat antibodies [HARAs]) is an intrinsic effect of murine mAbs, the available evidence indicates that they are not associated with any major safety issues.34,35 In fact, the development of HAMAs/HARAs can be associated with beneficial immunity and prolonged survival.34,36 A post-hoc analysis of the pivotal phase II/III trial demonstrated that there was a strong correlation between clinical outcome and humoral response, as measured by the detection of HAMAs 8 days after the fourth catumaxomab infusion.37 HAMA-positive and HAMA-negative catumaxomab-treated patients and control patients were analyzed separately for puncture-free survival, time to next puncture, and OS, and compared with each other. In the pooled population, patients who developed HAMAs after catumaxomab treatment showed significant improvement in all three clinical outcome measures compared with HAMA-negative patients: median puncture-free survival was 64 versus 27 days (p<0.0001; HR 0.330), median time to next therapeutic puncture was 104 versus 46 days (p=0.0002; HR 0.307), and median OS was 129 versus 64 days (p=0.0003; HR 0.433) (Figure 5). Similar differences were seen in the ovarian, nonovarian, and gastric cancer populations. The results showed that HAMA development may be a biomarker for catumaxomab response and patients who developed HAMAs sooner derived greater benefit from catumaxomab therapy.
EU Approval Procedure
Catumaxomab was developed and approved in the EU for the treatment of MA within 8 years. The dose-finding study commenced in November 2001, the pivotal study started in 2004 and reported in 2007, and the pharmacokinetic study plus three supporting studies were conducted between 2003 and 2007. The Committee for Human Medicinal Products (CHMP) provided scientific advice, particularly for the design of the pivotal study, including the selection of a suitable endpoint for MA, as no standards were available at the time of catumaxomab's clinical development. The most appropriate endpoint to show potential treatment benefits, taking into account the terminal nature of the disease, was identified as puncture-free survival, a combined endpoint of time to puncture or death, whichever occurs first.
A Marketing Authorization was compiled and submitted in December 2007 after successful completion of the pivotal study. CHMP review, which started in January 2008, included an assessment of the clinical data by the Scientific Advisory Group on Oncology. The CHMP reached a consensus decision that the risk-benefit profile of catumaxomab is positive and recommended authorization in February 2009. The European Commission followed the recommendation of the CHMP and approved catumaxomab (Removab®) in April 2009 for the i.p. treatment of MA in patients with EpCAM-positive carcinomas where standard therapy is not available or no longer feasible.
The approval of catumaxomab was unique for several reasons: it is the first drug approved specifically for the treatment of MA; to date, it is the only approved EpCAM-targeted antibody; it is the only approved agent based on the target antigen that is independent of the primary tumor type; and it is the first approved trAb.
Catumaxomab was awarded the Galen of Pergamon Prize, which recognizes pharmacological research for developing new and innovative drugs and diagnostics, in the specialist care category in 2010. The prize, which is awarded annually by Springer Medicine to honor excellence in pharmacological research in Germany, was founded in France in 1970.
Further Investigations in Malignant Ascites
Catumaxomab is being investigated in a number of clinical studies. CASIMAS (CAtumaxomab Study with Intraperitoneal infusion in Malignant AScites patients) is a randomized, phase lllb study of a 3-hour infusion of catumaxomab with corticosteroid premedication in an office-based setting. The study is intended to further optimize the administration of catumaxomab by reducing the infusion time from 6 to 3 hours. Repeated catumaxomab treatment cycles are being investigated in the SECIMAS (SEcond Cycle catumaxomab Intraperitoneal infusion Malignant Ascites Safety) study, a follow-on phase ll study to CASIMAS. Patients needing their first therapeutic puncture after treatment in CASIMAS are eligible for enrollment in SECIMAS to receive a second i.p. cycle of catumaxomab 10, 20, 50, and 150 µg. A non-interventional study (CARMA) is documenting treatment behavior.
Further Development Strategy for Catumaxomab
Intravenous infusion is being investigated as an additional route for administration in a phase I study that started at the beginning of 2011. Other indications under investigation for i.p catumaxomab include, for example, peritoneal carcinomatosis in gastric cancer. All carcinomas that express EpCAM could be future targets for catumaxomab therapy.
Conclusions
Catumaxomab's trifunctional mechanism of action utilizes the close proximity and local activation of T-cells and accessory cells against tumor cells. Its efficacy is dependent on the presence of immune effector cells, which confirms the importance of local immunostimulatory effects (e.g. cytokine release and physiological T-cell activation and proliferation) and their contribution to anti-tumor activity. Importantly, no additional activation of immune cells is necessary for effective tumor eradication by catumaxomab, which is thus a self-supporting system. The efficacy and safety of catumaxomab have been demonstrated in a pivotal phase II/III study and supporting phase I/II studies. It is administered as four intraperitoneal (i.p.) infusions of 10, 20, 50, and 150 µg on days 0, 3, 7, and 10, respectively. Treatment with catumaxomab significantly prolongs puncture-free survival, saves approximately five therapeutic punctures, and improves ascites-related symptoms, with a trend towards prolonging OS. Catumaxomab, which was approved in the EU in April 2009, is the first trAb to receive regulatory approval and the first drug in the world approved specifically for the treatment of MA. In 2010, catumaxomab was awarded the Galen von Pergamon Prize, which recognizes pharmacological research for developing new and innovative drugs and diagnostics, in the specialist care category. Clinical development is ongoing in a number of indications including MA and peritoneal carcinomatosis. Shorter administration times, additional routes of administration, and multiple dosing are under evaluation to fully utilize the therapeutic potential of catumaxomab in EpCAM-positive carcinomas.
Acknowledgements
The author thanks Kevin De-Voy for writing support (funded by Fresenius Biotech GmbH).
Conflict of Interest
Diane Seimetz is the Chief Scientific Officer and Executive Vice President of Fresenius Biotech GmbH.
References
1. Strome SE, Sausville EA, Mann D. A mechanistic perspective of monoclonal antibodies in cancer therapy beyond target-related effects. Oncologist. 2007;12:1084-95
2. Chames P, Baty D. Bispecific antibodies for cancer therapy. Curr Opin Drug Discov Devel. 2009;12:276-83
3. Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature. 1985;314:628-31
4. Ruf P, Gires O, Jäger M. et al. Characterisation of the new EpCAM-specific antibody HO-3: implications for trifunctional antibody immunotherapy of cancer. Br J Cancer. 2007;97:315-21
5. Zeidler R, Reisbach G, Wollenberg B. et al. Simultaneous activation of T-cells and accessory cells by a new class of intact bispecific antibody results in efficient tumour cell killing. J Immunol. 1999;163:1246-52
6. Zeidler R, Mysliwietz J, Csanady M. et al. The Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cells. Br J Cancer. 2000;83:261-6
7. Riesenberg R, Buchner A, Pohla H. et al. Lysis of prostate carcinoma cells by trifunctional bispecific antibodies (αEp-CAM × αCD3). J Histochem Cytochem. 2001;49:911-7
8. Schmitt M, Schmitt A, Reinhardt P. et al. Opsonization with a trifunctional bispecific (αCD3 × αEpCAM) antibody results in efficient lysis in vitro and in vivo of EpCAM positive tumour cells by cytotoxic T lymphocytes. Int J Oncol. 2004;25:841-8
9. Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007;18:945-9
10. Parsons SL, Watson SA, Steele RJC. Malignant ascites. Br J Surgery. 1996;83:6-14
11. Becker G, Galandi D, Blum HE. Malignant ascites: systematic review and guideline for treatment. Eur J Cancer. 2006;42:589-97
12. Mackey JR, Venner PM. Malignant ascites: demographics, therapeutic efficacy and predictors of survival. Can J Oncol. 1996;6:474-80
13. Parsons SL, Lang MW, Steel RJC. Malignant ascites: a 2-year review from a teaching hospital. Eur J Surg Oncol. 1996;22:237-9
14. Adam RA, Adam YG. Malignant ascites: past, present, and future. J Am Coll Surg. 2004;198:999-1011
15. Nagy JA, Masse EM, Herzberg KT. et al. Pathogenesis of ascites tumor growth: vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res. 1995;55:360-8
16. Nagy JA, Morgan ES, Herzberg KT. et al. Pathogenesis of ascites tumor growth: angiogenesis, vascular remodeling, and stroma formation in the peritoneal lining. Cancer Res. 1995;55:376-85
17. Tamsma J. The pathogenesis of malignant ascites. Cancer Treat Res. 2007;134:109-18
18. Davidson B, Risberg B, Kristensen G. et al. Detection of cancer cells in effusions from patients diagnosed with gynaecological malignancies: evaluation of five epithelial markers. Virchows Archiv. 1999;435:43-9
19. De Angelis M, Buley ID, Heryet A. et al. Immunocytochemical staining of serous effusions with the monoclonal antibody Ber-EP4. Cytopathology. 1992;3:111-7
20. Diaz-Arias AA, Loy TS, Bickel JT. et al. Utility of BER-EP4 in the diagnosis of adenocarcinoma in effusions: an immunocytochemical study of 232 cases. Diagn Cytopathol. 1993;9:516-21
21. Passebosc-Faure K, Li G, Lambert C. et al. Evaluation of a panel of molecular markers for the diagnosis of malignant serous effusions. Clin Cancer Res. 2005;11:6862-7
22. Freedman RS, Deavers M, Liu J. et al. Peritoneal inflammation - a microenvironment for epithelial ovarian cancer (EOC). J Transl Med. 2004;2:23
23. Kubicka U, Olszewski WL, Tarnowski W. et al. Normal human immune peritoneal cells: subpopulations and functional characteristics. Scand J Immunol. 1996;44:157-63
24. Burges A, Wimberger P, Kümper C. et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM × anti-CD3 antibody: a phase I/II study. Clin Cancer Res. 2007;13:3899-905
25. Heiss MM, Murawa P, Koralewski P. et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209-21
26. Belau A, Pfisterer J, Wimberger P. et al. Randomized, multicenter, two-dose level, open-label, phase IIa study with the intraperitoneally infused trifunctional bispecific antibody catumaxomab (anti-EpCAM × anti-CD3) to select the better dose level in platinum refractory epithelial ovarian cancer patients. J Clin Oncol. 2007;25(Suppl):Abstract5556
27. Ströhlein MA, Lordick F, Rüttinger D. et al. Immunotherapy of peritoneal carcinomatosis with the antibody catumaxomab in colon, gastric, or pancreatic cancer: an open-label, multicenter, phase I/II trial. Onkologie. 2011;34:101-8
28. Stroehlein M, Schemanski O, Jaeger M. et al. Intraoperative immunotherapy with the trifunctional antibody catumaxomab in patients with advanced gastric, colon, and pancreatic cancer: a pilot phase I study (Abstract 120). Orlando, Florida, USA: ASCO GI Cancers Symposium. 2008
29. Parulekar WR, Eisenhauer EA. Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J Natl Cancer Inst. 2004;96:990-7
30. Removab Summary of Product Characteristics. http://www.emea.europa.eu/humandocs/PDFs/EPAR/removab/H-972-PI-en.pdf
31. Ströhlein MA, Essing MM, Schmidt-Rimpler C. et al. Catumaxomab treatment significantly improves overall survival in patients with malignant ascites: follow-up results from a pivotal phase II/III study (Abstract AB1272). Paris, France: 22nd International Congress on Anti-Cancer Treatment. 2011
32. Jeyarajah DR, Thistlethwaite JRJr. General aspects of cytokine-release syndrome: timing and incidence of symptoms. Transplant Proc. 1993;25:16-20
33. Winkler U, Jensen M, Manzke O. et al. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood. 1999;94:2217-24
34. DeNardo GL, Bradt BM, Mirick GR. et al. Human antiglobulin response to foreign antibodies: therapeutic benefit? Cancer Immunol Immunother. 2003;52:309-16
35. Marmé A, Strauss G, Bastert G. et al. Intraperitoneal bispecific antibody (HEA125xOKT3) therapy inhibits malignant ascites production in advanced ovarian carcinoma. Int J Cancer. 2002;101:183-9
36. Azinovic I, DeNardo GL, Lamborn KR. et al. Survival benefit associated with human anti-mouse antibody (HAMA) in patients with B-cell malignancies. Cancer Immunol Immunother. 2006;55:1451-8
37. Ott MG, Lindhofer H, Linke RG. et al. The trifunctional antibody catumaxomab: correlation between immunological response and clinical outcome - new analysis of a pivotal phase II/III study. J Clin Oncol. 2010;28(Suppl):Abstract2551
Author contact
![]() Corresponding author: Fresenius Biotech GmbH, Frankfurter Ring 193a, D-80807 München, Germany. Tel.: +49 89 306593 47; Fax: +49 89 306593 77; E-mail: diane.seimetzcom
Corresponding author: Fresenius Biotech GmbH, Frankfurter Ring 193a, D-80807 München, Germany. Tel.: +49 89 306593 47; Fax: +49 89 306593 77; E-mail: diane.seimetzcom

 Global reach, higher impact
Global reach, higher impact