Impact Factor
ISSN: 1837-9664
J Cancer 2011; 2:401-412. doi:10.7150/jca.2.401 This volume Cite
Research Paper
In Situ Malignant Transformation and Progenitor-Mediated Cell Budding: Two Different Pathways for Breast Ductal and Lobular Tumor Invasion
1. Armed Forces Institute of Pathology and American Registry of Pathology, Washington, DC, USA
2. Department of Medical Oncology, Peking University Cancer Hospital and Institute, China
3. Surgical Oncology, Walter Reed Army Medical Center, Washington DC, USA
*Drs. Man and Izadjoo's new affiliation: Diagnostic and Translational Research Center, Henry Jackson Foundation, 401 Professional Drive, Gaithersburg, 20879.
Received 2011-6-14; Accepted 2011-7-19; Published 2011-7-20
Abstract
The human breast lobular and ductal structures and the derived tumors from these structures differ substantial in their morphology, microenvironment, biological presentation, functions, and clinical prognosis. Based on these differences, we have proposed that pre-invasive lobular tumors may progress to invasive lesions through “in situ malignant transformation”, in which the entire myoepithelial cell layer within a given lobule or lobular clusters undergoes extensive degeneration and disruptions, which allows the entire epithelial cell population associated with these myoepithelial cell layers directly invade the stroma or vascular structures. In contrast, pre-invasive ductal tumors may invade the stroma or vascular structures through “progenitor-mediated cell budding”, in which focal myoepithelial cell degeneration-induced aberrant leukocyte infiltration causes focal disruptions in the tumor capsules, which selectively favor monoclonal proliferation of the overlying tumor stem cells or a biologically more aggressive cell clone. Our current study attempted to provide more direct morphological and immunohistochemical data that are consistent with our hypotheses.
Keywords: Breast cancer, Tumor invasion, Tumor metastasis, Malignant transformation, Tumor cell budding, Myoepithelial cells, Tumor microenvironment, Tumor stem cells, lymphocytes
Introduction
The epithelial component of the human breast consists of lobular (or acinar) cells, which are arranged as grape-like structures responsible primarily for the production of milk, and the ductal system, which are arranged as branching, three-like structures responsible mainly for providing the drainage of the secretions [1-3]. Developmentally, the lobular cells are derived from “budding” cells of the terminal ducts during the early puberty [4-11]. The lobular cells undergo extensive proliferation, differentiation, molecular and biochemical changes during the entire lifespan of the female, more notably during pregnancy and lactation [4-11]. Following menopause, the lobular structures in both nulliparous and parous women start to regress and a majority of these structures are eventually replaced by fibrous tissues [4-11]. The ductal system starts at the terminal ducts, merges into larger ducts, and extends to the nipple orifices. Compared to the lobular (acinar) cells, ductal cells are relatively more stable during the entire lifetime of the subject [4-11].
Structurally, the lobular and ductal systems differ substantially in the following aspects:
1. The physical distribution and relationship to the myoepithelial cells
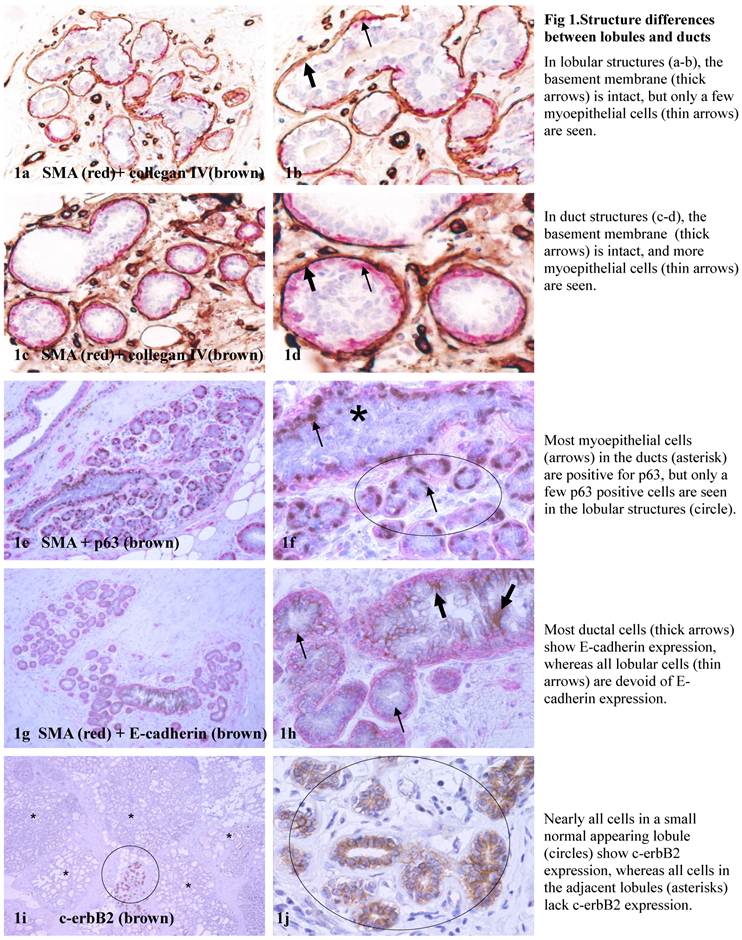
Both the lobular and ductal cells are physically separated from the stroma by a layer of basement membrane and a layer of myoepithelial cells. The basement membrane is structurally similar in both lobular and ductal systems. The myoepithelial cells in the ductal system generally form a continuous sheet that completely encircles all the ducal cells. In contrast, the myoepithelail cell layer in the lobular system is often discontinuous, defined as the lack of direct physical contact or the presence of small gaps (generally smaller than the size of two myoepithelial cells) among the neighboring myoepithelial cells [12,13] (Fig 1a-1d).
2. The expression pattern and frequency of tumor suppressors in the myoepithelial cell layer
The myoepithelial cell layer produces a number of tumor suppressors, including maspin, p63, and Wilms' tumor 1 (WT-1) that exert significant paracrine inhibition on proliferation and invasion of associated tumor cells [13-16]. In the normal ductal system, tumor suppressors are consistently expressed in all or nearly all morphologically distinct myoepithelial cells. In contrast, many morphologically distinct myoepithelial cells in the lobular system are often devoid of expression of these established tumor suppressors [17-20]. In some cases, many lobular clusters or an entire lobule show no or significantly reduced expression of p63 and WT-1 as compared to that of duct-associated [17-20] (Fig 1e-1f).
3. The expression pattern and frequency of cell surface adhesion molecules and c-erbB2
A number of cell surface adhesion molecules, including E-cadherin and ß-catenin, are strongly expressed in a vast majority of the ductal epithelial cells and their malignant derivatives, but are absent in the acinar counterpart, which, however, often harbors isolated lobules with aberrant c-erbB2 expression [21, 22] (Fig 1g-1j).
4. The size and length of the lumen
The lumen of the lobular units is very small with a single open end that leads to the terminal duct. Due to this structural feature, any substantially elevated lobular cell proliferation may over-stretch the associated basement membrane and myoepithelial cell layer, or even physically disrupt these two structures. In contrast, the lumen of the ducts is substantially larger and intercalated among ducts, which permits a longitudinal expansion of an increased volume of ductal cells and also preserves the physical integrity of the surrounding myoepithelial cell layer and the basement membrane.
Together, these structural features are apparently more favorable for proliferation, invasion, and metastasis of lobular tumor cells. Consistent with this speculation is the fact that invasive lobular cancers (ILC) tend to be significantly larger in size with a significantly higher rate of positive lymph nodes than stage-matched invasive ductal carcinoma (IDC) [23-25]. Although large tumor size and positive lymph node are two well-recognized risk factors for worse prognosis, patients with ILC have a substantially more favorable clinical outcomes compared to patients with IDC [23-27]. These contradictory impacts have been largely attributed to the unique features of ILC, including the lack of E-cadherin expression, higher expression of ER and PR, lower expression of HER-2, p53, EGFR, and lower S-phase fraction [28-31]. The trigger factor for the significant differences in clinical outcomes between stage-matched ILC and IDC, however, has not been identified.
We have hypothesized that the trigger factor for the significant differences in clinical outcomes between lobular and ductal tumors may result from their substantially different growth patterns during invasion. As the lobular cell population undergoes extensive proliferation and differentiation during puberty, pregnancy and lactation [6-11], most adult females may have largely exhausted or “used up” the residual stem cells, which have been suggested as the primary source of invasive and metastatic lesions [32-35]. In addition, the extensive proliferation of the epithelial cell population during these stages may have also caused the exhaustion of the residual stem cells in the myoepithelial cell population, which impairs the normal replenishment process, resulting in an aged myoepithelial cell population. Thus, pre-invasive lobular tumors may progress to invasive lesions through “in situ malignant transformation”, in which the entire myoepithelial cell layers within a given lobule or lobular clusters become degenerated and disrupted, which allows the entire tumor cell population to directly invade the stroma. In contrast, pre-invasive ductal tumors may invade the stroma or vascular structures through “progenitor-mediated cell budding”, in which focal myoepithelial cell degeneration-induced aberrant leukocyte infiltration causes focal disruptions of the tumor capsules, which selectively favor monoclonal proliferation of the overlying tumor stem cells or a biologically more aggressive cell clone. Our current study attempted to provide more morphological and immunohistochemical data supportive of our hypothesis.
Materials and Methods
Ten cases harboring large normal mammary ductal or acinar clusters or lobules with malignant features were selected from our previous studies [12,13,16-20]. All these samples were retrieved from the files of the Armed Forces Institute of Pathology with IRB approved protocols. Consecutive sections at 7-um thickness were cut and placed sequentially on positively charged slides. For each case, 300-500 sections were made. For each set of 10 consecutive sections, the first 3-4 sections were used for hematoxylin and eosin (H & E) staining and immunohistochemistry (IHC). The remaining sections were used for various molecular assays.
To identify cells with malignancy-associated alterations, sections were double immunostained for p53 (clone: D07, Dako, Carpinteria, CA) and smooth muscle actin (SMA; clone: 1A4; Sigma, St. Louis, MO). To differentiate between ductal and acinar cells, and to identify the potential impact of leukocytes on the physical integrity of myoepithelial cell layers, sections were double immunostained for E-cadherin (clone: 36B5; Lab Vision, Fremont, CA), and leukocyte common antigen (LCA, clone: 2B11+PD7/26), which is present in all normal hematopoietic cells and their neoplastic transformations. To identify disseminated or isolated epithelial cells within leukocyte aggregates, sections were double immunostained for LCA and cytokeratin (CK) AE1/3 (clone; AE1/AE3, Dako, Carpinteria, CA), which are expressed in all epithelium-derived cells.
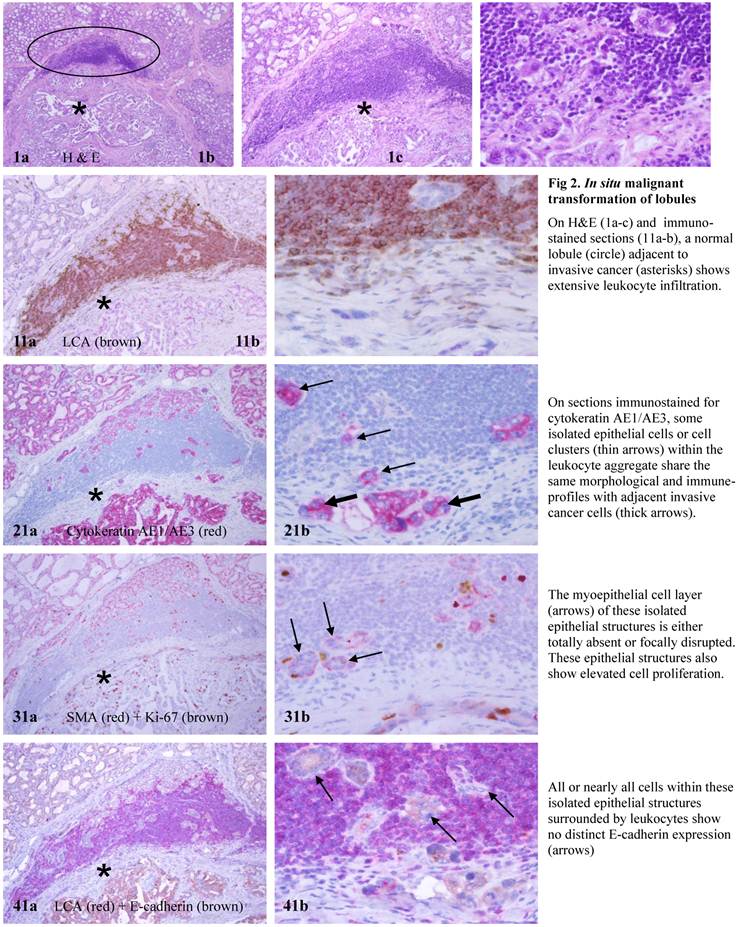
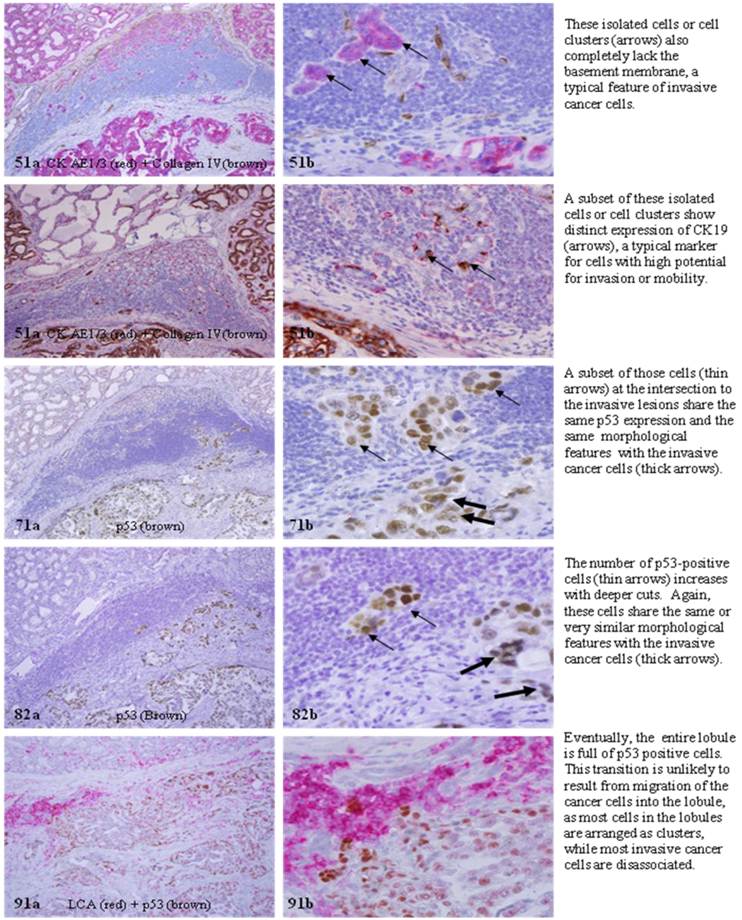
As our previous studies have suggested that aberrant leukocyte infiltration could trigger cell dissemination and malignant transformation in normal appearing lobules [36-40], the possible sign of “in situ malignant transformation ” was assessed by examining the morphological and immunohistochemical alterations of such lobules with infiltrated leukocyte aggregates in multiple consecutive sections, to determine: (1) whether cells with malignancy-associated changes can originate from normal lobules, (2) whether cells with malignancy-associated changes in the normal appearing lobules are eventually in physical continuity with clear-cut invasive lesions, (3) whether cells with malignancy-associated changes in normal appearing lobules share the same or similar morphological and immunohistochemical profile with their clear-cut malignant counterparts, and (4) whether leukocyte aggregates are exclusively or preferentially located at or near the intersection between these lobules and clear-cut invasive lesions.
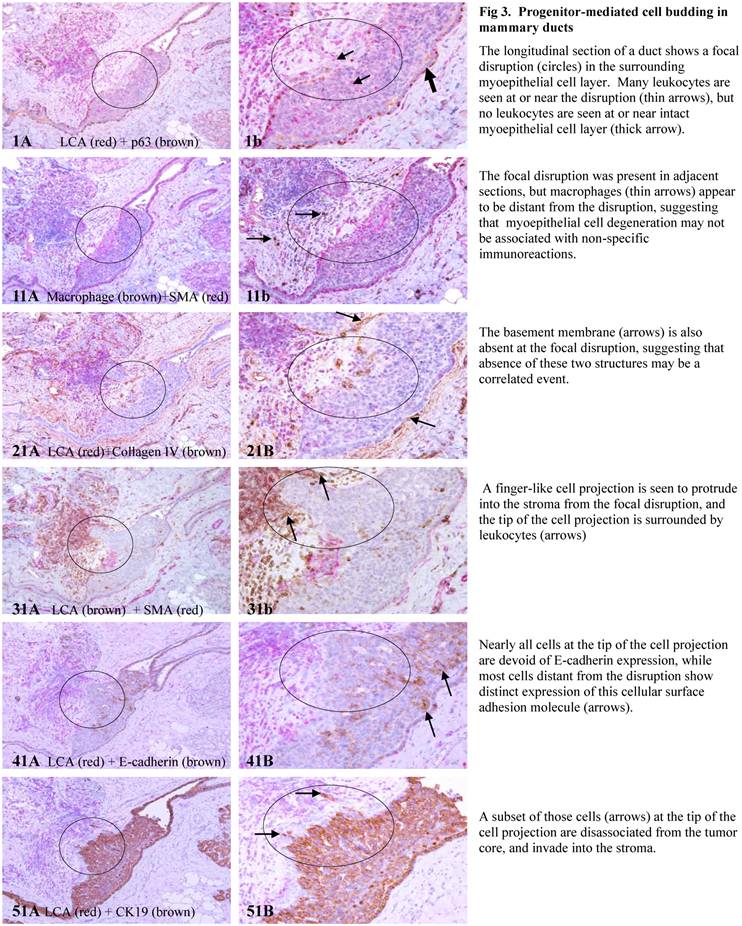
To identify signs of “progenitor-mediated cell budding”, the morphological and immunohistochemical alterations of hyperplastic or in situ tumors were examined in cross and longitudinal profile of consecutive sections, to determine: (1) whether morphologically and immunohistochemically different cell types co-exist within the same duct, (2) whether cell “budding” is exclusively seen at focally disrupted myoepithelial cell layers, (3) whether all “budding” cells share the same morphological and immunohistochemical profile, (4) whether “budding” cells are eventually in physical continuity with clear-cut invasive lesions, (5) whether “budding” cells share the same morphological and immunohistochemical profile with their clear-cut malignant and invasive counterparts.
Immunostaining was carried out using our published protocol with monoclonal mouse anti-human antibodies. The secondary antibody, ABC detection kit, and diaminobenzidine (DAB) chromogen kit were obtained from Vector (Burlingame, CA). The AP red-chromogen kit was purchased from Zymad (South San Francisco, CA). To assess the specificity of the immunostaining, different negative controls were used, including (1) the substitution of the primary antibody with the same isotype or pre-immune serum of the antibody; and, (2) omission of the secondary antibody. Immunostaining procedures were repeated at least twice using the same protocol and under the same conditions. Immunostained sections were independently evaluated by two investigators. A given cell was considered immunoreactive if distinct immunoreactivity was consistently seen in its cytoplasm, membrane, or nucleus, while all negative controls lacked distinct immunostaining.
Results
The findings of our current study are in total agreement with our hypothesis. Examinations of the normal appearing lobules with infiltrated leukocyte aggregate consistently revealed that: (1) cells with malignancy associated changes could originate from normal lobules, (2) cells with malignancy associated changes in the normal lobules were eventually in physical continuity with clear-cut invasive lesions, (3) cells with malignancy-associated changes in normal lobules shared the same or similar morphological and immunohistochemical profile with their clear-cut malignant counterparts, and (4) leukocyte aggregates were almost exclusively located at or near the intersection between these lobules and clear-cut invasive lesions (Fig 2).
Structure differences between lobules and ducts.
In situ malignant transformation of lobules.
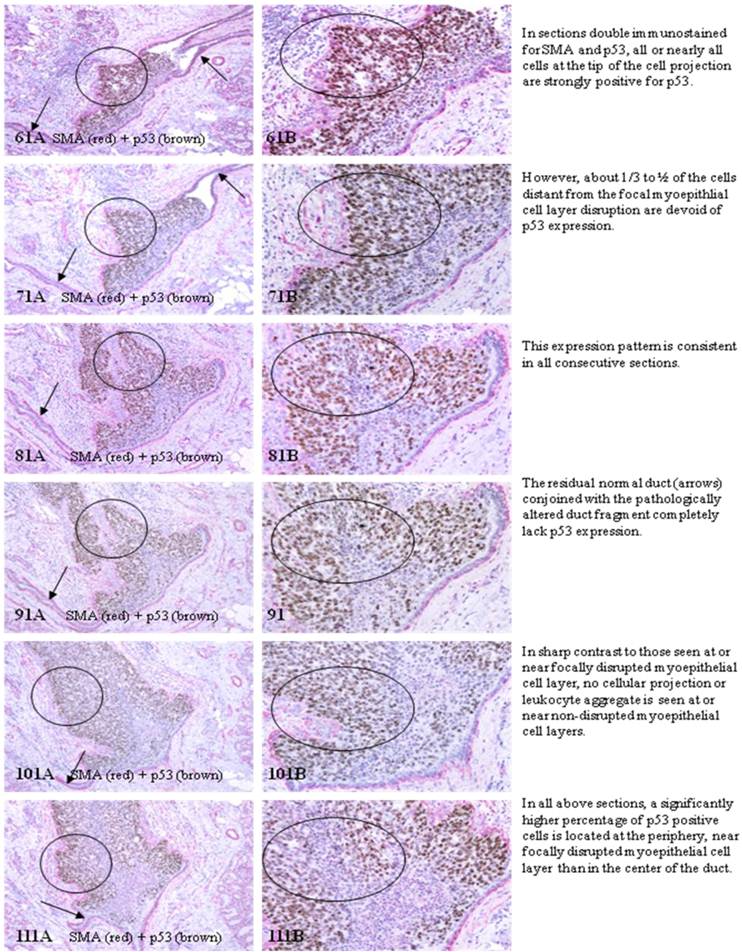
Progenitor-mediated cell budding in mammary ducts.
Multiple cell types within mammary ducts.
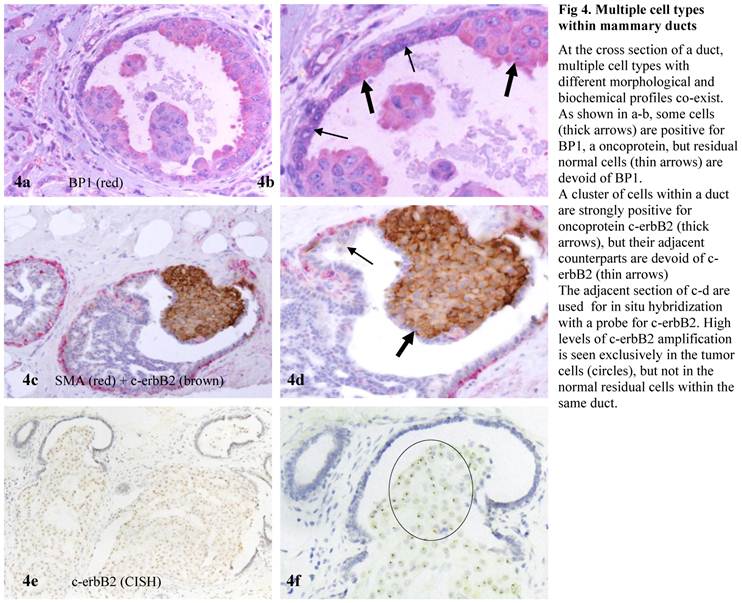
Examinations of the cross and longitudinal section profiles of hyperplastic and in situ breast tumors showed that: (1) morphologically and immunohistochemically different cell types co-existed within the same duct, (2) cell budding was exclusively seen at focally disrupted myoepithelial cell layers, (3) all budding cells shared the same morphological and immunohistochemical profile, (4) budding cells were eventually in physical continuity with clear-cut invasive lesions, and (5) budding cells shared a very similar profile with their clear-cut malignant counterparts (Figs 3-4).
Discussion
Based on our hypothesis, although the lobular cell population has a less suppressive microenvironment and growth advantage, it may retain fewer residual stem or progenitor cells, compared to its ductal counterpart. Therefore, lobular tumors may at a greater risk for invasion or metastasis, whereas the invasive or metastatic lobular tumor cells may have lower potential to form new tumor nests in new tissue sites. Consequently, lobular tumors may have substantially more favorable prognosis than their stage-matched ductal counterpart. Our speculation is in total agreement with a case control study of 37,692 ductal carcinomas in situ (DCIS) and 4,490 lobular carcinoma in situ (LCIS), which showed that patients with LCIS were 5.3-fold more likely than patients with DCIS to develop invasive lobular lesions [41]. Our speculation is also consistent with the pooled data of a number of epidemiological studies, which have shown that although invasive lobular tumors tends to be significantly larger in size with a significantly higher rate of positive lymph nodes than its stage-matched ductal counterpart, patients with invasive lobular tumors have a substantially more favorable clinical outcome [23-27]. Together, these findings suggest the exhaustion or “use-up” the stem population with a normal full term pregnancy or multiple pregnancies may represent an effective mean to reduce breast cancer risk [32-35].
In sharp contrast, as the epithelial component is normally devoid of blood vessels and lymphatic ducts and totally depends on the stroma for its metabolic needs and even survival, a focal myoepithelial cell layer disruption in a given duct could have a number of consequences, including: (a) a localized loss or reduction of tumor suppressors and the paracrine inhibitory functions, which allow the associated tumor cells to undergo elevated proliferation [42]; (b) focal alterations in the permeability for oxygen, which selectively triggers the exit of stem or progenitor cells from quiescence [43,44]; (c) a localized increase of leukocyte infiltration, which directly export growth factors to the associated epithelial cells through direct physical contact [45-47]; (d) the direct epithelial-stromal cell contact, which augments the expression of stromal MMP or represses the normal production and distribution of E-cadherin, and other cell adhesion molecules, facilitating epithelial-mesenchymal transition and cell motility [48-50]; (e) the direct exposure of the epithelial cells to different cytokines, which stimulate an aberrant expression of c-erbB2, which facilitates vasculogenic mimicry and tumor angiogenesis [51,52]; and, (f) the direct physical contact between newly formed cell clusters and stromal cells stimulates the production of tenascin and other invasion-associated molecules that facilitate the stromal tissue remodeling and angiogenesis, providing a favorable micro-environment for epithelial cell proliferation and migration [53,54]. Together, these alterations could selectively favor monoclonal proliferation of the overlying tumor progenitors or a biologically more aggressive cell clone. Thus, the invasive and metastatic cells derived from the duct system may have greater potential to form tumor nests in the new tissue sites, and consequently lead to worse prognosis.
If confirmed, our hypothesis would have a number of clinical implications. First, the application of double immunohistochemistry to identify normal appearing lobular clusters with malignancy-associated alterations and focal myoepithelial cell layer disruptions with “budding” tumor cells in clinical biopsies would significantly facilitate early detection of individuals at greater risk to develop invasive cancer or pending invasive lesions. Second, as if two independent mechanisms or pathways are responsible for lobular and ductal cancer invasion, the precursors of invasive lesions for these tumors are very likely to differ substantially in their morphological, molecular, and/or biochemical profiles. Consequently, micro-dissection of these potential precursors of invasive lesions for gene expression profiling may lead to identification of more specific molecules for differentiation and intervention of invasive lobular and ductal cancer. Third, as it has been well documented that invasive cancer cells derived from lobular cancer tend to be more ER (+), PR (+), and HER-2 (-), compared to their stage-matched ductal counterparts [1-6], invasive and metastatic lesions derived from these tumors may have different responses to the same therapeutic regimen. Therefore, the development of more specific reagents or detection methods to differentiate lobular and ductal cells and their malignant derivatives may have significant therapeutic value. More importantly, as leukocyte aggregates have been consistently seen at the junction between normal lobules harboring cells with malignancy-associated changes and invasive lesions, and also at or near focally disrupted myoepithelial cell layers with budding tumor cells, anti-inflammatory therapy may have significant clinical value for lobular cancers.
Acknowledgements
This study was supported in part by grants DAMD17-01-1-0129, DAMD17-01-1-0130, PC051308 from Congressionally Directed Medical Research Programs, BCTR0706983 from The Susan G. Komen Breast Cancer Foundation, 05AA from AFIP/ARP joint research initiative projects, grant 2008-02 from US Military Cancer Institute and Henry M. Jackson Foundation, and 2006CB910505 from the Ministry of Chinese Science and Technology Department.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Gerard J, Derrickson B. Principles of Anatomy and Physiology; 11th Edition. John Willey & Sons Inc. 2006: 1083.
2. Tot T. DCIS, cytokeratins, and the theory of the sick lobe. Virchows Arch. 2005;447(1):1-8
3. Hussain M, Cunnick GH. Management of lobular carcinoma in-situ and atypical lobular hyperplasia of the breast--a review. Eur J Surg Oncol. 2011;37(4):279-289
4. Tiede B, Kang Y. From milk to malignancy: the role of mammary stem cells in development, pregnancy and breast cancer. Cell Res. 2011;21(2):245-257
5. Russo J, Russo IH. Breast development, hormones and cancer. Adv Exp Med Biol. 2008;630:52-6
6. Russo J, Lynch H, Russo IH. Mammary gland architecture as a determining factor in the susceptibility of the human breast to cancer. Breast J. 2011;7(5):278-291
7. LaMarca HL, Rosen JM. Minireview: hormones and mammary cell fate--what will I become when I grow up? Endocrinology. 2008;149(9):4317-4321
8. Turashvili G, Bouchal J, Burkadze G, Kolar Z. Mammary gland development and cancer. Cesk Patol. 2005;41(3):94-101
9. Love SM, Barsky SH. Anatomy of the nipple and breast ducts revisited. Cancer. 2004;101(9):1947-1957
10. Tot T. The origins of early breast carcinoma. Semin Diagn Pathol. 2010;27(1):62-68
11. Tavassoli FA. Tumors of the mammary gland. Washington DC: American Registry of Pathology. 2009
12. Zhang R, Man YG, Vang RS. et al. A subset of morphologically distinct mammary myoepithelial cells lacks corresponding immunophenotypic markers. Breast Cancer Res. 2003;5:R151-156
13. Man YG, Sang QXA. The significance of focal myoepitehlial cell layer disruptions in breast tumor invasion: a paradigm shift from the “protease-centered” hypothesis. Exp Cell Res. 2004;301:103-118
14. Zou Z, Anisowicz A, Hendrix MJ. et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526-529
15. Barbareschi M, Pecciarini L, Cangi MG. et al. p63, a p53 homo-logue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol. 2001;25:1954-1960
16. Li JH, Man YG. Dual usages of single Wilms' tumor 1 immu-nohistochemistry in evaluation of breast tumors: A preliminary study of 30 cases. Cancer Biomarkers. 2009;5(3):109-116
17. Xu ZL, Wang W, Deng CX, Man YG. Aberrant p63 and WT-1 expression in myoepithelial cells of pregnancy-associated breast cancer: implications for tumor aggressiveness and inva-siveness. Int J Biol Sci. 2009;5(1):82-96
18. Hsiao YH, Siddiqui S, Man YG. Dual use of a single Wilms' tumor 1 immunohistochemistry in evaluation of ovarian tu-mors: a preliminary study of 20 cases. J Cancer. 2010;1:93-97
19. Zhang R, Man YG, Vang RS, Saenger JS, Barner R, Wheeler D. et al. A subset of morphologically distinct mammary myoepi-thelial cells lacks corresponding immunophenotypic markers. Breast Cancer Res. 2003;5:R151- 156
20. Hsiao YH, Su YA, Tsai HD, Mason JT, Chou MC, Man YG. Increased invasiveness and aggressiveness in breast epithelia with cytoplasmic p63 expression. Int J Biol Sci. 2010;6(5):428-442
21. Wahed A, Connelly J, Reese T. E-cadherin expression in pleo-morphic lobular carcinoma: an aid to differentiation from ductal carcinoma. Ann Diagn Pathol. 2002;6(6):349-351
22. Acs G, Lawton TJ, Rebbeck TR, LiVolsi VA, Zhang PJ. Diffe-rential expression of E-cadherin in lobular and ductal neop-lasms of the breast and its biologic and diagnostic implications. Am J Clin Pathol. 2001;115(1):85- 98
23. Wasif N, Maggard MA, Ko CY, Giuliano AE. Invasive lobular vs. ductal breast cancer: a stage-matched comparison of outcomes. Ann Surg Oncol. 2010;17(7):1862-1869
24. Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6(3):R149-56
25. Toikkanen S, Pylkkanen L, Joensuu H. Invasive lobular carci-noma of the breast has better short- and long- term survival than invasive ductal carcinoma. Br J Cancer. 1997;76(9):1234-1240
26. Crivellari D, Molino A. Small tumor size and node-negative HER2-positive breast cancer: a step forward for a better treat-ment? J Clin Oncol. 2010;28(16):e257
27. Foulkes WD, Reis-Filho JS, Narod SA. Tumor size and survival in breast cancer--a reappraisal. Nat Rev Clin Oncol. 7(6): 348-353, 2010. 2010
28. Korkola JE, DeVries S, Fridlyand J, Hwang ES, Estep AL, Chen YY. et al. Differentiation of lobular versus ductal breast carci-nomas by expression microarray analysis. Cancer Res. 2003;63(21):7167-7175
29. Lee JH, Park S, Park HS, Park BW. Clinicopathological features of infiltrating lobular carcinomas comparing with infiltrating ductal carcinomas: a case control study. World J Surg Oncol. 2010;8:34
30. Christgen M, Bruchhardt H, Hadamitzky C, Rudolph C, Stei-nemann D, Gadzicki D. et al. Comprehensive genetic and functional characterization of IPH-926: a novel CDH1-null tu-mor cell line from human lobular breast cancer. J Pathol. 2009;217(5):620-632
31. Tubiana-Hulin M, Stevens D, Lasry S, Guinebretiere JM, Bouita L, Cohen-Solal C. et al. Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol. 2006;17(8):1228-1233
32. Jordan I, Hebestreit A, Swai B, Krawinkel MB. Breast cancer risk among women with long-standing lactation and reproductive parameters at low risk level: a case-control study in Northern Tanzania. Breast Cancer Res Treat. 2010 [Epub ahead of print]
33. Russo J, Balogh GA, Russo IH. Full-term pregnancy induces a specific genomic signature in the human breast. Cancer Epidemiol Biomarkers Prev. 2008;17(1):51-66
34. Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K. et al. The CD44+/CD24- phenotype in enriched in basal-like breast tumors. Breast Cancer Res. 2008;10(3):R53
35. Guest I, IIic Z, Grant D, Glinsky G, Sell S. Direct and indirect contribution of bone marrow-derived cells to cancer. Int J Cancer. 2010;126(10):2308-2318
36. Man YG, Zhao CQ, Wang J. Breast tumor cell clusters and their budding derivatives show different immunohistochemical profiles during stromal invasion: implications for hormonal and drug therapies. Cancer Therapy. 2006;4:193-204
37. Man YG, Nieburgs HE. A subset of cell clusters with malignant features in morphologically normal and hyperplastic breast tissues. Cancer Detect Prev. 2006;30(3):239-247
38. Man YG. Focal degeneration of aged or injured myoepithelial cells and the resultant auto- immunoreactions are trigger factors for breast tumor invasion. Medical Hypotheses 69(6):1340-1357,2007 breast. Cancer Epi-demiol Biomarkers Prev. 2008;17(1):51-66
39. Man YG. Bad seeds produce bad crops: a single step-process of breast carcinogenesis and progression. Bioscience Hypotheses. 2008;1:147-155
40. Man YG, Mason J, Harley R, Kim YH, Zhu KM, Gardner WA. Leukocyte-facilitated tumor dissemination: a novel model for tumor cell dissociation and metastasis. J Cell Biochem. 2011 doi: 10.1002/jcb.23035
41. Li CI, Malone KE, Saltzman BS, Daling JR. Risk of invasive breast carcinoma among women diagnosed with ductal carcinoma in situ and lobular carcinoma in situ, 1988-2001. Cancer. 2006;106(10):2104-2012
42. Yanochko GM, Eckhart W. Type 1 insulin-like growth factor overexpression induce proliferation and anti- apoptotic sig-naling in a three-dimensional culture model of breast epithelial cells. Breast Cancer Res. 2006;8(2):R18-R23
43. Chakravarthy MV, Spangenhurg EE, Booth FW. Culture in low levels of oxygen enhances in vitro proliferation potential of satellite cells from old skeletal muscles. Cell Mol Life Sci. 2001;58:1150-1158
44. Studer L, Csete M, Lee SH. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowed oxygen. J Neurosci. 2000;20:7377-7383
45. Freeman MR, Schneck FX, Gognon ML. et al. Peripheral blood T-lymphocytes and lymphocytes infiltrating human cancers express vascular endothelianl growth factor: a potential role for T cells in angiogenesis. Cancer Res. 1995;55:4140-4145
46. Nienartowicz A, Sobaniee-Lotowska ME. et al. Mast cells in neoangiogenesis. Med Sci Monit. 2006;12(3):53- 56
47. Qu Z. Immunohistological detection of growth factors and cytokines in tissue mast cells. Methods Mol Biol. 2006;315:257-272
48. Kang Y, Massague J. Epithelial-mesenchymal transition: twist in development and metastasis. Cell. 2004;118:277-279
49. Sato T, Sakai T, Noguchi Y, Takita M, Hirakawa S, Ito A. Tumor-stromal cell contact promotes invasion of human uterine cervical carcinoma cells by augmenting the expression and ac-tivation of stromal matrix metalloproteinases. Gynecol Oncol. 2004;92:47-56
50. Strizzi L, Bianco C, Normanno N. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Criptol-1 transgenic mice. J Cell Physiol. 2004;201:266-276
51. Klos KS, Wyszomierski SL, Sun M. et al. c-erbB2 increases vas-cular endothelial growth factor protein synthesis via activation of mammalian target of rapamycin/p70S6K leading to in-creased angiogenesis and spontaneous metastasis of human breast cancer cells. Cancer Res. 2006;6(4):2028-2037
52. Carter WB, Hoying JB, Boswell C, Williams SK. HER2/neu over-expression induces endothelial cell retraction. Int J Cancer. 2001;91(3):295-299
53. Verstraeten AA, Mackie EJ, Hageman PC. et al. Tenascin ex-pression in basal cell carcinoma. Brit J Dermt. 1992;127:571-574
54. Ilunga K, Nishiura R, Inada H. et al. Co-stimulation of human breast cancer cells with transforming growth factor-beta and tenasicin-c enhances matrix metalloproteinase-9 expression and cancer cell invasion. Int J Exp Pathol. 2004;85:373-379
Author contact
![]() Corresponding author: Yan-gao Man, MD., PhD., Diagnostic and Translational Research Center, Henry Jackson Foundation, 401 Professional Drive, Gaithersburg, 20879. Phone: 301-326-6731; Fax: 301-879-0816; E-mail: ymanorg
Corresponding author: Yan-gao Man, MD., PhD., Diagnostic and Translational Research Center, Henry Jackson Foundation, 401 Professional Drive, Gaithersburg, 20879. Phone: 301-326-6731; Fax: 301-879-0816; E-mail: ymanorg

 Global reach, higher impact
Global reach, higher impact