Impact Factor
ISSN: 1837-9664
J Cancer 2012; 3:122-128. doi:10.7150/jca.4123 This volume Cite
Research Paper
Prostate Cancers Detected During 5α-Reductase Inhibitor Use Are Smaller, De-Differentiated, But Confined when Compared To Controls
1. Rochester Urology, PC, Rochester Hills, MI
2. Crittenton Hospital, Rochester Hills, MI
3. Department of Biostatistics and School of Public Health, University of Michigan, Ann Arbor, MI
Received 2012-1-19; Accepted 2012-3-4; Published 2012-3-7
Abstract
Rationale: To compare cancers detected during use of 5α-reductase inhibitors (5αRI) with cancers detected in untreated controls stratified for tumor size.
Methods: Prostate biopsies were performed on 235 consecutive patients “for cause” (elevated or rising PSA, positive digital rectal examination, or focal hypoechoic lesion). Fifty patients were excluded for a prior diagnosis of cancer, leaving 185 as the study group (5αRI=41, control=144). Patients in the 5αRI group had been treated for a mean of 3.5 years. Cancer was ultimately diagnosed in 114/185 patients.
Results: Cancer was diagnosed in 31/41 (76%) of patients treated with 5αRI and 83/144 (58%) of the control group (p=0.04). Control tumors were larger (14.3 mm) than those in 5αRI treated patients (9.4 mm, p=0.0007). No differences in mean PSA or PSA kinetics were detected between groups. For tumors less than 1.0 cm, the proportion of high grade cancers (Gleason 7-10 and Gleason 4+3-10) was higher in 5αRI subjects than in controls (p<0.05). Fewer 5αRI patients had proven extracapsular extension than controls, but this difference did not reach statistical significance (p=0.13). Normal DNA ploidy was more likely to be diagnosed in the 5αRI group versus controls, but this difference was not statistically significant (81% vs. 65%, p=0.14).
Conclusions: Cancers diagnosed in patients presenting “for cause” treated with 5αRI drugs are more likely to be de-differentiated compared to controls. However, these tumors are also smaller and less likely to have extracapsular extension and abnormal DNA ploidy than controls.
Keywords: Prostate Cancer, Benign Prostatic Hyperplasia, 5α-reductase inhibitors, Gleason score, DNA ploidy, cancer staging.
Introduction
Treatment of benign prostatic hyperplasia (BPH) with 5α-reductase inhibitors (5αRI) is now widespread (1,2). The 5αRI drugs block the conversion of testosterone into the more active dihydrotestosterone, leading to shrinkage of androgen sensitive tissues in the prostate. Besides the use for treatment of BPH, the anti-androgenic effects of 5αRI have been extensively explored for the chemoprevention of prostate cancer. In the largest studies to date, both finasteride and dutasteride were associated with a decrease in the overall number of prostate cancer cases (3,4). However, an unexpected finding was an increase in the rate of high grade cancer detected in the 5αRI groups compared to controls and raises questions about a possible association between 5αRI and biologically aggressive tumors (5).
Multiple studies have demonstrated a strong association between prostate cancer size, pathologic grade and curability. Before the era of 5αRI drugs, most tumors < 1 cm in size were of low Gleason grade, confined to the prostate, and considered “curable” (6-9). Small tumors with high Gleason grades and aggressive biologic behaviors were unusual. The motivation for this study was the recent anecdotal observation in our practice of an unexpected number of small prostate cancers with high Gleason grades in men treated with 5αRI drugs. Therefore, the purpose of this study was to retrospectively compare the characteristics of size matched cancers in patients treated with 5αRI vs. a control group consisting of cancers in untreated patients.
Materials and Methods
Between 1/1/2008 and 9/15/2010, 235 consecutive transrectal-ultrasound guided prostatic biopsies were performed by a single radiologist (FL) using a Hitachi model EUB 6000 (Hitachi Medical Systems, Terrytown, NY) with 5-7.5 MHz transducer with color flow Doppler. Indications for biopsy included: 1) an abnormal PSA (10) or digital rectal examination (DRE) and/or 2) a focal hypoechoic lesion detected by ultrasound (11,12). When a focal lesion was not visible but the PSA was elevated above predicted for gland volume, sextant biopsies were performed. When a focal hypoechoic lesion was visible, both targeted (2-3 cores) and sextant biopsies were performed (13). Mean tumor size was calculated by the formula (Width + Height + Length)/3 (11). Tumors were assigned to one of four groups based on mean tumor size (<5 mm, 5-9 mm, 10-15 mm, >15 mm). Tumors that were diagnosed only by sextant biopsies (i.e. no hypoechoic lesion was visible) were placed in the < 5 mm group (9). Tumors were grouped into two size categories (<1.0 cm and ≥1.0 cm for the purposes of statistical comparisons. Retrospective chart review included DRE, PSA level and kinetics, length of time on 5αRI, and prostate gland volume. The pathologic results included tumor size in millimeters, Gleason score and extracapsular extension determined by biopsies of neurovascular bundles and seminal vesicles (14). DNA ploidy was obtained and considered abnormal if tetraploid and aneuploid phases were detected (15,16).
Statistical Analysis
For all statistical analysis results reported, two-sided p-values of 0.05 or less were considered to indicate statistical significance. Two-sample t-tests, ANOVA, Wilcoxon-Mann-Whitney tests and Kruskal-Wallis tests were conducted for continuous variables, and Chi-square tests or Fisher's exact tests for associations were performed for contingency tables. Relative risks were used to compare the risks of high-grade cancer or abnormal pathologic results between the 5αRI treated group and controls. For our study, Mantel-Haenszel estimates adjusting for tumor size were used to calculate all relative risks and associated confidence intervals; for other 5αRI related studies we considered, logarithm transformation was used to calculate the confidence intervals of relative risks.
RESULTS
Study group: Of the 235 patients who underwent prostatic biopsies during the study period, 50 were excluded due to a previous diagnosis of prostate cancer. Of the remaining 185 subjects, 41 were being treated with a 5αRI at the time of biopsy (21 on finasteride alone, 12 on dutasteride alone, and 8 on both), and 144 were not. Prostate cancer was diagnosed in a total of 114 of the 185 subjects, and these 114 patients constitute the study group. When stratified for 5αRI use, 31/41 (76%) of patients treated with 5αRI and 83/144 (58%) of the control group had cancer (p=0.04). Among the 31 5αRI treated subjects with cancer, the mean duration (standard deviation) of 5αRI use was 3.5 (2.3) years. The treatment durations were not significantly different between subjects with different tumor sizes [p=0.53].
Patient ages in the 5αRI and control groups were not statistically different, with a mean (standard deviation) of 66.5 (8.6) years and 65.8 (8.8) years, respectively [p=0.70]. Gland volumes of subjects in the 5αRI group were significantly larger than controls, with a mean (standard deviation) 53.7 (30.4) and 45.4 (24.3) cubic centimeters, respectively [p=0.13].
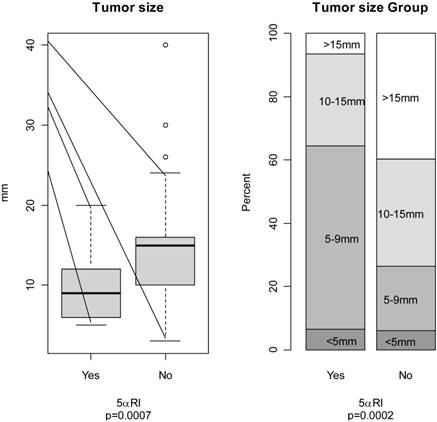
Tumor sizes: The distribution of tumor sizes between groups was significantly different, with larger tumor diameters found in controls (14.3 [7.0] mm) vs. the 5αRI subjects (9.4 [4.0] mm) [p=0.0007]. In particular, there was a significantly higher proportion of small prostate cancers (< 1 cm) in the 5αRI group when compared to controls, Figure 1 [64.5% vs. 26.5%, p=0.0002].
Tumor sizes Distributions. : Distribution of tumor size by 5αRI use. Left: Boxplot for tumor size in millimeters; Right: Percentage of tumor sizes <5mm, 5-9mm, 10-15mm, and >15mm.
Physical examination findings: Digital rectal examination overall was not as likely to be positive in 5αRI patients (16% positive) as controls (41% positive, p=0.01). The likelihood of a positive DRE increased as tumor size increased for both 5αRI and controls. For tumors < 1 cm, DRE was positive in 15% and 9%, and in tumors ≥ 1 cm DRE positive tumors increased to 18% and 52% for 5αRI patients and controls, respectively [p=0.04]. After adjusting for tumor sizes, the relative risk of a positive DRE between 5αRI and no 5αRI groups was 0.60, with 95% C.I. (0.23, 1.56).
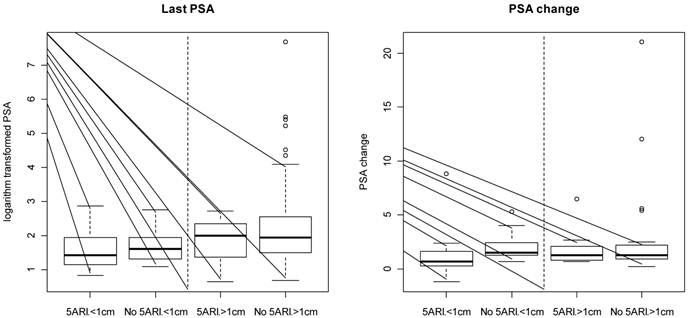
Prostate specific antigen: PSA was either elevated (≥4) or increased in the majority of patients in both the 5αRI and control groups, regardless of tumor size (Table 1). No significant differences were detected between groups for either total PSA or PSA kinetics (Figure 2).
Gleason scores: Table 1 summarizes the distribution of histologic high grade cancer (Gleason 7-10 or Gleason 4+3-10) stratified by tumor size and 5αRI use. Overall, patients on 5αRI drugs diagnosed with cancer had a higher likelihood of having a high grade tumor, but this difference did not quite reach statistical significance (Gleason 7-10: 77% vs. 64%, p=0.17; Gleason 4+3-10: 45% vs. 30%, p=0.13). For small (< 1 cm) tumors, the proportion of high grade cancers (Gleason 7-10 and Gleason 4+3-10) was higher than in controls [85% vs. 27% (p=0.0002) and 55% vs. 5% (p=0.0003), respectively].
Relative risk of high grade prostate cancer: The relative risk of high-grade cancer for 5αRI patients in our study is presented in Table 2 along with the results of other trials examining 5αRI use in chemoprevention. The studies listed include: Finnish Prostate Cancer Screening Trial (17), Prostate Cancer Prevention Trial (PCPT) (3), Radical Prostatectomy (RP) Cases of PCPT (18) and Effect of Dutasteride on the Risk of Prostate Cancer (REDUCE) (4). For the relative risks of Gleason 7-10 and associated confidence intervals, our result of 1.54 with 95% C.I. (1.06, 2.24) was consistent with those from the Finnish study, 7-year PCPT, RP cases of PCPT, as well as REDUCE at 3-4 years. Our relative risk of Gleason 4+3 to 10 was 2.84 with 95% C.I. (1.28, 6.27) was also similar to the relative risk of RP cases of PCPT (where only results of Gleason 8-10 are available), and similar to results from the REDUCE study (3-4 years).
Extracapular extension: Analysis of systematic biopsy was available in 7/31 (23%) of 5αRI subjects, and 45/83 (54%) of controls (p=0.003). Among patients whose systematic biopsy results were available, a higher proportion of control patients had positive extracapsular extension, but this difference did not quite meet statistical significance (14% vs. 44%, p=0.13, 5αRI and control subjects respectively). In particular, for tumor size ≥1 cm, 0/5 of 5αRI subjects and 20/42 (48%) of non-5αRI subjects had positive ECE (p=0.06).
DNA Ploidy: Analysis of DNA ploidy was available in 26/31 (84%) of 5αRI subjects, and 75/83 (90%) of controls. The remaining 13 patients had insufficient tissue for evaluation. The proportion of cancers with normal DNA in the 5αRI group was higher than in controls, but this difference was not statistically significant [81% vs. 65%, p=0.14, Table 2]. Adjusting for tumor sizes, the relative risk of abnormal DNA ploidy in the 5αRI group patients was 0.73 with 95% C.I. (0.27, 2.02).
PSA kinetics. Distribution of last PSA measurement (left) and PSA measurement change (right) by 5αRI use and tumor size.
PSA Kinetics and Gleason Scores of Prostate Cancers.
| TRUS Tumor Size | Group | 1st PSA ≥ 4 or change > 0 | PSA Change Median (Range) | Gleason 7-10 | Gleason 4+3 to 10 | Total |
|---|---|---|---|---|---|---|
| <1 cm | 5αRIs | 18 (90%) | 0.8 (0.2) | 17 (85.0%) | 11 (55.0%) | 20 |
| No 5αRIs | 20 (90.9%) | 1.5 (0.7) | 6 (27.3%) | 1 (4.5%) | 22 | |
| ≥1 cm | 5αRIs | 11 (100%) | 1.3 (0.7) | 7 (63.6%) | 3 (27.3%) | 11 |
| No 5αRIs | 55 (90.2%) | 1.3 (0.2) | 47 (77.0%) | 24 (39.3%) | 61 | |
| Combined | 5αRIs | 29 (93.5%) | 1.3 (0.2) | 24 (77.4%) | 14 (45.2%) | 31 |
| No 5αRIs | 75 (90.4%) | 1.5 (0.2) | 53 (63.9%) | 25 (30.1%) | 83 | |
| Subtotal | 104 (91.2%) | 1.3 (0.2, 21) | 77 (67.5%) | 39 (34.2%) | 114 | |
Relative Risk of High-Grade Cancer in 5αRI patients.
| Study | Relative Risk (95% C.I.) | |
|---|---|---|
| Gleason 7-10 vs. Gleason ≤ 6 | Gleason 4+3-10 vs. Gleason ≤ 3+4 | |
| 1. Current study | 1.54 (1.06, 2.24) | 2.84 (1.28, 6.27) |
| 2. Finnish study17 | 1.59 (1.01, 2.50) | - |
| 3. PCPT (All Cancer)3 | 1.69 (1.46, 1.96) | 2.43 (1.75, 3.36)* |
| 4. PCPT (RPs)18 | 1.68 (1.30, 2.17) | 3.26 (1.87, 5.69)* |
| 5. REDUCE (1-2 years)4 | 1.09 (0.91, 1.31) | 1.19 (0.81, 1.75) |
| 6. REDUCE (3-4 years)4 | 1.67 (1.21, 2.34) | 5.09 (2.27, 11.40) |
| 7. REDUCE (1-4 years)4 | 1.22 (1.04, 1.42) | 1.68 (1.21, 2.34) |
*: Relative risk between Gleason 8 to 10 vs. Gleason ≤ 7.
Tumor Characteristics.
| Extracapsular extension | DNA ploidy | ||||
|---|---|---|---|---|---|
| Tumor Size | Group | Positive | Total | Normal | Total |
| <1 cm | 5αRIs | 1 | 2 | 14 (77.8%) | 18 |
| No 5αRIs | 0 | 3 | 14 (82.4%) | 17 | |
| ≥1 cm | 5αRIs | 0 | 5 | 7 (87.5%) | 8 |
| No 5αRIs | 20 (47.6%) | 42 | 35 (60.3%) | 58 | |
| Combined | 5αRIs | 1 (14.3%) | 7 | 21 (80.8%) | 26 |
| No 5αRIs | 20 (44.4%) | 45 | 49 (65.3%) | 75 | |
| Subtotal | 21 (40.4%) | 52 | 70 (69.3%) | 101 | |
Discussion
The randomized controlled trials investigating the potential chemopreventive attributes of 5αRI drugs published to date were designed primarily to detect a difference in the incidence of prostate cancer in a large population treated with 5αRI drugs. As a result, routine biopsies performed at fixed time intervals (without a particular clinical indication) were a critical component of the trial design. The results of these timed biopsies demonstrated a decrease in the overall number of cancers detected in 5αRI-treated subjects compared to untreated controls. This decreased number of detected cancers led the authors to conclude that 5αRI drugs were chemopreventive against prostate cancer. By way of contrast, our study is a retrospective analysis of a series of prostatic biopsies performed for specific clinical indications (an abnormal physical examination, rising or abnormal PSA, or a hypoechoic lesion detected at ultrasound), i.e. “for cause.” Therefore, the focus of our study is on those patients that already have met the criteria to undergo prostatic biopsy, not an epidemiologic study of the effect of 5αRI treatment on a population. In this setting, the positive biopsy rate was slightly higher for patients treated with 5αRI when compared to controls (76% vs. 58%, p=0.04). We believe that biopsying “for cause” such as in this study, is representative of most clinical practices, and is best used to inform physicians and patients when management decisions are being made. Interestingly, in the two randomized studies of 5αRI for chemoprevention of prostate cancer published to date (PCPT and REDUCE), biopsies performed "for cause” found no significant difference in prostate cancer detection rates between groups (3,4).
Assuming that treatment of a population with 5αRI decreases the number of cancers detected, the overall salutatory effect of this result on a population bears close scrutiny in light of other data from the trials and the results of this study. Prior to the 5αRI era, virtually all small (<1cm) prostate cancers were of low histologic grade, only rarely had extracapsular extension, and were highly curable (6-9, 11,12). Prior pathologic studies strongly suggest that 5αRI drugs either shrink or inhibit the growth of many prostate cancers (18,19). The results of our study support this hypothesis--65% of patients treated with 5αRI had tumors <1cm as compared to only 27% in untreated controls. The question that remains is whether shrinking or inhibiting tumor growth ultimately changes biologic behavior. In the absence of a long-term longitudinal randomized controlled trial, examination of surrogate markers of tumor aggressiveness lead to a mixed picture. For example, in this study, Gleason scores were higher in patients treated with 5αRI compared to controls. This finding was even more dramatic in small tumors where 85% of patients in the 5αRI group were found to have aggressive tumors (Gleason 7-10) compared to 27% in the control group. The relative risk of having an aggressive tumor in this study was 1.54, a comparable result to other studies where this ranged from 1.1-1.7 (Table 2). Thus, while the overall number of tumors may be decreased, the histologic aggressiveness of the remaining tumors appears to be increased.
Questions have been raised as to whether the high grade cancers detected during PCPT and REDUCE were a result of artifactual morphologic changes that could result in an over-estimation of the biologic potential of high-grade cancers in patients treated with 5 alpha-reductase inhibitors (20). Lucia, et al. evaluated the pathology of cancers found during PCPT and concluded that finasteride did not induce histomorphologic changes in prostate cancer, but may have contributed to the increase in high-grade cancers (18). Civantos, et al. observed that finasteride could induce foci of low grade prostate cancer to resemble high grade cancer (21). In response to this controversy, the FDA Oncologic Drugs Advisory Committee had the histopathology from the PCPT and REDUCE trials re-evaluated by multiple urologic pathologists. Results of this analysis demonstrated that insignificant histopathologic differences were detected between cancers in patients treated with 5 alpha-reductase inhibitors and placebo, and intra-observer variability between Gleason scores among pathologists was low (22,23).
The association of low volume tumors and high Gleason scores found in this study most likely is a reflection of the time our patients had been on 5αRI drugs prior to diagnosis (mean 3.5 years). Despite the fact that our study is limited by data collection at a single time point, the longitudinal data from other studies demonstrates similar results in patients who have been on 5aRI drugs for ≥ 4 years. For example, a higher proportion of high grade cancers were detected in the later data (but not at earlier time points) in the Finnish (17) and REDUCE (4) studies. Throughout the seven years of the PCPT (3) high grade cancers occurred. However the end of study biopsies at year 7 discovered 38% (211/557) of these high grade cancers (23). This raises the possibility that 5αRI drugs selectively inhibit the growth of hormonally sensitive low grade cancers by reducing the levels of intracellular dihydrotestosterone within the prostate. In the case of high grade or heterogeneous cell populations, 5aRIs may selectively allow growth of only the hormonal refractory high grade components.
Besides Gleason score, other markers of biologic activity include extracapsular extension of tumor and tumor ploidy (14,15,16). In this study, the risk of extracapsular extension was most dependent on tumor size rather than Gleason score. In fact, the overall risk of extracapsular extension was less in 5αRI treated patients vs. untreated controls, likely reflecting the smaller tumor size in 5αRI patients. The etiology of decreased extracapsular extension in this series may be similar to earlier studies where adjuvant treatment of large prostate cancers prior to radiation therapy with androgen deprivation shrunk tumors and decreased overall mortality (24,25). Based on the results of this study, it could be argued that 5αRI is protective against extracapsular invasion in patients ultimately diagnosed with cancer, despite higher Gleason scores. The other marker of aggressive biologic behavior examined in this study, tumor ploidy, demonstrated a more normal DNA appearance in 5αRI treated patients vs. controls, but this difference was not statistically significant (p=0.14). Therefore, it appears as if patients treated with 5αRI who are eventually proven to have prostate cancer tend to have higher grade (but smaller) tumors with less evidence of extracapsular extension vs. controls, and more normal (but not statistically significant) appearing DNA. These findings correlate with the findings of the PCPT where high grade cancers were associated with lower surrogate findings of tumor aggression in patients treated with Finasteride (18). Whether this translates into a survival difference compared to untreated prostate cancer remains to be seen.
The main limitation of this study is the retrospective consecutive case-series design and the lack of randomized controlled data. However, while this is a weakness in determining the impact of 5αRI treatment on a population, it is advantageous when applying the findings to a clinical practice where the target population is patients who have undergone a positive biopsy and are being treated with 5αRI drugs. An additional limitation is our use of ultrasound to stratify patients according to tumor size. We have proven the accuracy of tumor size measurements using ultrasound in our practice (11,12,14), however, it is unclear whether this method is generalizable to all practitioners. In the interest of completeness, we have also included conglomerate data based on sextant biopsies similar to studies where targeted ultrasound techniques were not available (13). The data on extracapsular extension was also obtained using targeted biopsies according to our previously published methods (14). This could lead to an undersampling bias since generally only tumors >1 cm received extra-capsular biopsies. Lastly, this study was limited to a 32 month period and the mean duration of 5αRI use was only 3.5 years. Whether treatment with 5αRI over longer time periods would change the results is not known, but a longer term study will be needed to answer these questions and determine any impact on survival.
In summary, our study and others suggest that prostate cancer diagnosed in patients who are treated with 5αRI over extended periods are associated with increased tumor grade, even in small tumors < 1cm. Extracapsular extension of cancer and abnormal tumor ploidy appear to be decreased in patients on 5αRI drugs. Therefore, besides Gleason score, the other surrogate markers of tumor aggressiveness suggest that small tumors (even if high grade) in 5αRI-treated patients may still be low risk if detected when small. This information suggests the need for an aggressive screening strategy for patients who are expected to remain on 5aRI drugs for long time periods.
Acknowledgements
This work was supported in part by an unrestricted grant from Crittenton Hospital, Rochester Hills, MI, and Grants 5P50CA069568 and 5U01CA157224 from the National Cancer Institute.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Debruyne F, Barkin J, van Erps P, Reis M, Tammela TLJ, Roehrborn C. Efficacy and safety of long-term treatment with the dual 5α-Reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. European Urology. 2001;46:488-95
2. McConnell JD, Roehrborn CG, Bautista OM. et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387-98
3. Thompson IM, Goodman PJ, Tangen CM. et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215-24
4. Andriole GL, Bostwick DG, Brawley OW. et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192-202
5. Walsh PC. Chemoprevention of prostate cancer. N Engl J Med. 2010;362:1237-8
6. McNeal JE. Origin and development of carcinoma in the prostate. Cancer. 1969;23:24
7. McNeal JE. Cancer volume and site of origin of adenocarcinoma in the prostate: Relationship to local and distant spread. Hum Pathol. 1992;23:258
8. Culp OS, Meyer JJ. Radical prostatectomy in the treatment of prostatic cancer. Cancer. 1973;32:1113
9. Stamey TA, Freiha FS, McNeal JE, Redwine EA, Whittemore AS, Schmid HP. Localized prostate cancer. Cancer. 1993;71(S3):933-38
10. Lee F, Littrup PJ, Loft-Christensen L, Kelly BS. et al. Predicted prostate specific antigen results using transrectal ultrasound gland volume differentiation of benign prostatic hyperplasia and prostate cancer. Cancer. 1992;70(S1):211
11. Lee F, Torp-Pedersen S, Littrup PJ. et al. Hypoechoic lesions of the prostate: Clinical relevance of tumor size, digital rectal examination, and prostate-specific antigen. Radiology. 1989;170:29-32
12. Mettlin CJ, Murphy GP, Babaian RJ. et al. Observations on the early detection of prostate cancer from the American Cancer Society national prostate cancer detection project. Cancer. 1997;90:1814-1817
13. Onur R, Littrup PJ, Pontes JE, Bianco FJ Jr. Contemporary impact of transrectal ultrasound lesions for prostate cancer detection. J Urol. 2004;172:512-4
14. Lee F, Bahn DK, Siders DB, Green C. The role of TRUS-guided biopsies for determination of internal and external spread of prostate cancer. Sem Urol Onc. 1998;16:129-36
15. Montgomery BT, Nativ O, Blute ML. et al. Stage B prostate adenocarcinoma: Flow cytometric nuclear DNA ploidy analysis. Arch Surg. 1990;125:327-31
16. Seay TM, Blute ML, Zincke H. Long-term outcome in patients with pTxN+ adenocarcinoma of prostate treated with radical prostatectomy and early androgen ablation. J Urol. 1998;159:357-64
17. Murtola TJ, Tammela TL, Maattanen L, Ala-Opas M, Stenman UH, Auvinen A. Prostate cancer incidence among finasteride and alpha-blocker users in the finnish prostate cancer screening trial. Br J Cancer. 2009;101:843-8
18. Lucia MS, Epstein JI, Goodman PJ. et al. Finasteride and high-grade prostate cancer in the prostate cancer prevention trial. J Natl Cancer Inst. 2007;99:1375
19. Gleave M, Qian J, Andreou C. et al. The effects of the dual 5α-reductase inhibitor dutasteride on localized prostate cancer - Results from a 4-month pre-radical prostatectomy study. Prostate. 2006;66:1674
20. Andriole GL, Humphrey PA, Serfling RF, Grubb RL. High-grade prostate cancer in the prostate cancer prevention trial: Fact or artifact? J Natl Cancer Inst. 2007;99:1355-56
21. Civantos F, Watson RF, Pinto JE, Korman HG, Soloway MS. Finasteride effect on prostatic hyperplasia and prostatic cancer. J Urol Path. 1997;6:1-13
22. FDA Briefing Document. Oncologic Drugs Advisory Committee Meeting, December 1, 2010 (NDA180/S034); Proscar (Finasteride 5 mg). Merck and Co, Inc. Page 22.
23. FDA Briefing Document. Oncologic Drugs Advisory Committee Meeting, Decvember 1, 2010 (NDA21319/S0024); Avodart (Dutasteride). GlaxoSmithKline, Inc. Page 19.
24. Bolla M, de Reijke TM, Van Tienhoven G. et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516-27
25. Widmark A, Klepp O, Solberg A. et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomized phase III trial. Lancet. 2009;373:301-08
Author contact
![]() Corresponding author: Fred Lee, MD., Rochester Urology, PC, 1202 Walton Boulevard, Suite 211, Rochester Hills, MI 48307. Tel: 248-650-4699, Fax: 248-650-4696 fleecom
Corresponding author: Fred Lee, MD., Rochester Urology, PC, 1202 Walton Boulevard, Suite 211, Rochester Hills, MI 48307. Tel: 248-650-4699, Fax: 248-650-4696 fleecom

 Global reach, higher impact
Global reach, higher impact