Impact Factor
ISSN: 1837-9664
J Cancer 2012; 3:226-230. doi:10.7150/jca.4091 This volume Cite
Case Report
A Case Report: Lobular Carcinoma In Situ in a Male Patient with Subsequent Invasive Ductal Carcinoma Identified on Screening Breast MRI
Montefiore Medical Center, Greene Medical Arts Pavilion, 3400 Bainbridge Avenue. Bronx, NY 10467, USA
Received 2012-1-14; Accepted 2012-3-2; Published 2012-5-22
Abstract
Lobular carcinoma in situ is a form of in situ neoplasia that develops within the terminal lobules of the breast. It is an extremely rare finding in males due to the lack of lobular development in the male breast. The authors herein report an unusual case of incidentally discovered lobular carcinoma in situ in a male patient with recurrent bilateral gynecomastia who was subsequently diagnosed with invasive ductal carcinoma of the left breast. The pathology of lobular carcinoma in situ in a male as well as screening MRI surveillance of male patients at high risk for breast cancer are discussed, emphasizing the importance of screening and imaging follow up in men who are at high risk for breast cancer.
Keywords: Lobular carcinoma in situ, male, breast cancer, MRI, screening and imaging
Introduction
Lobular carcinoma in situ (LCIS) is a form of in situ neoplasia that develops within the terminal lobules of the breast. It is extremely rare in males due to the lack of lobular development in the male breast. Furthermore, there is scarce data on the utility of screening MRI for male patients who are known to have high risk lesions.
We herein report a rare case of LCIS in a male breast discovered incidentally on pathologic analysis of the breast tissue, which had been removed during breast reduction surgery for gynecomastia. Invasive ductal carcinoma developed in the ipsilateral breast two years later, as was detected on screening MRI.
Case Report
The patient is a 55-year-old African American male who reported a brief history of anabolic steroid use and no family history of breast cancer. The patient had a history of multiple surgical procedures for recurrent gynecomastia over the course of many years. The patient presented to our institution for bilateral breast reduction for cosmetic purposes. Pathologic evaluation demonstrated a few foci of LCIS within the left breast along with atypical duct cell hyperplasia in a background of gynecomastia. Due to the highly unusual finding of LCIS in this male patient, genetic analysis was performed and the male XY genotype was confirmed.
The patient was subsequently referred to our breast imaging center for a screening bilateral breast MRI. This demonstrated mild diffuse background enhancement bilaterally without suspicious enhancing signal abnormalities in either breast. Screening annual breast MRI was recommended in view of his highly unusual diagnosis of LCIS.
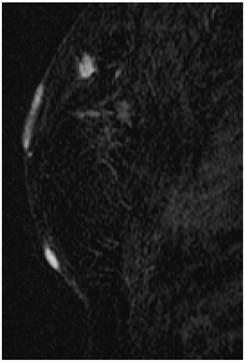
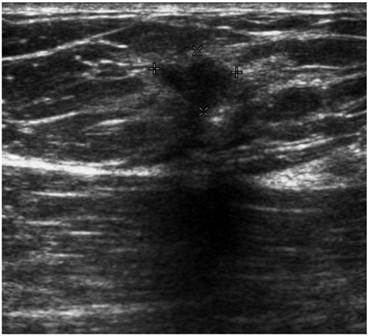
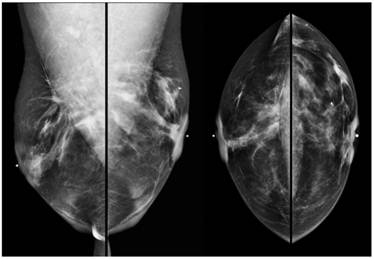
Screening MRI of both breasts performed 15 months later demonstrated interval development of a 1.1 cm enhancing ill-defined mass at the 1 o'clock axis of the left breast (Figure 1). A corresponding solid hypoechoic mass with angulated margins was seen on targeted left breast ultrasound (Figure 2). Mammography demonstrated heterogeneously dense breast tissue with post surgical changes bilaterally. No dominant mass or suspicious clustered microcalcifications were identified in either breast (Figure 3). Ultrasound guided core biopsy yielded moderately differentiated invasive ductal carcinoma.
MR image demonstrating a 1.1 cm suspicious enhancing mass at the left breast 1 o'clock axis for which targeted ultrasound was recommended.
Targeted ultrasound of the left breast demonstrating a solid hypoechoic lesion at the 1 o'clock axis which corresponded to the enhancing lesion on the MRI.
Digital bilateral mammogram demonstrating heterogeneously dense breast tissue and post surgical changes bilaterally. A microclip is seen at the 1 o'clock axis of the left breast, marking the site of ultrasound guided core biopsy.
The patient was referred for surgical evaluation. Physical exam at that time revealed that his gynecomastia had recurred and the patient was noted to have C-cup sized breasts. He had significant hypertrophic circumareolar and inframammary scars on both breasts from his previous breast surgeries. BRCA testing was negative. Bilateral mastectomies were performed.
Pathology
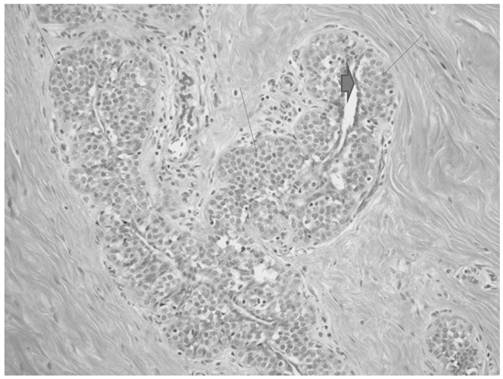
The breast reduction specimen consisted of 426 grams of tissue from the right breast and 490 grams of tissue from the left breast. Gross examination revealed 80% fibrous tissue and 20% adipose tissue without a discrete mass. Extensive histopathologic sampling of the left breast revealed a few foci of lobular carcinoma in situ which was confirmed with a negative E-cadherin immunostain (Figures 4 and 5). Atypical duct cell hyperplasia, cribriform and micropapillary type was also present in a few foci on the left (Figure 6). Both right and left breasts revealed gynecomastia, florid phase.
The ultrasound guided core biopsy specimen demonstrated invasive ductal carcinoma and subsequent bilateral mastectomy specimens revealed a 1.4 x 1.2 x 1.0 cm irregular hard mass at the 1 o'clock position of the left breast. No discrete mass was seen in the right mastectomy specimen. The breast tissue was about 60% fibrous bilaterally. Histopathologic examination of the left breast mass revealed a 1.3 cm well differentiated invasive ductal cancer with a Nottingham Score of 5 of 9, including a tubule score of 2, nuclear pleomorphism score of 2 and mitotic count score of 1 (Figure 7). The carcinoma was strongly and diffusely positive for both estrogen and progesterone receptors and negative for Her-2/Neu. Lobular carcinoma in situ was present bilaterally as was florid phase gynecomastia. Sentinel lymph nodes were negative.
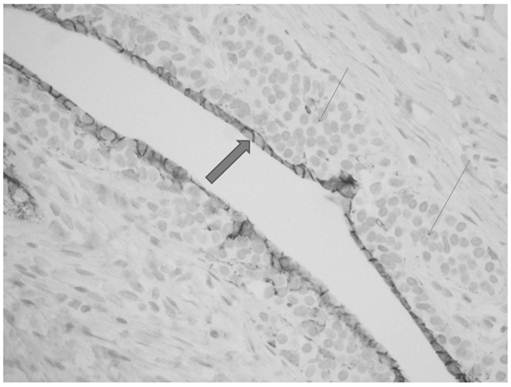
Monotonous small cells of lobular carcinoma in situ (thin arrows) show pagetoid extension along ducts with undermining of normal ductal epithelium (thick arrow).
E-cadherin immunostain shows absence of staining in cells of lobular carcinoma in situ (thin arrows) with positive staining in residual benign ductal cells (thick arrow).
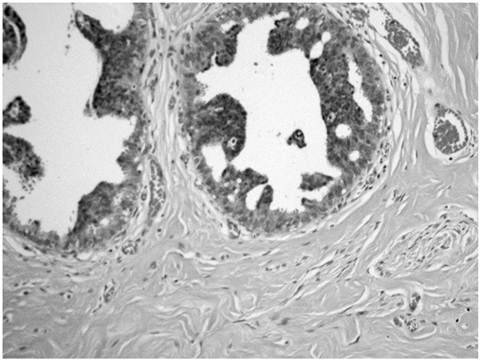
Atypical ductal hyperplasia showing hyperchromatic cells with micropapillary type.
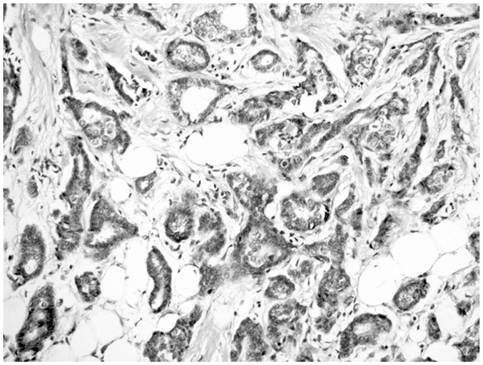
Invasive ductal carcinoma showing a mixture of infiltrating tubules and cords of cells.
Discussion
Breast carcinoma in men is an uncommon disease, representing approximately 1% of all breast cancers and 1% of all malignancies in men; although based on current statistics, the incidence of male breast cancer is increasing (1,2). While the etiology of male breast cancer is uncertain, risk factors include genetic predisposition, prior radiation exposure, alterations of the estrogen-testosterone ratio, and occupational hazards (3). To date, there is no evidence linking gynecomastia with male breast cancer (4,5).
Male breast cancers are predominantly of ductal origin due to the lack of terminal lobules within the male breast. As a result, LCIS and infiltrating lobular carcinoma are extremely unusual in male patients (6). Nance et al reported the first case of LCIS in a phenotypic and apparently genotypic male in 1989 in association with a large infiltrating lobular carcinoma (7); and in fact, only a limited number of cases of infiltrating lobular carcinoma of the male breast have been reported (8).
Over the past decade, there has been an increase in the number of imaging studies performed in male patients. These are largely performed in patients who present with complaints of a breast lump and/or breast pain. Although there are no standardized protocols in evaluating the male breast, mammography is usually the initial study and is followed by ultrasound as needed (9). Occasionally, MRI may be obtained for further evaluation, and it has been shown that the diagnostic criteria used in the evaluation of the female breast may be applied to the male breast as well (10). However, there are no guidelines regarding screening mammography in asymptomatic men at any age due to the rarity of male breast cancer. In the absence of screening, most male patients present with clinical symptoms and more advanced disease (11). Current National Comprehensive Cancer Network guidelines for men with BRCA mutations recommend consideration of baseline mammography followed by annual mammography in those men who are shown to have gynecomastia on the baseline study (12).
The role of screening MRI even in female patients with LCIS is not well established despite the fact that LCIS is known to represent a high risk marker lesion. In fact, lifetime risk estimates for patients with incidentally diagnosed LCIS range from 10 to 20%, imparting a significant lifetime risk for the development of invasive ductal or lobular carcinoma in either breast (13,14). In 2007, a retrospective study evaluated screening MRI in asymptomatic female patients with LCIS, demonstrating a small increase in early cancer detection (15). Subsequently, the 2007 American Cancer Society guidelines for screening breast MRI advised that there was insufficient evidence to recommend for or against screening MRI in patients with a known diagnosis of LCIS and only recommended annual screening breast MRI for patients with a lifetime risk of greater than 20-25% (16). The 2009 National Comprehensive Cancer Network guidelines, however, advised consideration of annual breast MR imaging as an adjunct to mammography and clinical examination in these patients (17). More recently, two additional retrospective studies specifically studied screening breast MRI in asymptomatic female patients with LCIS and concluded that screening breast MRI is a useful adjunctive tool to mammography in this high risk population (18,19). As such, one may extrapolate this information to males with a known diagnosis of LCIS and recommend screening MRI, as was done in this case.
This case report is, to the best of our knowledge, the first reported case of a genotypic and phenotypic male patient without a BRCA mutation, who was found to have incidental LCIS which was unrelated to a lobular carcinoma. In addition, this is the first reported case of a male patient with LCIS to be screened with annual MRI surveillance and in whom the MRI detected a mammographically occult stage I invasive ductal carcinoma. This case highlights the importance of imaging management and the potential for an improved prognosis in men who are at high risk for breast cancer.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyl GN. Breast carcinoma in men: a population based study. Cancer. 2004;101:51-57
2. Pant K, Dutta U. Understanding and management of male breast cancer: a critical review. Med Oncol. 2008;25:294-298
3. Johansen Taber KA, Morisy LR, Osbahr AJ, Dickinson BD. Male breast cancer: risk factors, diagnosis, and management (review). Oncology Reports. 2010;24:1115-1120
4. Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367:595-604
5. Cardenosa G. Breast imaging; 1st ed Chapter 9, The male breast; p299-312. Philadelphia: Lippincott Williams & Wilkins. 2004
6. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyl GN. Breast carcinoma in men: a population based study. Cancer. 2004;101:51-57
7. Nance KV, Reddick RL. In situ and infiltrating lobular carcinoma of the male breast. Human Pathology. 1989;20:1220-1222
8. Mariolis-Sapsakos T, Theodoropoulos G, Flessas II, Orfanos F, Orfanos N, Konstadinou E. et al. Lobular breast cancer in men: case report and review of the literature. Onkologie. 2010;33:698-700
9. Iuanow E, Kettler M, Slanetz PJ. Spectrum of disease in the male breast. AJR. 2011;196:247-259
10. Morakkabati-Spitz H, Schild HH, Leutner CC, von Falkenhausen M, Lutterbey G, Kuhl CK. Dynamic contrast-enhanced breast MR imaging in men: preliminary results. Radiology. 2006;238:438-445
11. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyl GN. Breast carcinoma in men: a population based study. Cancer. 2004;101:51-57
12. Korde LA, Zujewski J, Kamin L, Giordano S. et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. Journal of Clinical Oncology. 2010;28:2114-2122
13. Simpson PT, Gale T, Fulfor LG. et al. Pathology of atypical lobular hyperplasia and lobular carcinoma in situ. Breast Cancer Res. 2003;5:258-262
14. Arpino G, Laucirica R, Elledge RM. Premalignant and in situ breast disease: biology and clinical implications. Ann intern Med. 2005;143(6):446-457
15. Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Annals of Surgical Oncolog. 2007;14:1051-1057
16. Saslow D, Boetes C, Burke E. et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75-89
17. Bevers TB, Anderson BO, Bonaccio E. et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060-1096
18. Friedlander LC, Orel Roth S, Gavenonis SC. Results of MR imaging screening for breast cancer in high-risk patients with lobular carcinoma in situ. Radiology. 2011 doi:10.1148/radiol.11103516
19. Sung JS, Malak SF, Punam B. et al. Screening breast MR imaging in women with a history of lobular carcinoma in situ. Radiology. 2011 doi:10.1148/radiol.11110091
Author contact
![]() Corresponding author: Phone: (718) 920-5400; Fax: (718) 324-1156; tkoenigsorg
Corresponding author: Phone: (718) 920-5400; Fax: (718) 324-1156; tkoenigsorg

 Global reach, higher impact
Global reach, higher impact