Impact Factor
ISSN: 1837-9664
J Cancer 2012; 3:241-245. doi:10.7150/jca.2586 This volume Cite
Short Research Communication
FDG PET/CT Response Evaluation in Malignant Pleural Mesothelioma Patients Treated with Talc Pleurodesis and Chemotherapy
1. Department of Medical Oncology, IRCCS Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST), Meldola, Italy;
2. Department of Nuclear Medicine, Morgagni-Pierantoni Hospital, Forlì, Italy;
3. Department of Radiology, Morgagni Pierantoni Hospital; Forlì, Italy;
4. Unit of Biostatistics and Clinical Trials, IRCCS Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST), Meldola, Italy;
5. Department of Thoracic Surgery, Morgagni-Pierantoni Hospital, Forlì, Italy;
6. Department of Pneumology, Morgagni-Pierantoni Hospital, Forlì, Italy;
7. Department of Radiology, IRCCS Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST), Meldola, Italy;
Received 2012-1-25; Accepted 2012-4-10; Published 2012-6-1
Abstract
Purpose: Talc pleurodesis (TP) is employed worldwide for the management of persistent pneumothorax or pleural effusion, particularly of malignant origin. However, there are very little available data on 18F-fluorodeoxyglucose positron-emission tomography/computed tomography (18F FDG PET/CT) response evaluation in malignant pleural mesothelioma (MPM) patients treated with TP and chemotherapy.
Methods: Patients with histologically confirmed MPM underwent TP and FDG PET/CT staging and restaging after 3-4 courses of chemotherapy. All patients fasted and received a dose of 5.18 MBq 18F-FDG per kilogram of body weight. Whole-body emission scans were acquired with and without Ordered Subset Expectation Maximization (OSEM) iterative reconstruction algorithm.
Results: From January 2004 to March 2010, 8 patients with biopsy confirmed MPM (7 epithelial, 1 biphasic), with a median age of 65 years (range: 54-77), were evaluated. Median follow-up was 31 months (range: 4-44). After TP treatment, there was a mean interval of 14 days (range: 9-22) and 125 days (range: 76-162) between FDG PET/CT staging and restaging. According to modified RECIST and EORTC criteria, there was a concordance between the radiologic and metabolic SUVmean and SUVmax responses in 6 (75%) and 3 (37.5%) patients, respectively.
Conclusion: TP produces an increased FDG PET uptake which may interfere with the post-chemotherapy disease evaluation. In our case series, the metabolic response measured by SUVmean seems to be in better agreement with the radiologic response compared to the SUVmax.
Keywords: 18F-FDG PET-CT scan, fluorodeoxyglucose, SUV, talc pleurodesis, malignant pleural mesothelioma.
Introduction
Talc pleurodesis (TP) is a procedure first described by Bethune [1] in 1935 as a means to anchor the lung during lobectomy [2]. Ever since then, TP has been employed worldwide in the management of recurrent pneumothorax and malignant pleural effusion, with a success rate in excess of 90% [3-5]. TP is performed by instillation of sterile talc into the pleural cavity (chemical pleurodesis), with chemical irritation leading to pleuritis, and ultimately to pleural fibrosis. Computed tomography (CT) and 18F-fluorodeoxy-glucose (FDG) positron-emission tomography (PET) are important imaging techniques for the detection and staging of cancers that, used together, may provide a more accurate tumor staging tool [6]. However, TP treatment has been reported to increase FDG uptake in thickened pleura, making it difficult to distinguish between benign inflammatory processes and malignancies, with the potential for false positive results [7-16].
Malignant pleural mesothelioma (MPM) is a thoracic neoplasm originating from the pleural lining, where an increased FDG uptake has been observed [17], and several studies have reported on the use of PET to monitor chemotherapeutic response [18-21]. In this setting, the concomitant presence of a disease such as MPM, and the application of a procedure like TP, may produce an increase in SUV that underestimates the tumor response to chemotherapy. In clinical practice, the distinction of responding patients by those with stable or progressive disease has a prognostic implications and this is confirmed by some reports [22, 23]. The distinction of these two categories of disease may allow to recognize patients with better prognosis which may benefit by trimodality therapy by those with a resistant disease where it may be suggested a conservative approach.
The aim of this retrospective MPM case series was to analyze TP-induced pleural changes by FDG PET/CT and to verify the concordance between metabolic and radiologic response after chemotherapy.
Subjects and Methods
Clinical data
Inclusion criteria was biopsy confirmed MPM performed during TP, followed by FDG PET/CT staging. All patients subsequently underwent three or four courses of Pemetrexed based chemotherapy before FDG PET/CT restaging. Written informed routinary consent was obtained from each patient before each examination, as standard quality of our institution require.
FDG PET/CT evaluation
All data were acquired on a combined PET/CT in-line system (Biograph Sensation 16, Siemens). Patients were instructed to fast for at least 6 h prior to scanning, which started 50-60 min after injection of 5.18 MBq FDG per kilogram body weight. The glucose level was checked before radiotracer injection and it always was lower than 160 mg/dL. All patients received a non-enhanced CT scan with 80mA, 120 kV parameters. Scans were acquired in the head and neck area, the thorax and the lower abdomen during shallow breathing, and the upper abdomen during non-forced expiration. Immediately after CT, the PET scan was performed using an acquisition time of 3 min per bed position. The patient remained in the same supine position on the PET/CT table throughout the procedure, and acquisition times ranged from 18 to 24 min. CT data were used for attenuation correction, and PET images were reconstructed by standard 2D-iterative algorithm (ordered subset expectation maximization). Images were derived from an isocontour Volume Of Interest (VOI) drawn over the corresponding hemithorax region using a commercially available workstation (Siemens Esoft 2004). VOI margins were checked to cover the whole area of interest in all three planes. The kidneys and the myocardium were spared from analysis by tracing a second VOI positioned over the heart or kidneys. The values from the second VOI were then subtracted from the first VOI. The minimal SUV within the first VOI was set to a level of 2.5 to eliminate any physiological liver uptake, that may have influenced MPM measurements. An example of PET/CT scan with recognized VOI is represented in Figure 1.
All patients were radiologically staged according to Brigham and Women's Hospital's revised MPM surgical staging system which considers tumor histology, resectability and nodal status [24]. Pleural biopsies obtained during TP procedures were analyzed to determine the histologic MPM subtypes [25].
Image and response evaluations
CT scans were assessed by a thoracic radiologist, experienced in MPM measurement and unaware of patient outcome; radiologic response to chemotherapy was assessed using the modified MPM RECIST criteria [26].
Qualitative and semiquantitative evaluations of PET/CT images were performed by drawing a VOI around the pleural thickness; SUVmax and SUVmean depended on the initial dose administered and the body mass index. Criteria for therapeutic response assessment were according to the European Organization for Research and Treatment of Cancer (EORTC) that defined guidelines for the use of PET using FDG. These guidelines stated a 25% reduction in the maximum standardized uptake value (SUVmax) as threshold for definition of partial response [27].
PET/CT scan with recognisement of VOI.
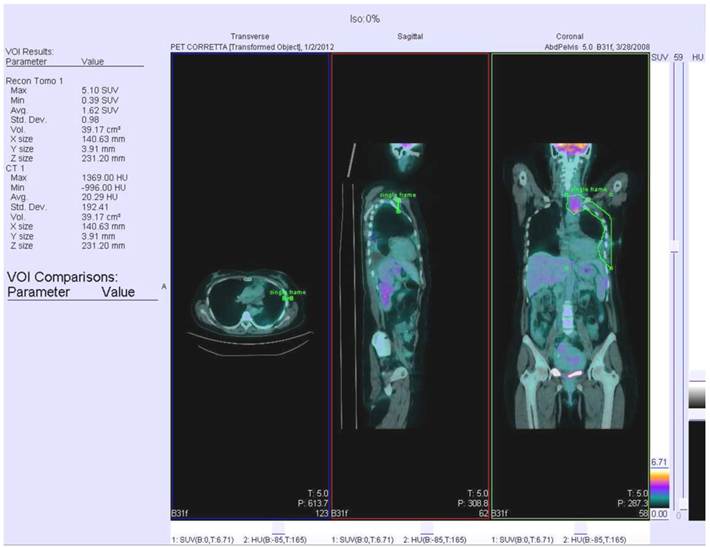
Statistical analysis
Frequency tables were prepared for all categorical variables. Continuous variables were presented using the median and range. All analyses were carried out by SAS statistical software for Windows Version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Eight patients were included in our study, 6 (75%) males and 2 (25%) females, with a median age of 65 years (range: 54-77). Histologic examination revealed an epitheliod subtype in 7 (88%) cases and a mixed subtype in 1 (12%), 5 (63%) of which were located on the left side and 3 (37%) on the right. Overall, 7 (88%) stage I and 1 (12%) stage II diseases were reported. All patients underwent Pemetrexed-based chemotherapy (3 patients in combination with Carboplatin, 4 with Cisplatin, and 1 with Pemetrexed only) with a median of 3.5 courses (range: 3-4) before restaging. Patient characteristics are summarized in Table 1.
After TP treatment, there was a mean interval of 14 days (range: 9-22) and 125 days (range: 76-162) between FDG PET/CT staging and restaging, respectively, and, on average, 26 days (range: 6-46) between the last course of chemotherapy and the FDG PET/CT restaging.
SUV mean and SUV max before and after treatment is summarized in the table 2.
We compared radiological and metabolic response, as showed in the table 3. The concordance between two kinds of responses was respectively 37.5% (3 out of 8 patients) and 75% (6 out of 8 patients).
Comparing radiologic and metabolic response, expressed as SUVmax and SUVmean (see Table 2 and Table 3), the concordance was 37.5% (3 out of 8 patients) and 75% (6 out of 8 patients), respectively.
Patient Features.
| Features | No. of patients (%) (N = 8) |
|---|---|
| Gender | |
| Male | 6 (75) |
| Female | 2 (25) |
| Median age | 65 (Range: 54-77) |
| Stage | |
| I | 7 (88) |
| II | 1 (12) |
| Histology | |
| Epithelioid | 7 (88) |
| Mixed | 1 (12) |
| Localization | |
| Left | 5 (63) |
| Right | 3 (37) |
| Median cycles of chemotherapy | 3.5 (Range: 3-4) |
| Type of chemotherapy | |
| Carboplatin with Pemetrexed | 3 (38) |
| Cisplatin with Pemetrexed | 4 (50) |
| Pemetrexed only | 1 (12) |
Summary of SUV mean and SUV max before and after treatment.
| Pt No. | Pre treatment SUVmax | Pre treatment SUVmean | Post treatment SUVmax | Post treatment SUVmean |
|---|---|---|---|---|
| 1 | 4,6 | 2,78 | 5,3 | 3,15 |
| 2 | 5,14 | 2,97 | 7.07 | 2,45 |
| 3 | 3,21 | 2,28 | 4,2 | 2,29 |
| 4 | 3,8 | 1,91 | 2,4 | 2,1 |
| 5 | 3,2 | 2,3 | 0 | 2,23 |
| 6 | 3,8 | 2,8 | 5.38 | 2,48 |
| 7 | 4,69 | 2,61 | 5.36 | 2,23 |
| 8 | 2,97 | 3,36 | 4,88 | 2,3 |
Radiological and metabolic evaluation.
| Patient No. | Radiologic response | Metabolic response | |
|---|---|---|---|
| SUVmax | SUVmean | ||
| 1 | SD | SD | SD |
| 2 | SD | PD | SD |
| 3 | SD | PD | SD |
| 4 | PR | PR | SD |
| 5 | SD | CR | SD |
| 6 | SD | PD | SD |
| 7 | SD | SD | SD |
| 8 | SD | PD | PR |
CR = complete response; PR = partial response; SD = stable disease; PD = progression disease.
Discussion
Talc pleurodesis is used to treat patients with persistent pleural effusions or pneumothorax not amenable to other therapies [1-5]. Pathologic studies have shown that granulomatous inflammation occurs in the visceral and parietal pleural immediately after TP, resulting in pleural inflammation. For several years, this has been presumed to be the cause of increased FDG activity, leading to the difficulty in distinguishing between benign and malignant disease [7, 8, 10]. MPM is a malignancy arising from the pleural sierosa with an FDG-avidity [17], and this characteristic has been proposed to evaluate chemotherapeutic response [18-21]. Pleural inflammation, induced by TP and MPM, produces pleural thickening or nodularity detected by radiologic examination, and the resulting increase in SUV uptake leads to biases in the post-chemotherapy evaluation. Increased FDG uptake, with an associated increase in the thickness of non calcified pleural lesions, or new FDG-avid pleural lesions without associated calcification, are suspicious for malignancy, whereas FDG uptake in calcified pleural lesions suggests benign TP-induced changes.
Currently, little clinical data is available on which is the best metabolic index to reduce chemotherapy evaluation bias in MPM patients previously treated with TP. In our experience, considering that morphologic changes shown by CT-scan predict response to chemotherapy [26], we observed that the metabolic response measured by SUVmean seems to be in better agreement with the radiologic evaluation compared to the SUVmax, but without reaching statistical significance. This may probably be due to the relatively small number of cases in our study, and therefore a larger prospective study is required to confirm our hypothesis.
Acknowledgements
We thank Grainne Tierney for assistance with the English editing. We are grateful to all the colleagues and members of GIPO (Gruppo Interdisciplinare di Pneumo-Oncologia).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bethune N. Pleural poudrage: a new technique for the deliberate production of pleural adhesions as a preliminary to lobectomy. J Thorac Surg. 1935;4:251-61
2. Weissberg D. Talc pleurodesis: a matter of priority. AJR Am J Roentgenol. 1997;169:911
3. Weissberg D, Ben-Zeev I. Talc pleurodesis. Experience with 360 patients. J Thorac Cardiovasc Surg. 1993;106:689-95
4. Kennedy L, Sahn SA. Talc pleurodesis for the treatment of pneumothorax and pleural effusion. Chest. 1994;106:1215-22
5. Rodriguez-Panadero F, Antony VB. Pleurodesis: state of the art. Eur Respir J. 1997;10:1648-54
6. Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A. et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44:1200-9
7. Murray JG, Erasmus JJ, Bahtiarian EA, Goodman PC. Talc pleurodesis simulating pleural metastases on 18F-fluorodeoxyglucose positron emission tomography. AJR Am J Roentgenol. 1997;168:359-60
8. Ahmadzadehfar H, Palmedo H, Strunk H, Biersack HJ, Habibi E, Ezziddin S. False positive 18F-FDG-PET/CT in a patient after talc pleurodesis. Lung Cancer. 2007;58:418-21
9. Al-Sarraf N, Doddakula K, Wedde T, Young V. Positron emission tomography staging of pleural deposits following Monaldi's procedure: an accurate reflection or a false staging? Interact Cardiovasc Thorac Surg. 2007;6:260-1
10. Peek H, van der Bruggen W, Limonard G. Pleural FDG Uptake More Than a Decade after Talc Pleurodesis. Case Report Med. 2009;2009:650864
11. Bury T, Paulus P, Dowlati A, Corhay JL, Rigo P, Radermecker MF. Evaluation of pleural diseases with FDG-PET imaging: preliminary report. Thorax. 1997;52:187-9
12. Erasmus JJ, McAdams HP, Rossi SE, Goodman PC, Coleman RE, Patz EF. FDG PET of pleural effusions in patients with non-small cell lung cancer. AJR Am J Roentgenol. 2000;175:245-9
13. Gupta NC, Rogers JS, Graeber GM, Gregory JL, Waheed U, Mullet D. et al. Clinical role of F-18 fluorodeoxyglucose positron emission tomography imaging in patients with lung cancer and suspected malignant pleural effusion. Chest. 2002;122:1918-24
14. Kwek BH, Aquino SL, Fischman AJ. Fluorodeoxyglucose positron emission tomography and CT after talc pleurodesis. Chest. 2004;125:2356-60
15. Weiss N, Solomon SB. Talc pleurodesis mimics pleural metastases: differentiation with positron emission tomography/computed tomography. Clin Nucl Med. 2003;28:811-4
16. Nguyen NC, Tran I, Hueser CN, Oliver D, Farghaly HR, Osman MM. F-18 FDG PET/CT characterization of talc pleurodesis-induced pleural changes over time: a retrospective study. Clin Nucl Med. 2009;34:886-90
17. Benard F, Sterman D, Smith RJ, Kaiser LR, Albelda SM, Alavi A. Metabolic imaging of malignant pleural mesothelioma with fluorodeoxyglucose positron emission tomography. Chest. 1998;114:713-22
18. Steinert HC, Santos Dellea MM, Burger C, Stahel R. Therapy response evaluation in malignant pleural mesothelioma with integrated PET-CT imaging. Lung Cancer. 2005;49(Suppl 1):S33-5
19. Ceresoli GL, Chiti A, Zucali PA, Rodari M, Lutman RF, Salamina S. et al. Early response evaluation in malignant pleural mesothelioma by positron emission tomography with [18F]fluorodeoxyglucose. J Clin Oncol. 2006;24:4587-93
20. Francis RJ, Byrne MJ, van der Schaaf AA, Boucek JA, Nowak AK, Phillips M. et al. Early prediction of response to chemotherapy and survival in malignant pleural mesothelioma using a novel semiautomated 3-dimensional volume-based analysis of serial 18F-FDG PET scans. J Nucl Med. 2007;48:1449-58
21. Veit-Haibach P, Schaefer NG, Steinert HC, Soyka JD, Seifert B, Stahel RA. Combined FDG-PET/CT in response evaluation of malignant pleural mesothelioma. Lung Cancer. 2010;67:311-7
22. Byrne MJ, Davidson JA, Musk AW, Dewar J, van Hazel G, Buck M. et al. Cisplatin and gemcitabine treatment for malignant mesothelioma: a phase II study. J Clin Oncol. 1999;17:25-30
23. Hillerdal G, Sorensen JB, Sundstrom S, Riska H, Vikstrom A, Hjerpe A. Treatment of malignant pleural mesothelioma with carboplatin, liposomzed doxorubicin, and gemcitabine. A phase II study. J Thorac Oncol. 2008Nov;3(11):1325-31
24. Sugarbaker DJ, Norberto JJ, Swanson SJ. Surgical staging and work-up of patients with diffuse malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 1997;9:356-60
25. Corson JM. Pathology of diffuse malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 1997;9:347-55
26. Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257-60
27. Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA. et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773-82
Author contact
![]() Corresponding author: Genestreti Giovenzio MD; Department of Medical Oncology, IRCCS Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori, Via Maroncelli 40, Meldola 47014 (FC), Italy. Tel: +39 0543 739100, Fax: +39 0543 739123, e-mail: g.genestretiemr.it
Corresponding author: Genestreti Giovenzio MD; Department of Medical Oncology, IRCCS Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori, Via Maroncelli 40, Meldola 47014 (FC), Italy. Tel: +39 0543 739100, Fax: +39 0543 739123, e-mail: g.genestretiemr.it

 Global reach, higher impact
Global reach, higher impact