Impact Factor
ISSN: 1837-9664
J Cancer 2012; 3:322-327. doi:10.7150/jca.4716 This volume Cite
Research Paper
Primary Refractory and Relapsed Classical Hodgkin Lymphoma - Significance of Differential CD15 Expression in Hodgkin-Reed-Sternberg Cells
1. Departments of Pathology, Soroka University Medical Center, Israel;
2. Department of Desert Ecology, Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Israel;
3. Departments of Oncology, Soroka University Medical Center, Israel;
4. Department of Microbiology-Immunology, Faculty of Health Sciences, Ben-Gurion University of the Negev and Mitrani, Israel.
5. Departments of Hematology, Soroka University Medical Center, Israel.
Received 2012-6-10; Accepted 2012-7-21; Published 2012-7-24
Abstract
We recognized a few possible complications of classical Hodgkin lymphoma therapy in a cohort of 209 patients: 8 developed a primary refractory disease (primary progression), 36 showed an early relapse and 21 showed a late relapse. Sialyl-CD15 expression in Hodgkin-Reed-Sternberg cells was significantly more positive in primary refractory Hodgkin lymphoma, which confirms our previously published findings. Bcl-2 showed a significantly lower level of expression in primary refractory disease than in the other follow-up groups. This is in contrast with a previous finding of Bcl-2, associated with a poor prognosis in primary refractory illness. Another category of variables, old age and advanced stages, was significantly different in the various complications but this finding is probably to be expected. We could not demonstrate a difference between the sequels and the control group with regard to several clinical and immunohistochemical markers. Sialyl-CD15 and Bcl-2 expression, in contrast, were confirmed as prognostic factors, mainly of tumor progression into primary refractory disease.
Keywords: Primary refractory, relapse, classical Hodgkin lymphoma, CD15, Bcl-2.
Introduction
Classical Hodgkin lymphoma (cHL) is relatively rare, but it is one of the most curable malignancies. Primary progressive cHL occurs during the induction of treatment and up to 90 days after its completion. The criteria for progressive disease include one of the following: either a 25% increase in the volume of lymph nodes, or the appearance of a new lesion during therapy or less than 90 days after the end of therapy [1]. Progression occurs in 70% of these patients during primary treatment. Patients who did not achieve temporary remission with the first-line therapy are found to have an 18% lower survival rate than the remainder [1]. Prognostic markers evaluated by immunohistochemistry showed that Bcl-2 and CD20 were independent prognostic factors in refractory cHL [2]. The rate of relapsing disease depends on the tumor stage: it has been described in 5% of early stages and may reach up to 35% in advanced cHL [3]. Patients who relapse following chemotherapy can present a cure rate of 20% with conventional salvage therapy [4-6]. Provencio et al. [7] retrospectively studied relapse cases that became evident at a later stage of the disease: 150 (28%) cases were seen within 2 years, 30 relapses occurred 5 years after the termination of therapy. In 7 out of the 30 relapse patients, the histological HL type was different from that at the primary diagnosis. Of the 12 markers examined by immunohistochemistry, including CD30, CD15, CD20 and Bcl-2, none were associated with relapse [7, 8].
We attempted to define the characteristics of primary refractory cHL and of early and late relapse as they presented in our cohort of cHL patients. Our analysis included the evaluation of CD15 in its differential expression [9] as it relates to refractory and relapsed cHL.
Materials and Methods
Patients
The cohort described in this study was previously included in a publication on apoptosis in cHL [10]. Among these patients, we identified those with primary refractory (PR) cHL as described by Josting et al. [1] and by Canioni et al. [2]. We could not find a difference in the definition between primary progression and PR cHL. We singled out cHL cases that reflected an early relapse (ER) as those that were detected less than 5 years after diagnosis and segregated them from those with late relapse (LR) identified after more than 5 years [7]. Patients who did not relapse were defined as the control group. These four study groups (PR, ER, LR and control) were the 4 levels of our main (categorical) dependent variable: the "group".
In this study, we used whole tissue sections. Fixation for CD15 was in B5 fixative.
Immunohistochemical studies
We used the avidin-biotin peroxidase complex method with the Vectastain kit from Vector Laboratories (Burlingame, CA, USA), following pretreatment in citrate. The antibodies used in this analysis are presented in Table 1. A case was considered positive for any one of the markers if 10% or more of the Hodgkin-Reed-Sternberg cells stained positive [11]. This was often the case with non-sialyl CD15 immunostains of HRS cells, while CD30 stained a majority of tumor cells.
Statistical analysis
We used contingency tables to study the association between clinical and biological (categorical) variables and the cHL "groups" (a contingency table displays the frequency distribution of the levels of two categorical variables). Using Fisher's exact test, we examined the hypothesis that the proportions of patients in the different columns vary significantly between the rows (or vice versa). We first tested a set of 20 hypotheses, corresponding to the 20 clinical/biological variables. A rejection of any of the hypotheses meant that there was a significant association between the “group” and the given variable. Because no post-hoc test is available for contingency tables, we selected the variables that were significantly associated with a "group" (sequel) and established new contingency tables in which we included only one level of the "group" and the control. Some data were missing in part of the cases.
Antibodies used in the study.
| Antigen | Antibody Clone | Source | Dilution |
|---|---|---|---|
| CD15 | LeuM1, monoclonal | Dako, Copenhagen, Denmark | 1:50 |
| CD15 | 80H5, monoclonal | Immunotech,Marseille, France | 1:50 |
| Sialyl-CD15 | KM93, monoclonal | Millipore,Billerica,MA, USA | 1:40 |
| CD30 | BerH2, monoclonal | Dako | 1:100 |
| LMP1 | CS1-4, monoclonal | Dako | 1:50 |
| CD20 | L26, monoclonal | Dako | 1:100 |
| Bcl-2 | Clone 124, monoclonal | Dako | 1:100 |
| p53 | DO-7, monoclonal | Dako | 1:200 |
| MDM2 | Polyclonal | Dako | 1:200 |
| Rb | Polyclonal | Sigma-Aldrich, Israel | 1:300 |
| L39/22 | Monoclonal anti-NP-MV | Contributed by Schneider-Schaulies | 1:100 |
| L77 | Monoclonal anti-H-MV | Contributed by Schneider-Schaulies | 1:100 |
| NP3MV | H14, polyclonal anti-H-MV | Inhouse development | 1:50 |
H-MV, hemagglutinin of measles virus; NP-MV, nucleoprotein of measles virus.
We used Kaplan-Meier curves for right-censored data combined with log-rank tests to examine the difference in overall survival between pairs of "groups". We also used the same procedure to examine the differences in survival between positive and negative n-s-CD15 as well as s-CD15 cases in two steps: first, we analyzed the overall patient population. Then, we excluded the control group and repeated the analysis. In addition we used the Cox proportional hazard analysis to test the effect of age, stage, sialyl-CD15, non sialyl-CD15 and Bcl-2 on the risk to die from cHL.
All statistical analyses were conducted using the R version (R Development Core Team, 2011). We used the “survival” package (version 2.36; Threneau 2011) for Kaplan-Meier and Cox proportional hazard analyses. We used alpha <0 .05 as the threshold of significance.
Results
We studied 209 cHL patients, 8 of whom were defined as primary refractory patients, 36 of whom showed early relapse and 21 who were described as late relapse patients. The remaining 144 showed no evidence of any of the above complications and therefore they were consistent with a control group. The entire study population was comprised of 89 female and 120 male patients. Of the 20 variables evaluated (Table 2), 6 were found to be significantly associated with a cHL sequel.
In Table 3, we compared primary refractory disease with the control group. The patients in the refractory group were older (p= 0.01); they had either died of cHL or showed significant evidence of active disease at the last follow up (p<0.001); sialyl-CD15 was more strongly expressed than in the control (p= 0.03) and Bcl-2 was predominantly negative when compared to the control group (p= 0.01).
Early relapse was found more often in males (p= 0.017); in older patients (p= 0.002) and in advanced stages (p= 0.004) and the outcome was poorer (p<0.001) in comparison with the control group (Table 4).
Late relapse differed from the control group by more advanced stages (p= 0.0032) and by a poorer outcome (p<0.001) (data not shown).
We also compared the features of primary refractory cHL and late relapse disease. The only varying characteristic noted was in the differential CD15 expression. While primary refractory cHL was associated with a more positive sialyl-CD15 (p= 0.007), late relapsed disease was more associated with positive non-sialyl-CD15 expression (p= 0.017) (Table 5).
Contingency table on the association between 20 clinical and biological variables and the probability of them belonging to a certain group. Each association represents an independent hypothesis.
| Variable | Level | PR | ER | LR | CONTROL | p value |
|---|---|---|---|---|---|---|
| SEX | F | 4 | 9 | 9 | 67 | 0.12 |
| M | 4 | 27 | 12 | 77 | ||
| AGE | old | 6 | 19 | 6 | 36 | 0 |
| young | 1 | 8 | 9 | 67 | ||
| STAGE | I-IIA | 0 | 4 | 2 | 58 | 0 |
| IIB-IV | 3 | 17 | 13 | 45 | ||
| BULKY | no | 2 | 18 | 9 | 78 | 0.15 |
| yes | 1 | 2 | 6 | 33 | ||
| OUTCOME | NED | 0 | 1 | 0 | 126 | 0 |
| ED | 7 | 27 | 16 | 12 | ||
| RADIOTHERAPY | no | 5 | 16 | 8 | 78 | 0.27 |
| yes | 0 | 7 | 7 | 31 | ||
| CHEMTHERAPY | classic | 1 | 14 | 9 | 89 | 1 |
| other | 0 | 1 | 1 | 11 | ||
| TYPE | else | 4 | 16 | 8 | 61 | 0.93 |
| ns | 4 | 20 | 13 | 83 | ||
| RB | neg | 2 | 6 | 3 | 21 | 0.64 |
| pos | 1 | 13 | 7 | 47 | ||
| n-s-CD15 | neg | 3 | 3 | 0 | 16 | 0.05 |
| pos | 5 | 31 | 20 | 125 | ||
| Sialyl-CD15 | neg | 3 | 25 | 20 | 98 | 0.02 |
| pos | 3 | 4 | 0 | 12 | ||
| CD20 | neg | 6 | 29 | 17 | 117 | 0.73 |
| pos | 2 | 4 | 2 | 23 | ||
| P53 | neg | 2 | 4 | 5 | 28 | 0.62 |
| pos | 6 | 29 | 16 | 110 | ||
| BCL2 | neg | 8 | 15 | 14 | 66 | 0.01 |
| pos | 0 | 20 | 7 | 63 | ||
| MDM2 | neg | 2 | 10 | 10 | 45 | 0.53 |
| pos | 3 | 16 | 7 | 65 | ||
| LMP1 | neg | 4 | 28 | 13 | 99 | 0.36 |
| pos | 4 | 8 | 8 | 45 | ||
| MV | neg | 2 | 7 | 5 | 23 | 0.63 |
| pos | 3 | 15 | 10 | 72 | ||
| NP3MV | neg | 6 | 14 | 7 | 58 | 0.17 |
| pos | 0 | 9 | 8 | 38 | ||
| CD30 | neg | 1 | 5 | 2 | 19 | 0.96 |
| pos | 7 | 30 | 19 | 117 | ||
| AI | high | 3 | 9 | 5 | 52 | 0.74 |
| low | 4 | 15 | 10 | 61 |
ER - early relapse cHL; LR - late relapse cHL; PR - primary refractory cHL.
Association between clinical and biological features in patients with primary refractory disease compared to those in the control group.
| Variable | Prim refract α | Control | p value | |
|---|---|---|---|---|
| Age | 15-34 | 1(14.3) | 67(65) | |
| >45 | 6(85.7) | 36(35) | 0.01 | |
| Stage B | I-IIA | 0 | 58(56) | |
| IIB-IVB | 3(100) | 45(44) | 0.09 | |
| Outcome | NED γ | 0 | 126(91) | |
| AWD&DOD δ | 7(100) | 12(9) | 0.000001 | |
| n-s-CD15 β | Neg | 3(37.5) | 16(11) | |
| Pos | 5(62.5) | 125(89) | 0.07 | |
| Sialyl-CD15 | Neg | 3(50) | 98(89) | |
| Pos | 3(50) | 12(11) | 0.03 | |
| Bcl-2 | Neg | 8(100) | 66(51) | |
| Pos | 0 | 63(49) | 0.01 |
α - Prim refract: primary refractory disease.
β - n-s-CD15: non-sialylated CD15.
γ - NED: no evidence of disease.
δ - AWD & DOD: alive with disease and dead of disease.
Early relapsed as compared with uncomplicated classical Hodgkin lymphoma (clinical and biological features).
| Variables | Early relapse | Control | p-value | |
|---|---|---|---|---|
| Age | 15-34 | 8(29.6) | 67(65) | |
| >45 | 19(70.4) | 36(35) | 0.0019 | |
| Stage B | I-IIA | 4(19) | 58(56) | |
| IIB-IVB | 17(81) | 45(44) | 0.004 | |
| Outcome | NED α | 1(3.6) | 126(91) | |
| AWD&DOD β | 27(96.4) | 12(9) | 0.00000 | |
| n-s-CD15 γ | Neg | 3(8.8) | 16(11) | |
| Pos | 30(91.2) | 125(89) | 1.00 | |
| Sialyl-CD15 | Neg | 25(86.2) | 98(89) | |
| Pos | 4(13.8) | 12(11) | 0.74 | |
| Bcl-2 | Neg | 15(42.9) | 66(51) | |
| Pos | 20(57.1) | 63(49) | 0.45 |
α NED - no evidence of disease.
β AWD & DOD - alive with disease and dead of disease.
γ n-s-CD15 - non-sialyl-CD15.
The Bcl-2 expression was significantly less positive in primary refractory cHL compared to early relapsed disease (p<0.001). No significant difference was found between early and late relapse concerning the variables investigated. When comparing primary refractory disease with a combination of both types of relapse (data not shown), non-sialyl-CD15 (n-s-CD15) was more significantly expressed in the joint relapse group (p= 0.025) whereas sialyl-CD15 (s-CD15) was less positive for the relapse patients (p= 0.01). In addition, a negative Bcl-2 expression was predominant in primary refractory cHL (p= 0.02).
Table 6 shows an association between non-sialyl-CD15 and sialyl-CD15 as it appeared in the cohort as a whole. The vast majority were both n-s-CD15 positive and s-CD15 negative. An overlap is evident in a small minority.
The Kaplan-Meier analysis on the overall cohort showed that there were no significant differences in survival between positive and negative n-s-CD15, as well as between positive and negative s-CD15 (data not shown). However, when excluding the control group, it became evident that patients with positive n-s-CD15 survive longer than patients with negative n-s-CD15 and that patients with negative s-CD15 survive longer than patients with positive s-CD15 (Fig 1A and Fig 1B, respectively).
Clinical and biological features of primary refractory (prim refract) compared with those of late relapsed classical Hodgkin lymphoma.
| Variables | Prim refract | Late relapse | p value | |
|---|---|---|---|---|
| Age | 15-35 | 1(14.3) | 9(60) | |
| >45 | 6(85.7) | 6(40) | 0.07 | |
| Stage B α | I-IIA | 0 | 2(13) | |
| IIB-IVB | 3(100) | 13(87) | 1.00 | |
| Outcome | NED β | 0 | 0 | |
| AWD&DOD γ | 7(100) | 16 (100) | 1.00 | |
| n-s-CD15 δ | Neg | 3(37.5) | 0 | |
| Pos | 5(62.5) | 20(100) | 0.017 | |
| Sialyl-CD15 | Neg | 3(50) | 20(100) | |
| Pos | 3(50) | 0 | 0.007 | |
| Bcl-2 | Neg | 8(100) | 14(67) | |
| Pos | 0 | 7(33) | 0.14 |
α Stage B - stage and systemic symptoms.
β NED - no evidence of disease.
γ AWD & DOD - alive with disease and dead of disease.
δ n-s-CD15 - non-sialyl-CD15.
Differential CD15 expression in the whole cohort of classical Hodgkin lymphoma patients.
| Total | S-CD15 α pos | S-CD15 neg | p value | |
|---|---|---|---|---|
| n-s-CD15 β pos | 146 | 13 | 133 | |
| n-s-CD15 neg | 17 | 6 | 11 | 0.006 |
| Total | 163 | 19 | 144 |
α - S-CD15 - sialyl-CD15.
β - n-s-CD15 - non-sialyl-CD15.
Kaplan-Meier survival analysis on positive vs. negative ns-CD15 (A) and s-CD15 (B), performed with the exclusion of control patients. Significance was calculated with the Log-rank test.
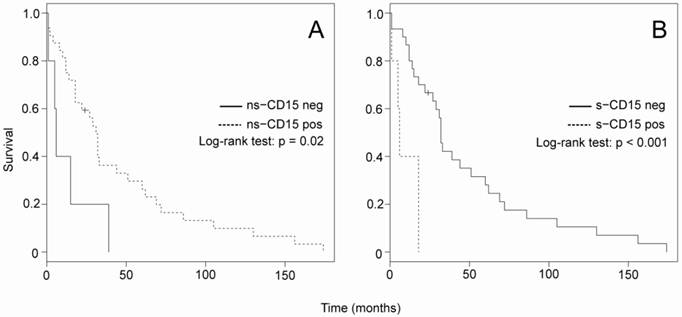
The Kaplan-Meier analysis also indicated, as expected (data not shown), that the overall survival of patients with primary refractory cHL was markedly reduced compared to the control group (log-rank test P < 0.0001) and that overall survival rate in early relapsed disease compared to primary refractory disease was reduced ( log-rank test P < 0.0001). In addition, late relapsed cHL patients had a significant better overall survival than primary refractory (log-rank test P < 0.0001). Finally, Cox proportional hazard analysis showed that stage and sialyl-CD15 expression had an independent effect on survival (Table 7).
Cox proportional hazard analysis model on the association of prognostic factors with dying of HD in 74 patients.
| Factor | Regression coefficient | 95% Confidence intervals | pcoefficient |
|---|---|---|---|
| Stage α | 3.81 | 2.52-801.79 | 0.01 |
| Age β | 0.67 | 0.8-4.81 | 0.14 |
| n-s-CD15γ | 0.59 | 0.35-9.48 | 0.481 |
| Sialyl-CD15δ | 3.36 | 1.19-695.5 | 0.039 |
| Bcl-2ε | 0.29 | 0.52-3.4 | 0.551 |
α. Stages IIB-IVB as compared with stages IA-IIA.
β. Age >45 as compared with age 15-34.
γ. non-sialyl CD15 expression - negative versus positive.
δ. sialyl-CD15 expression - positive as compared with negative.
ε. Bcl-2 expression - negative as compared with positive.
Discussion
We compared the properties of the potential complications of cHL therapy with those of uncomplicated disease. It was found that many of these features could be anticipated but not all of them [12, 13].
We assessed the clinical and biological aspects of primary refractory cHL, early relapsed and late relapsed disease compared to those of a cHL control group [14].
Three categories of attributes recognized upon the primary diagnosis were investigated. In a small majority, no statistically significant differences were evident between the complications and the control groups. They included the following: gender, bulky disease, nature of primary therapy and histological type of cHL. They also comprised the biological markers CD30, CD20, LMP1/EBV, p53, mdm-2, and the apoptotic index. Another subset included clinical aspects with a statistically significant difference: older age (>45) and advanced stages, which may explain the development of sequels. However, this association was largely anticipated [1].
The last category was limited and comprised the differential CD15 and the Bcl-2 expressions. While a positive non-sialyl-CD15 (LeuM1 and 80H5) expression showed a near significant (p= 0.05) association with the complications and the control groups, sialyl-CD15 expression was significantly more positive in primary refractory cHL. We sustained these findings, by excluding the control group and using the Kaplan-Meier analysis, but not at the level of the entire cohort. The Cox analysis confirmed sialyl-CD15 as an independent negative prognostic factor.
We have previously described the negative prognostic significance of sialyl-CD15 expression in cHL [9]. While in the earlier study, the expression of n-s-CD15 and s-CD15 were mutually exclusive, in the present analysis an overlap was found. A possible explanation for this discrepancy is that we used in the first study two clones each of the two CD15 forms, while we used two clones of n-s-CD15 and only one clone of s-CD15 in the second. Since the publication of this s-CD15 paper, some authors have confirmed the value of LeuM1 (non-sialyl-CD15) as a positive prognostic factor, but our findings of the negative prognostic significance of sialyl-CD15 have not been studied further nor were they replicated.
The expression of Bcl-2 was significantly more negative in primary refractory disease than in the other follow-up groups. Positive Bcl-2 expression has been previously associated with a poor outcome, but it was then studied in an entire cohort of cHL patients rather than in a subgroup like primary progression [15]. Our conclusions regarding Bcl-2 expression do not support the recent finding of high Bcl-2 expression as a strong negative prognostic factor in refractory cHL [2]. The theory involving Bcl-2 expression in apoptosis deregulation in cHL has not been completely clarified. Moreover, the role of apoptosis in Reed-Sternberg cells in cHL has not been completely clarified at this point [10]. Therefore, we cannot present a definite explanation for our discrepant results.
Several authors have maintained that the disease status at the time of relapse or of autologous stem cell transplantation seems to be the most important prognostic factor [16, 17], but these data were not available for our patients.
In summary, in addition to old age and advanced stages, which are expected properties for disease progression, we found that sialyl-CD15 and Bcl-2 expression had a prognostic significance for the development of the sequels investigated, mainly of primary refractory cHL.
Acknowledgements
The study was supported in part by Kibbutz Sde Boker, Israel Ministry of Health, and the Richard H. Holzer Foundation. However, none of the above institutions was involved in the conception, data collection or writing of the manuscript. We thank Eugenia Mejirovski for her excellent technical work.
The study was approved by an Investigational Review Board: SOR-0276-11.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Josting A, Rueffer U, Franklin J, Sieber M, Diehl V, Engert A. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood. 2000;96:1280-1286
2. Canioni D, Deau-Fischer B, Taupin P. et al. Prognostic significance of new immunohistochemical markers in refractory classical Hodgkin lymphoma: a study of 59 cases. PLoS One. 2009;4:e6341
3. Canellos GP. Relapsed and refractory Hodgkin lymphoma: new avenues? Hematology Oncology Clinics of North America. 2007;21:929-941
4. Tesch H, Sieber M, Diehl V. Treatment of advanced stage Hodgkin disease. Oncology. 2001;60:101-109
5. Byrne BJ, Gockerman JP. Salvage therapy in Hodgkin lymphoma. Oncologist. 2007;12:156-167
6. Younes A. Novel treatment strategies for patients with relapsed classical Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2009:507-519
7. Provencio M, Salas C, Milan I, Cantos B, Sanchez A, Ballas C. Late relapses in Hodgkin lymphoma: a clinical and immunohistochemistry study. Leukemia & Lymphoma. 2010;51:1686-1691
8. Sibon D, Brice P. Optimal treatment for relapsing patients with Hodgkin lymphoma. Expert Review of Hematology. 2009;2:285-295
9. Benharroch D, Dima E, Levy A. et al. Differential expression of sialyl and non-sialyl-CD15 antigens on Hodgkin-Reed-Sternberg cells: significance in Hodgkin's disease. Leukemia & Lymphoma. 2000;39:185-195
10. Benharroch D, Einav I, Feldman A, Levy A, Ariad S, Gopas J. Apoptosis of Hodgkin-Reed-Sternberg cells in classical Hodgkin lymphoma revisited. APMIS. 2010;118:339-345
11. Benharroch D, Shemer-Avni Y, Myint Y-Y. et al. Measles virus: evidence of an association with Hodgkin's disease. British Journal of Cancer. 2004;91:572-579
12. Diehl V, Stein H, Hummel M, Zollinger R, Connors JM. Hodgkin lymphoma: biology and treatment strategies for primary refractory, and relapsed disease. Hematology Am Soc Hematol Educ Program. 2003:225-249
13. Cerci JJ, Pracchia LF, Linard CC. et al. 18F-FDG PET after two cycles of ABVD predicts event free survival in early and advanced Hodgkin lymphoma. Journal of Nuclear Medicine. 2010;51:1337-1343
14. Kuruvilla J, Keating A, Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. 2011;117:4208-4217
15. Rassidakis GZ, Medeiros LJ, Vassilakopoulos TP. et al. BCL-2 expression in Hodgkin and Reed-Sternberg cells of classical Hodgkin disease predicts a poorer prognosis in patients treated with ABVD or equivalent regimens. Blood. 2002;100:3935-3941
16. Brice P, Bastion Y, Divine M. et al. Analysis of prognostic factors after the first relapse of Hodgkin disease in 187 patients. Cancer. 1996;78:1293-1299
17. Martin A, Fernandez-Jimenez MC, Caballero MD. et al. Long-term follow-up in patients treated with Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin's disease. British Journal of Haematology. 2001;113:161-171
Author contact
![]() Corresponding author: Prof. Daniel Benharroch, Department of Pathology, Soroka University Medical Center, P.O. Box 151, Beer-Sheva 84101, Israel. E-mail: benarochac.il. Tel: 972-8-6400920; Fax: 972-8-6232770;
Corresponding author: Prof. Daniel Benharroch, Department of Pathology, Soroka University Medical Center, P.O. Box 151, Beer-Sheva 84101, Israel. E-mail: benarochac.il. Tel: 972-8-6400920; Fax: 972-8-6232770;

 Global reach, higher impact
Global reach, higher impact