Impact Factor
ISSN: 1837-9664
J Cancer 2013; 4(3):210-216. doi:10.7150/jca.5839 This issue Cite
Review
Serum-Based DNA Methylation Biomarkers in Colorectal Cancer: Potential for Screening and Early Detection
1. Department of Pathology and Laboratory Services, Walter Reed National Military Medical Center, Bethesda, MD, USA.
2. Georgetown University Hospital, Washington, DC, USA.
3. Department of Surgery, Division of Surgical Oncology, Rabin Medical Center, Beilinson Hospital, Petah Tiqva, Israel.
4. United States Military Cancer Institute, Washington, DC, USA.
5. International Consortium of Research Excellence (INCORE) of the Theodor-Billroth-Academy®, Munich, Germany
6. California Oncology Research Institute, Santa Monica, CA, USA.
7. John Wayne Cancer Institute, Santa Monica, CA, USA.
8. Clinic of Abdominal, Endocrine, and Transplantation Surgery, Clinical Center of Vojvodina, Novi Sad, Serbia.
9. University of Novi Sad - Medical Faculty, Novi Sad, Serbia.
10. Sylvia Lawry Center for MS Research, Munich, Germany.
11. Department of Surgery, Uniformed Services University of the Health Sciences, Bethesda, MD, USA.
12. Bon Secours Cancer Institute, Richmond, VA, USA.
Received 2013-1-14; Accepted 2013-2-8; Published 2013-3-1
Abstract
Colorectal cancer (CRC) is the third most common cause of cancer-related death in the United States. Early identification and treatment of pre-cancerous colorectal lesions, or node-negative CRC are highly effective interventions that substantially reduce disease-specific mortality. Colonoscopy remains a highly effective primary screening tool based on its excellent diagnostic accuracy, and its ability to remove pre-cancerous lesions. However, the nature of the procedure limits compliance with colonoscopy intended for population-based CRC screening. A significant advance in the screening and care of these patients could be realized by blood-based biomarkers, which could accurately identify patients at-risk for CRC development whom might benefit from early and/or more frequent surveillance for disease. We reviewed and herein discuss the potential for serum based DNA methylation biomarkers for screening and early detection of CRC.
Keywords: Colorectal cancer, Biomarker: Early detection, Screening, Serum, DNA methylation.
Introduction
Four decades ago a “war” was declared on cancer with the passing of the National Cancer Act of 1971, but only recently has the overall cancer-related death rate begun to slowly decline as the death rate for other diseases has plummeted.1,2 For the most part, this relatively minor decline in mortality has been attributed to increased disease prevention efforts, improved treatments for advanced malignancy, and early detection and treatment of localized cancers.3 Notwithstanding, cancer remains a major cause of mortality in the United States (U.S.); in fact, it was the second leading cause of death for all ages, genders, and races in 2009 and is expected to top the list this year.2
Advances are urgently needed from the key stakeholders in translational cancer research, which will impact cancer-related mortality in the near-term. It is felt that these advances will most likely come from studies focusing on early detection of cancer rather than the prevention or treatment of cancer, as the latter require a fundamental understanding of the underlying causes and complex mechanisms of cancer.3 Numerous recent trials have already demonstrated an improvement in survival for patients with breast, colon, prostate and lung cancer when the disease is identified and treated early.4-7
Colorectal cancer (CRC) is the third most common cause of cancer-related death in the US.1 The colorectal adenoma-carcinoma sequence is well established. For CRC, the identification and treatment of early stage, pre-malignant lesions are highly effective interventions that substantially reduce CRC-specific mortality. Due to its excellent diagnostic accuracy, coupled with its ability to remove pre-cancerous lesions, colonoscopy continues to be the primary screening tool. However, the nature of the procedure limits its effectiveness as a screening method.8 A significant advance in the screening and care of these patients could be realized by blood-based biomarkers, which could accurately identify patients at-risk for CRC development whom might benefit from early and/or more frequent surveillance for disease. Extensive cancer research over the last decade has uncovered numerous genomic alterations in CRC - epidermal growth factor receptor (EGFR), BRAF, tumor MSI-H expression (defects in DNA mismatch repair, MSI phenotype), 18q AI expression, p53 expression and KRAS mutation. But, these are poor targets for early detection biomarkers for CRC due to their lack of global expression across all CRC molecular subtypes. Alongside these advances in the detection and understanding of genomic modifications in CRC over the past decade, an appreciation for the importance of epigenetic modifications has emerged.
Epigenetic modifications refer to changes in gene expression and cellular phenotype without corresponding changes in the DNA sequence i.e. DNA methylation and histone modification. An important epigenetic mechanism for silencing tumor suppressor genes during carcinogenesis is the hyper-methlyation of CpG islands. This methylation of the base cytosine in CpG islands is under intense investigation and it has been reported, that in certain cancers such as colorectal, small bowel, and endometrial cancers, loss of function of the DNA mismatch repair gene hMLH1 by hyper-methylation of its promoter can be observed.9
DNA methylation studies have demonstrated that dense methylation of promoter regions is associated with transcriptional silencing and that this silencing, especially of tumor suppressor genes, is important in tumorigenesis. Furthermore, DNA methylation profiles are reportedly distinct between diseased and benign tissues and may be useful in cancer screening. The aim of our review is to discuss the potential for serum-based DNA methylation biomarkers for screening and early detection of CRC.
Serum-based Cancer Biomarkers
The National Institute of Health (NIH) defines a biological marker (biomarker) as a biological molecule found in blood, other body fluids, or tissues that is an objective indicator of normal or abnormal process, or of a condition or disease10 Cancer biomarkers would therefore signal the presence of cancer, and some might expand upon the above definition in defining cancer biomarkers to include any substance or process that is indicative of the presence of cancer in the body. A cancer biomarker might be either a molecule secreted by the tumor itself, or it could be a specific response of the body to the presence of cancer. A biomarker can exist as related to DNA, RNA, micro-RNA, epigenetic changes, protein and even antibody expression.
Body fluids, especially blood, contain molecules from many tissues of the body, which have been released into the fluid for various reasons. These molecules may signal the presence of cancer and thus could be potential cancer biomarkers. Much work has been conducted on blood in the pursuit of novel biomarkers, of which proteins are probably the most investigated. A number of first generation protein biomarkers have been associated with CRC including CEA, CA19-9 and CA125; however none have possessed sufficient specificity and sensitivity for the purpose of CRC screening and early detection.11
Nucleic acids represent obvious potential targets for biomarker development, especially with the relative ease in which they can be amplified today. DNA-based biomarkers developed using cell-free circulating DNA (cfcDNA) in blood have been used successfully for pre-natal diagnosis, with applications for cancer detection and diagnosis, and monitoring of treatment efficacy starting to emerge.11 Micro-RNA (miRNA)-based biomarkers are also emerging in the realm of CRC after having demonstrated their superior stability in aqueous solutions as compared to RNA, which has been shown to be chemically unstable and therefore suboptimal for study.11 Biomarker discovery is increasing with the modern throughput of medical research and related technology in genomics and proteomics. New biomarkers are building upon growing information at the same time that high throughput research tools are becoming less expensive, robust, and more efficient.
Optimal Cancer Biomarker Test Criteria
In order to detect and diagnose cancer through the analysis of peripheral blood, the test must identify the origin, or site of disease, as well as the nature of the disease (benign neoplasm, inflammatory condition, malignant neoplasm).11 In determining the nature of the disease, different results for benign or malignant processes must be produced by the test, which have been based upon well-defined diagnostic parameters, that recognize the differences in the diseases.11 Ultimately, thresholds must be established to define the diagnostic parameters. Unfortunately, by their very nature, such thresholds will probably be artificial and only remotely related to the underlying biological complexity of developing cancer.11
The efficacy of a biomarker test is determined by its sensitivity and specificity and these terms take on precise meanings in the development of biomarker tests for population-based screening.12 Sensitivity of a biomarker refers to the proportion of case subjects (with disease) who test positive for the biomarker assay, while the specificity refers to the proportions of control subjects (without disease) who test negative for the biomarker assay. Clinical sensitivity is a composite of 1) the marker prevalence in the tumor; 2) the efficiency of transfer of the marker to the remote media being tested; and, 3) the analytical sensitivity of the assay.12 In the development of a robust cancer diagnostic tool, both sensitivity and specificity are significant parameters. Ultimately, biomarkers must show clinical utility, specificity, and most importantly - an ultimate reduction in mortality.
cfcDNA and epigenetics
It was demonstrated more than 25 years ago that cancer patients have increased levels of free DNA in their serum, or cell free circulating DNA (cfcDNA), which is considered to be released from apoptotic or necrotic tumor cells or actively secreted from proliferating cells.13,14 Initially it was thought that simply a measurement of the cfcDNA level could be used as a cancer marker since high concentrations of cfcDNA were identified in some cancer patients.11 Unfortunately, high variability in abnormal cfcDNA concentrations prevented it from becoming more than a secondary biomarker, and it was subsequently determined that not only cancer, but trauma, inflammation, stroke, and even extensive exercise could significantly increase circulating concentrations of cfcDNA.11, 15-20
With the discovery of tumor-specific mutations found in cfcDNA, the advent of early cancer diagnostic tests based upon mutation analysis in cfcDNA seemed possible.11, 21 However, this possibility currently remains unrealized due to low representation of mutated sequences early in disease development, the inability of mutation detection to indicate tumor location, and technical problems with mutation discovery.11 However, cfcDNA carries not only tumor-specific mutations in its sequences, but also distinctive epigenetic marks, namely DNA methylation in certain GC-rich fragments.
DNA methylation
For decades, we have known and taught that the coding potential for life comprises the varying combinations of the four bases adenine, cytosine, guanine, and thymine. Yet in recent history, the list has expanded and now includes 5-methylcytosine - the “fifth base”. 5-methylcytosine arises from the enzymatic methylation of cytosine, which tends to occur in mammals in GC-rich fragments known as CpG dinucleotides. These GC-rich fragments are usually located near the 5' ends of genes within the promoters and first exons, comprising CpG islands. CpG islands were first described by Adrian Bird, and are currently defined as a contiguous window of DNA of at least 500 base pairs with a G:C content of at least 55%, and an observed over expected CpG frequency of at least 0.65.12,22
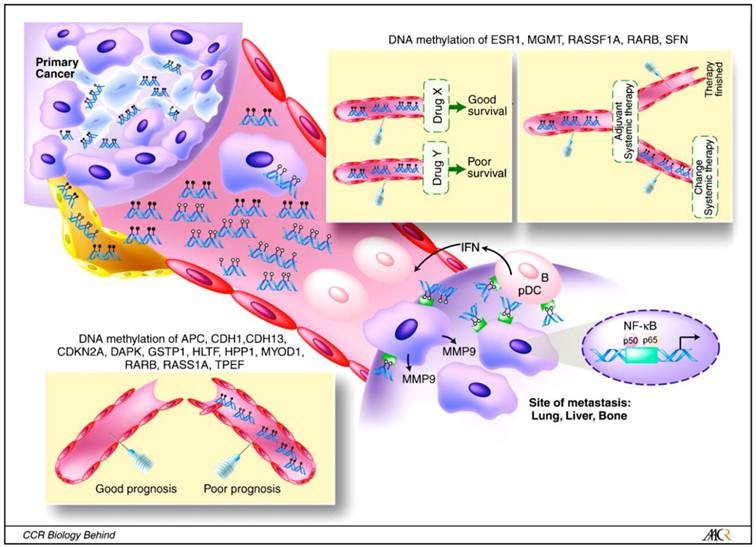
DNA hypermethylation is defined as an increased level of DNA methylation in a DNA sample at either an individual CpG dinucleotide or a CpG island relative to a reference DNA sample that is usually derived from normal tissue.12 DNA hyper-methylation is associated with gene silencing, as it causes the DNA's double helix to fold even tighter upon itself preventing transcription. Hyper-methylation of CpG islands is a common event in carcinogenesis and is the most frequent mechanism for gene inactivation in cancer. Cancer-specific DNA methylation patterns can be found in detached tumor cells in body fluids and biopsies, and they can be detected in free floating DNA that is released from dead cancer cells.23 Analysis of DNA methylation test results from cfcDNA in CRC patients could facilitate the development of accurate biomarkers for early detection and diagnosis of CRC, prognosis and clinical decision support (Figure 1). Currently, evaluation for SEPT9 methylated DNA in peripheral blood is one such marker which has demonstrated feasibility as a blood-based biomarker for all stages and locations of CRC.24,25
Potential for Development of DNA Methylation-based Biomarker Tests for CRC Screening
Analysis of DNA methylation using cfcDNA can facilitate the development of very accurate biomarkers for screening and early detection if researched and developed appropriately. The comparatively high frequency of aberrant DNA methylation found in tumors creates an advantage of using methylation loci over other DNA-type abnormalities (e.g. mutations) as markers to identify various epithelial malignancies (Table 1).12 Additionally, organ-specific benign and inflammatory disease have distinct methylation patterns within cfcDNA, which are different from the pattern of a malignant tumor of the same organ.19,26 In establishing DNA methylation-based tests, sensitivity and specificity must be optimized through the identification of markers that show the highest differences in methylation between the cancer and the background. This can be achieved by comparing the degree of DNA methylation in the target cancer with that in a healthy tissue sample from the same organ, healthy blood samples, and organs from which cells could be present in the bloodstream as a result of disease conditions associated with the tested population.12,19,26
Blood - based detection of cancer using DNA methylation markers (source: Peter W. Laird. The power and the promise of DNA methylation markers. Nature Reviews Cancer 3, 253-266 (April 2003).
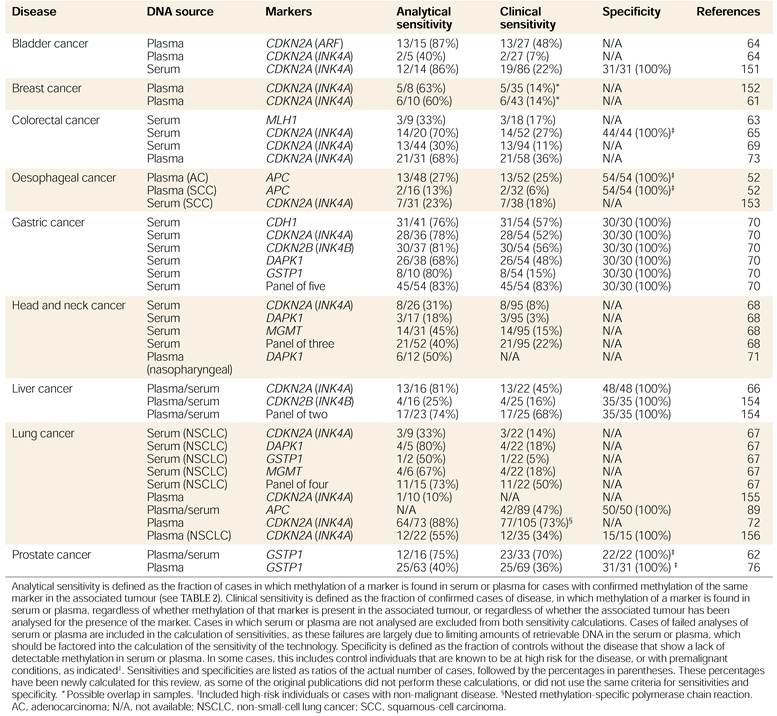
The advantages of utilizing DNA methylation-based tests in screening and early CRC detection also include the ability to compare test results to absolute reference points (completely methylated or completely unmethylated DNA), which simplifies the design of internal references for methylation assays.12 Additionally, methylation assays for individual markers tend to be universal, just like gene-expression markers. Lastly, DNA methylation profiles represent a more chemically and biologically stable source of molecular diagnostic information with patterns that remain fairly stable over time, and do not fluctuate in response to short term stimuli, unlike gene expression profiles.12
The development of DNA methylation-based tests for the early detection of CRC also faces significant challenges. The search for early-stage diagnostic methylation markers hinges on identifying methylation loci with high sensitivity in the early, pre-invasive stages of CRC, such as adenomas and premalignant polyps. However, the majority of DNA methylation markers reported in the literature are associated with later tumorigenic stages rather than even earlier tumor stages.12 These markers are therefore useful in prognosis and measuring therapeutic response, but not in early detection and screening of CRC (Figure 1). Even if pre-invasive markers are identified, isolating and detecting cfcDNA in complex samples like blood in patients with low-to-no tumor burden is a challenge. Additional challenges to the discovery of such biomarkers are technological and encompass a lack of uniform standards across technologies, which has prevented cross validation studies, as well as the difficulty inherent in analyzing heterogeneous clinical samples.11 Moreover, sample preparation is time- and labor-intensive, adding considerable expense to the diagnostic assay. Lastly, the discovery of differentially methylated markers typically produces large numbers of potential candidates complicating the selection processes critical for identifying clinically relevant markers that have the properties necessary to perform adequately in future tests.
Potential role of blood-based DNA methylation in cancer, prognosis and prediction (source: Widschwendter M, Menon U. Clin Cancer Res 2006;12:7205-7208; ©2006 by American Association for Cancer Research. Potential biological role and possible clinical use of CpG DNA in cancer. IFN, interferon; MMP, matrix metalloproteinase; pDC, plasmacytoid dendritic cells; B, B cells.
Current available DNA methylation tests for colorectal carcinoma.
| Biomarker (s) | Application | Specimen | Test Name (Company) |
|---|---|---|---|
| - Methylated SEPT9 | Early Detection of CRC | PB | Epi proColon® 1.0 (Epigenomics) |
| - Methylated SEPT9 | Aid in detection of CRC | PB | ColoVantage® (Quest Diagnostics) |
| - Methylated SEPT9 | Detection of CRC | PB | Real Time mS9 (Abbott) |
| - Methylated BMP3 and NDRG4 - Mutant KRAS, beta actin - Fecal hemoglobin | Early detection of advanced adenomatous polyps and CRC | Stool | sDNA-MT (Exact Sciences)* |
PB = Peripheral Blood * = currently still investigational.
Conclusion
DNA methylation-based tests appear to have a promising role in early CRC detection and screening. Identifying and treating early stage, pre-malignant lesions has been shown to substantially reduce CRC-specific mortality.8 The feasibility of using DNA methylation based biomarkers for early detection of cancer has been shown, as has the use of site-specific assays, which can detect specific methylation patterns for particular tumors. A list of current assays specific to CRC is presented in Table 2. An accurate, non-invasive, early detection method would increase adherence with CRC screening guidelines and reduce the number of patients reluctant to be screened. However, many obstacles still must be surpassed in order to make blood-based DNA methylation screening for CRC a reality and a useful component of standard clinical practice.
Contributing Author Declaration
We certify that all individuals who qualify as authors have been listed; each has participated in one or more of the following areas: conception and design of this work, the acquisition and/or analysis of data, the writing, and/or critical revision of the document, and supervision of this cooperative research effort. All contributing authors approve of the submission of this version of the manuscript and assert that the document represents valid work. If information derived from another source was used in this manuscript, we obtained all necessary approvals to use it and made appropriate acknowledgements in the document. All contributing authors take public responsibility for this work.
Disclaimer
The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense or the United States Government.
Copyright protection
Some of the contributing authors are military service members (or employees of the U.S. Government: TS, AS), and this work was prepared as part of their official duties. Title 17 U.S.C. 105 provides the “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: a cancer journal for clinicians. 2011;61:212-36
2. Heron M. Deaths: Leading causes for 2009. National Vital Statistics Report. 2012;61(7):1-95
3. Brooks JD. Translational genomics: The challenge of developing cancer biomarkers. Genome Res. 2012;22:183-187
4. Glass AG, Lacey JV Jr, Carreon JD, Hoover RN. Breast cancer incidence,1980-2006: Combined roles of menopausal hormone therapy,screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99:1152-1161
5. Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D. et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595
6. Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M. et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328
7. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM. et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409
8. De Paoli VM, Chen C-H, Chang Y-W. Are DNA methylation markers ready for colorectal cancer detection? Cancer Biology & Therapy. 2010;9(11):872-874
9. Brücher BLDM, Geddert H Langner C, Höfler H Fink U, Siewert JR Sarbia M. Hypermethylation of hMLH1, HPP1, p14ARF, p16INK4A, and APC in primary adenocarcinomas of the small bowel. Int J Cancer. 2006;119:1298-1302
10. De Gruttola VG, Clax P, DeMets DL. et al. Considerations in the evaluation of surrogate endpoints in clinical trials. summary of a National Institutes of Health workshop. Controlled clinical trials. 2001;22:485-502
11. Levenson VV. DNA methylation as a universal biomarker. Expert Rev Mol Diagn. 2010;10(4):481-488
12. Laird PW. The power and the promise of DNA methylation markers. Nature Reviews. 2003;3:253-266
13. Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646-650
14. Matei DE, Nephew KP. Epigenetic therapies for chemoresensitization of epithelial ovarian cancer. Gynecol. Oncol. 2010;116:195-201
15. Levenson VV, Melnikov AA. DNA methylation as clinically useful biomarkers - Light at the end of the tunnel. Pharmaceuticals. 2012;5:94-113
16. Atamaniuk J, Vidotto C, Kinzlbauer M, Bachl N, Tiran B, Tschan H. Cell-free plasma DNA and purine nucleotide degradation markers following weightlifting exercise. Eur J Appl Physiol. 2010;110:695-701
17. Lo YM, Rainer TH, Chan LY, Hjelm NM, Cocks RA. Plasma DNA as a prognostic marker in trauma patients. Clin Chem. 2000;46:319-323
18. Giacona MB, Ruben GC, Iczkowski KA, Roos TB, Porter DM, Sorenson GD. Cell-free DNA in human blood plasma: Length measurements of cell-free circulating DNA among patients with pancreatic cancer and healthy controls. Pancreas. 1998;17:89-97
19. Liggett T, Melnikov A, Yi QL, Replogle C, Brand R, Kaul K, Talamonti M, Abams RA, Levenson V. Differential methylation of cell-free circulating DNA among patients with pancreatic cancer versus chronic pancreatitis. Cancer. 2010;116:1674-1680
20. Rainer TH, Wong LK, Lam W, Yuen E, Lam NY, Metreweli C, Lo YM. Prognostic use of circulating plasma nucleic acid concentrations in patients with acute stroke. Clin Chem. 2003;49:562-569
21. Goebel G, Zitt M, Zitt M, Muller HM. Circulating nucleic acids in plasma or serum (CNAPS) as prognostic and predictive markers in patients with solid neoplasms. Dis Markers. 2005;21:105-120
22. Bird A. DNA methylationpatterns and epigenetic memory. Genes Dev. 2002;16:6-21
23. Sidransky D. Emerging molecular markers of cancer. Nature Rev Cancer. 2002;2:210-219
24. Warren JD, Xiong W, Bunker AM, Vaughn CP, Furtado LV, Roberts WL, Fang JC, Samowitz WS, Heichman KA. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011Dec14;9:133
25. Tóth K, Sipos F, Kalmár A, Patai AV, Wichmann B, Stoehr R, Golcher H, Schellerer V, Tulassay Z, Molnár B. Detection of Methylated SEPT9 in Plasma Is a Reliable Screening Method for Both Left- and Right-Sided Colon Cancers. PLoS One. 2012;7(9):e46000
26. Liggett T, Melnikov A, Yi Q, Replogle C, Hu W, Rotmensch J, Kamat A, Sood AK, Levenson V. Distinctive DNA methylation patterns of cell-free plasma DNA in women with malignant ovarian tumors. Gynecol Oncol. 2011;120:113-120
Author contact
![]() Corresponding author: Thomas Summers, MD; Department of Anatomic Pathology; Walter Reed National Military Medical Center, 8901 Wisconsin Avenue; Bethesda, MD 20889-5600. Thomas.A.Summersmil.
Corresponding author: Thomas Summers, MD; Department of Anatomic Pathology; Walter Reed National Military Medical Center, 8901 Wisconsin Avenue; Bethesda, MD 20889-5600. Thomas.A.Summersmil.

 Global reach, higher impact
Global reach, higher impact