Impact Factor
ISSN: 1837-9664
J Cancer 2013; 4(6):502-513. doi:10.7150/jca.6503 This issue Cite
Research Paper
Comparison of Clinical Characteristics and Survival after Surgery in Patients with Non-B and Non-C Hepatocellular Carcinoma and Hepatitis Virus-Related Hepatocellular Carcinoma
1. Departments of Gastroenterology and Hepatology, Osaka Red Cross Hospital, 5-30 Fudegasaki-cho, Tennoji-ku, Osaka 543-0027, Japan;
2. Departments of Surgery, Osaka Red Cross Hospital, 5-30 Fudegasaki-cho, Tennoji-ku, Osaka 543-0027, Japan;
3. Departments of Pathology, Osaka Red Cross Hospital, 5-30 Fudegasaki-cho, Tennoji-ku, Osaka 543-0027, Japan.
Received 2013-4-18; Accepted 2013-7-10; Published 2013-7-18
Abstract
Background and aims: We compared clinicopathologic data and long-term clinical outcomes among patients with non-B and non-C hepatocellular carcinoma (NBNC-HCC) who underwent curative resection (group A, n=129), those with hepatitis B virus-related HCC (group B, n=62) and those with hepatitis C virus-related HCC (group C, n=284).
Methods: Clinicopathologic characteristics and cumulative overall survival (OS) and recurrence-free survival (RFS) after curative resection were compared among the three groups.
Results: The proportion of patients with non-liver cirrhosis (LC) or diabetes mellitus in group A was significantly higher than that in group B or group C. The mean maximum tumor size in group A was significantly larger than that of group B or group C. Cumulative 3-year OS rates after resection were 76% in group A, 79% in group B and 72% in group C (A vs. B, P=0.638; A vs. C, P=0.090; B vs. C, P=0.091; overall significance, P=0.088). The corresponding RFS rates after resection were 38% in group A, 36% in group B and 36% in group C (A vs. B, P=0.528; A vs. C, P=0.281; B vs. C, P=0.944; overall significance, P=0.557). In subgroup analyses in patients with LC, in those without LC and in those who satisfied the Milan criteria, similar results were obtained, i.e., the difference among the three groups did not reach significance in terms of OS and RFS.
Conclusion: Long-term clinical outcomes in patients NBNC-HCC after curative resection were comparable to those in patients with hepatitis virus-related HCC after curative resection.
Keywords: Non-B and non-C hepatocellular carcinoma, overall survival, recurrence-free survival, surgery, prognostic factor.
Introduction
Hepatocellular carcinoma (HCC) is a common and deadly cancer. The prevalence of HCC is increasing in developed countries. In general, the prognosis for untreated HCC is poor, and curative treatments include surgical resection and liver transplantation [1-4]. HCC frequently recurs after curative resection, leading to high mortality, though recurrence occurs only at intrahepatic sites in 68-96% of HCC patients [5, 6]. Stringent follow-up of HCC patients after resection is therefore essential and postoperative surveillance using imaging should be intensified.
Most cases of HCC are attributable to hepatitis virus-related chronic liver disease such as chronic infection by the hepatitis B virus (HBV) and hepatitis C virus (HCV). Nevertheless, a substantial proportion of HCC patients are negative for markers of HBV surface antigen (HBsAg) and HCV antibody (HCVAb), i.e., non-B and non-C HCC (NBNC-HCC). The frequency of NBNC-HCC has been reported to be 5-15% and the number and ratio of NBNC-HCC in Japan has been increasing gradually [7-12].
Clinical characteristics and outcomes in patients with HBV-related HCC (B-HCC) or HCV-related HCC (C-HCC) have been examined fully in previous decades. Owing to improvements in screening programs for HCC development in high-risk populations, small HCCs have been detected with increasing frequency, especially in areas in which the disease is endemic. [2, 3] However, few data regarding clinical characteristics and outcomes in NBNC-HCC are available. This is despite the fact that several investigators reported that NBNC-HCC patients had a poorer prognosis than hepatitis virus-related HCC patients because HCCs in NBNC-HCC patients were often detected at an advanced stage incidentally without follow-up [3, 7, 8, 9, 13]. Thus, there is an urgent need for investigation of the clinical characteristics and outcomes in patients with NBNC-HCC.
The aims of the present study were to compare clinicopathologic data and long-term clinical outcomes between patients with NBNC-HCC who underwent curative resection and those with B-HCC/C-HCC who underwent curative resection.
Patients and methods
Written informed consent was obtained from all patients before resection. The study comprised a retrospective analysis of patient records, and all treatments were conducted in an open-label manner. The Ethics Committee of Osaka Red Cross Hospital (Osaka, Japan) approved the study protocol. The study protocol complied with all of the provisions of the Declaration of Helsinki. The need for written informed consent was waived because the data were analyzed retrospectively and anonymously.
Patients
Patients were selected for resection based on assessments of tumor characteristics, liver function, remnant liver volume and general status through discussion with experienced surgeons, radiologists and physicians. Patients who did not wish to undergo surgery, those with intractable ascites, and those with poor liver function were excluded from consideration for resection.
Curative resection was carried out in 497 treatment-naïve HCC patients at the Department of Surgery, Osaka Red Cross Hospital (Osaka, Japan) between December 1999 and December 2012. Curative surgery was defined as resection of all tumors detectable using imaging. Of these subjects, we excluded 6 patients with surgery-related death, 6 with positive for both HBsAg and HCV Ab, 1 with autoimmune hepatitis, 1 with primary biliary cirrhosis, and 8 with alcoholic cirrhosis. Therefore, 475 HCC patients who underwent resection were assessed. The study cohort comprised: 129 patients with non-B and non-C virus HCC (NBNC-HCC; group A) who were negative for HBsAg and HCVAb; 62 patients with HBV-related HCC (B-HCC; group B) who were positive for HBsAg and negative for HCVAb; and 284 patients with HCV-related HCC (C-HCC; group C) who were negative for HBsAg and positive for HCVAb.
All surgical procedures were carried out by one of four surgeons with ≥10 years of experience of resection. Anatomical resection was defined as resection in which tumors were removed completely anatomically on the basis of the Couinaud classification (segmentectomy, sectionectomy, and hemihepatectomy or extended hemihepatectomy). Non-anatomical partial resection was carried out as a limited resection or tumor enucleation.
Anatomical resection was undertaken in 73 patients in group A, 30 in group B and 98 in group C. Non-anatomical resection was carried out in 56 patients in group A, 32 in group B and 186 in group C.
We compared baseline clinical characteristics, pathological findings and clinical outcomes including overall survival (OS) and recurrence-free survival (RFS) among these three groups.
HCC and the diagnosis of liver cirrhosis (LC)
HCC was diagnosed using abdominal ultrasound and dynamic computed tomography (CT; hyper-attenuation during the arterial phase in all or some part of the tumor and hypo-attenuation in the portal-venous phase) and/or magnetic resonance imaging (MRI) based mainly on the recommendations of the American Association for the Study of Liver Diseases [14]. Arterial- and portal-phase dynamic CT images were obtained at approximately 30 s and 120 s, respectively, after the injection of contrast material. HCC stage was determined using the staging system set by the Liver Cancer Study Group of Japan [15]. HCC was confirmed by pathological means in resected specimens at surgery except in patients with complete necrosis due to pretreatment transcatheter arterial chemoembolization (TACE; n=31). LC was diagnosed by pathological means in resected specimens.
Follow-up
Follow-up after surgery comprised periodic blood tests and monitoring of the tumor markers α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP) using chemiluminescent enzyme immunoassays (Lumipulse PIVKAII Eisai; Eisai, Tokyo, Japan). Dynamic CT images and/or MRI were carried out every 3-4 months after resection. Chest CT, whole abdominal CT, brain MRI, and bone scintigraphy were done if recurrence of extrahepatic HCC was suspected.
Statistical analyses
Data were analyzed using univariate and multivariate analyses. Continuous variables were compared using unpaired t-tests. Categorical variables were compared using the Fisher exact test. Time-to-recurrence was defined as the interval between surgery and the first confirmed recurrence. For analyses of RFS, follow-up ended at the time of first recurrence; other patients were evaluated at their last follow-up visit or the time of death from any cause without recurrence. For analyses of OS, follow-up ended at the time of death from any cause, and the remaining patients were evaluated at the last follow-up visit. The prevalence of cumulative OS and RFS was calculated using the Kaplan-Meier method, and tested using the log-rank test. The Cox proportional hazards model was used for multivariate analyses of factors considered significant in univariate analyses or close to it (P<0.1). These statistical methods were used to estimate the interval from surgery. Data were analyzed using SPSS software (SPSS, Chicago, IL, USA) for Microsoft Windows. Data are the means ± standard deviation (SD). P<0.05 was considered significant.
Results
Clinical characteristics among the three groups
The baseline clinical characteristics of the three groups are shown in Table 1. The mean observation periods in the three groups were 3.8 ± 2.9 years in group A, 4.1 ± 3.0 years in group B and 4.1 ± 2.9 years in group C.
Baseline characteristics among three groups (group A, B and C).
| Group A (n=129) | Group B (n=62) | Group C (n=284) | P-value A vs. B | P-value A vs. C | |
|---|---|---|---|---|---|
| Gender (male/female) | 104/25 | 42/20 | 194/90 | 0.068a | 0.013a |
| Age (years) | 68.6±8.8 | 57.7±12.3 | 69.1±7.8 | <0.001b | 0.559b |
| Hepatectomy | |||||
| Anatomical/non-anatomical | 73/56 | 30/32 | 98/186 | 0.351a | <0.001a |
| HCC stage (I/II/III/IV) | 8/74/39/8 | 5/37/19/1 | 26/164/76/18 | 0.565a | 0.724a |
| Presence of LC (yes/no) | 46/83 | 35/27 | 181/103 | 0.008a | <0.001a |
| Tumor number (single/multiple) | 84/45 | 44/18 | 186/98 | 0.511a | >0.999a |
| Maximum tumor size (cm) | 5.5±3.2 | 4.0±2.2 | 4.1±2.3 | 0.001b | <0.001b |
| Body mass index (kg/m2) | 24.1±4.0 | 22.5±3.7 | 22.8±3.6 | 0.008b | 0.002b |
| Diabetes mellitus (yes/no) | 63/66 | 7/55 | 79/204 | <0.001a | <0.001a |
| AST (IU/l) | 62.9±52.1 | 49.9±43.5 | 55.7±32.9 | 0.092b | 0.093b |
| ALT (IU/l) | 53.4±39.1 | 54.1±69.5 | 52.2±42.8 | 0.931b | 0.795b |
| ALP (IU/l) | 347.9±174.8 | 299.0±133.3 | 335.9±147.6 | 0.053b | 0.469b |
| GGT (IU/l) | 150.9±174.7 | 105.4±193.8 | 89.4±84.4 | 0.106b | <0.001b |
| Serum albumin (g/dl) | 4.00±0.49 | 4.04±0.39 | 3.75±0.49 | 0.555b | <0.001b |
| Total bilirubin (mg/dl) | 0.82±0.41 | 0.86±0.48 | 0.85±0.40 | 0.607b | 0.516b |
| Prothrombin time (%) | 91.5±15.8 | 89.7±16.9 | 87.1±13.2 | 0.472b | 0.004b |
| Platelets (×104/mm3) | 17.2±7.9 | 13.7±8.5 | 12.8±5.6 | 0.005b | <0.001b |
| AFP (ng/ml) | 1726.1±11663.8 | 2450.1±7702.4 | 2470.3±12094.2 | 0.610b | 0.558b |
| DCP (mAU/ml) | 8573.2±45695.6 | 2913.2±13229.8 | 3330.9±14306.1 | 0.196b | 0.204b |
| HCC histology | |||||
| Well/moderate/poorly/necrosis | 10/76/42/1 | 4/24/25/9 | 24/137/102/21 | <0.001a | 0.021a |
| Fibrous capsule (yes/no) | 94/35 | 53/9 | 245/39 | 0.066a | 0.001a |
| Microscopic vascular invasion (yes/no) | 55/74 | 25/37 | 92/192 | 0.876a | 0.047a |
| Microscopic surgical margin (yes/no) | 31/98 | 14/48 | 78/206 | 0.858a | 0.473a |
HCC: hepatocellular carcinoma; LC: liver cirrhosis; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; GGT: gamma glutamyl transpeptidase; AFP: alpha-fetoprotein; DCP: des-γ-carboxy prothrombin; a Fisher,s exact test; b unpaired t-test.
The proportion of patients with non-LC in group A was significantly higher than that in group B (P=0.008) or group C (P<0.001). The mean value of the platelet count in group A was significantly higher than that in group B (P=0.005) or group C (P<0.001), suggesting that subjects in group A had better liver function than those in groups B or C. The maximum tumor size in group A was significantly larger than that in group B (P=0.001) or group C (P<0.001). The mean value of the body mass index (BMI) and the proportion of patients with diabetes mellitus (DM) in group A were significantly higher than those in group B (BMI, P=0.008; DM, P<0.001) or group C (BMI, P=0.002; DM, P<0.001), suggesting that group-A patients had a higher prevalence of metabolic disorders as compared with group-B or group-C patients. With regard to tumor histology, significantly better tumor differentiation in group A was observed than in group B (P<0.001) or group C (P=0.021).
OS among the three groups
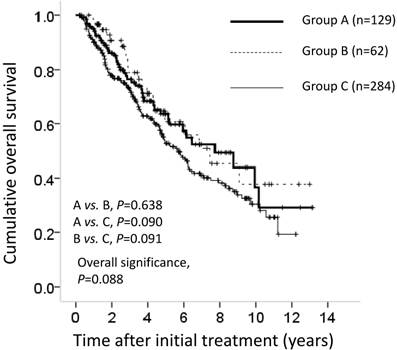
The cumulative 1-, 3- and 5-year OS rates after resection in each group were 96%, 76% and 63%, respectively, in group A; 98%, 79% and 63%, respectively, in group B; and 90%, 72% and 53%, respectively, in group C. In terms of OS, there was no overall significant difference among these three groups (A vs. B; P=0.638; A vs. C; P=0.090; B vs. C; P=0.091, overall significance; P = 0.088) (Figure 1).
Cumulative overall survival (OS) for all cases (n=475). The cumulative 1-, 3- and 5-year OS rates after resection in each group was 96%, 76% and 63%, respectively, in group A (n=129), 98%, 79% and 63%, respectively, in group B (n=62) and 90%, 72% and 53%, respectively, in group C (n=284). In terms of OS, there was no overall significant difference among these three groups (A vs. B, P=0.638; A vs. C, P=0.090; B vs. C, P=0.091; overall significance; P=0.088).
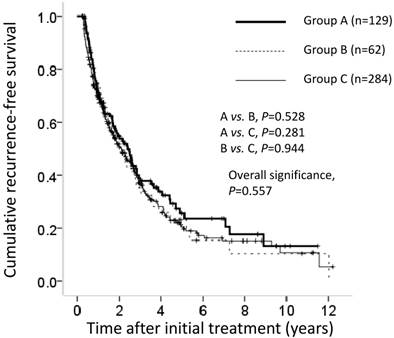
Cumulative recurrence-free survival (RFS) for all cases (n=475). The cumulative 1-, 3- and 5-year RFS rates after resection in each group was 68%, 38% and 26%, respectively, in group A, 68%, 36% and 22%, respectively, in group B and 68%, 36% and 20%, respectively, in group C. In terms of RFS, there was no overall significant difference among these three groups (A vs. B, P=0.528; A vs. C, P=0.281; B vs. C; P=0.944; overall significance, P=0.557).
RFS among the three groups
The cumulative 1-, 3- and 5-year RFS rates after resection in each group were 68%, 38% and 26%, respectively, in group A; 68%, 36% and 22%, respectively, in group B; and 68%, 36% and 20%, respectively, in group C. In terms of RFS, there was no overall significant difference among these three groups (A vs. B, P=0.528; A vs. C, P=0.281; B vs. C, P=0.944; overall significance, P=0.557) (Figure 2).
Univariate and multivariate analyses of factors contributing to OS and RFS for all cases (n=475)
Univariate analyses was used to identify the following as significant factors linked to OS: HCC stage (P<0.001); maximum tumor size ≥4 cm (P=0.001); tumor number (P<0.001); LC (P<0.001); serum albumin ≥3.9 g/dL (P<0.001); alkaline phosphatase (ALP) ≥300 IU/L (P=0.013); gamma glutamyl transpeptidase (GGT) ≥80 IU/L (P=0.026); prothrombin time (PT) ≥88% (P=0.006); AFP ≥20 ng/mL (P<0.001); DCP ≥200 mAU/mL (P=0.003); and microscopic vascular invasion (MVI) (P<0.001) as significant factors contributing to OS (Table 2). Multivariate analyses involving 12 factors with P<0.1 in the univariate analysis identified HCC stage (P=0.039), maximum tumor size ≥4 cm (P=0.029), LC (P=0.006), serum albumin ≥3.9 g/dL (P=0.002), AFP ≥20 ng/mL (P=0.001) and MVI (P=0.003). The hazard ratios (HRs) and 95% confidence intervals (CIs) for these factors are detailed in Table 3.
Univariate analyses identified HCC stage (P<0.001), maximum tumor size ≥4 cm (P=0.025), tumor number (P<0.001), LC (P<0.001), serum albumin ≥3.9 g/dL (P=0.044), ALP ≥300 IU/L (P=0.001), GGT ≥80 IU/L (P<0.001), PT ≥88 % (P=0.033), BMI ≥23 kg/m2 (P=0.022), AFP ≥20 ng/mL (P<0.001), DCP ≥200 mAU/mL (P=0.005) and MVI (P<0.001) as significant factors associated with RFS (Table 2). Multivariate analyses involving 15 factors with P<0.1 in the univariate analysis identified LC (P=0.020), GGT (P=0.008), BMI ≥23 kg/m2 (P=0.022), AFP ≥20 ng/mL (P=0.006) and MVI (P<0.001) as significant factors contributing to RFS. The details of HRs and 95% CIs for these factors are shown in Table 3.
Univariate analysis of factors contributing to overall survival (OS) and recurrence-free survival (RFS) after surgical resection for all cases (n=475).
| OS | RFS | ||
|---|---|---|---|
| Variables | n | P-valuea | P-valuea |
| Age ≥70 years (yes/no) | 223/252 | 0.153 | 0.867 |
| Gender (male/female) | 340/135 | 0.452 | 0.317 |
| Cause of liver disease | |||
| Hepatitis B/hepatitis C/nonB nonC | 62/284/129 | 0.088 | 0.557 |
| HCC stage (I, II / III, IV) | 314/161 | <0.001 | <0.001 |
| Maximum tumor size ≥4 cm (yes/no) | 229/246 | 0.001 | 0.025 |
| Tumor number (single/multiple) | 314/161 | <0.001 | <0.001 |
| Presence of LC (yes/no) | 262/213 | <0.001 | <0.001 |
| Total bilirubin ≥ 0.8 mg/dl (yes/no) | 236/239 | 0.208 | 0.995 |
| Serum albumin ≥3.9 g/dl (yes/no) | 255/220 | <0.001 | 0.044 |
| AST ≥50 IU/l (yes/no) | 214/261 | 0.137 | 0.095 |
| ALT ≥50 IU/l (yes/no) | 182/293 | 0.389 | 0.097 |
| ALP ≥ 300 IU/l (yes/no) | 237/238 | 0.013 | 0.001 |
| GGT ≥80 IU/l (yes/no) | 207/268 | 0.026 | <0.001 |
| Platelets ≥13×104/mm3 (yes/no) | 249/226 | 0.228 | 0.078 |
| Prothrombin time ≥88 % (yes/no) | 259/216 | 0.006 | 0.033 |
| Diabetes mellitus (yes/no) | 149/325 | 0.307 | 0.982 |
| Body mass index ≥23 kg/m2 (yes/no) | 222/253 | 0.542 | 0.022 |
| AFP ≥20 ng/ml (yes/no) | 237/238 | <0.001 | <0.001 |
| DCP ≥200 mAU/ml (yes/no) | 240/235 | 0.003 | 0.005 |
| Microscopic vascular invasion (yes/no) | 172/303 | <0.001 | <0.001 |
| Microscopic surgical margin (yes/no) | 123/352 | 0.405 | 0.685 |
HCC; hepatocellular carcinoma, LC; liver cirrhosis, AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, AFP; alpha-fetoprotein, DCP; des-γ-carboxy prothrombin, a; Log-rank test.
Multivariate analysis contributing to overall survival (OS) and recurrence-free survival (RFS) for all cases (n=475).
| OS | RFS | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P valuea | HR | 95% CI | P valuea |
| Cause of liver disease | ||||||
| Non B non C | 1.000 | |||||
| Hepatitis B | 1.095 | 0.763-1.572 | 0.623 | |||
| Hepatitis C | 0.841 | 0.487-1.451 | 0.533 | |||
| HCC stage | ||||||
| Stage I or II | 1.000 | 1.000 | ||||
| Stage III or IV | 0.517 | 0.277-0.968 | 0.039 | 0.702 | 0.437-1.127 | 0.143 |
| Maximum tumor size | ||||||
| ≥ 4cm | 0.695 | 0.502-0.963 | 0.029 | 0.901 | 0.696-1.166 | 0.428 |
| < 4cm | 1.000 | 1.000 | ||||
| Tumor number | ||||||
| Single | 1.097 | 0.590-2.042 | 0.770 | 1.364 | 0.856-2.174 | 0.191 |
| Multiple | 1.000 | 1.000 | ||||
| Presence of LC | ||||||
| Yes | 0.642 | 0.468-0.880 | 0.006 | 0.713 | 0.536-0.948 | 0.020 |
| No | 1.000 | 1.000 | ||||
| Serum albumin | ||||||
| ≥3.9 g/dl | 1.620 | 1.188-2.210 | 0.002 | 1.191 | 0.926-1.532 | 0.174 |
| <3.9 g/dl | 1.000 | 1.000 | ||||
| AST | ||||||
| ≥50 IU/l | 1.000 | |||||
| <50 IU/l | 1.099 | 0.806-1.499 | 0.549 | |||
| ALT | ||||||
| ≥50 IU/l | 1.000 | |||||
| <50 IU/l | 0.964 | 0.696-1.336 | 0.827 | |||
| ALP | ||||||
| ≥300 IU/l | 1.000 | 0.990 | 0.770-1.273 | 0.937 | ||
| <300 IU/l | 1.095 | 0.799-1.500 | 0.573 | 1.000 | ||
| GGT | ||||||
| ≥80 IU/l | 0.782 | 0.579-1.056 | 0.109 | 0.723 | 0.568-0.920 | 0.008 |
| <80 IU/l | 1.000 | 1.000 | ||||
| Platelet | ||||||
| ≥13×104/mm3 | 1.000 | |||||
| <13×104/mm3 | 0.992 | 0.749-1.314 | 0.956 | |||
| Prothrombin time | ||||||
| ≥88 % | 1.196 | 0.894-1.600 | 0.229 | 1.089 | 0.859-1.381 | 0.480 |
| <88 % | 1.000 | 1.000 | ||||
| Body mass index | ||||||
| ≥23 kg/m2 | 0.767 | 0.611-0.962 | 0.022 | |||
| <23 kg/m2 | 1.000 | |||||
| AFP | ||||||
| ≥20 ng/ml | 0.618 | 0.461-0.829 | 0.001 | 0.728 | 0.579-0.915 | 0.006 |
| <20 ng/ml | 1.000 | 1.000 | ||||
| DCP | ||||||
| ≥ 200 mAU/ml | 0.979 | 0.702-1.365 | 0.900 | 0.962 | 0.739-1.254 | 0.777 |
| < 200 mAU/ml | 1.000 | 1.000 | ||||
| MVI | ||||||
| Yes | 0.629 | 0.463-0.855 | 0.003 | 0.629 | 0.507-0.826 | <0.001 |
| No | 1.000 | 1.000 | ||||
HCC, hepatocellular carcinoma; LC, liver cirrhosis; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transpeptidase; AFP, alpha-fetoprotein; DCP, des-γ-carboxy prothrombin; MVI, microscopic vascular invasion; HR, hazard ratio; CI, confidence interval; a, Cox proportional hazard model.
HCC recurrence in the three groups
HCC recurrence during the follow-up period was observed in 81 (62.8%) patients in group A, in 44 patients (71.0%) in group B and in 199 patients (70.1%) in group C. The patterns of HCC recurrence after resection in group A were: single HCC recurrence in the liver in 30 patients; multiple HCC recurrences in the liver in 40 patients; and multiple HCC recurrences in the liver with extrahepatic metastases in 11 patients. The patterns of HCC recurrence after resection in group B were: single HCC recurrence in the liver in 20 patients; multiple HCC recurrences in the liver in 19 patients; and multiple HCC recurrences in the liver with extrahepatic metastases in 5 patients. The patterns of HCC recurrence after resection in group C were: single HCC recurrence in the liver in 76 patients; multiple HCC recurrences in the liver in 108 patients; multiple HCC recurrences in the liver with extrahepatic metastases in 14 patients; and single brain metastasis in 1 patient.
Treatment methods for the first recurrence in group A were: resection in 6 patients; radiofrequency ablation (RFA) in 34 patients; TACE in 23 patients; systemic chemotherapy in 4 patients; radiation therapy in 1 patient and no specific treatment in 13 patients. The treatment methods used for the first recurrence in group B were: resection in 3 patients; RFA in 21 patients; TACE in 11 patients; percutaneous ethanol injection (PEI) in 2 patients; systemic chemotherapy in 2 patients, radiation therapy in 2 patients and no specific treatment in 3 patients. The treatment methods used for the first recurrence in group C were: resection in 15 patients; RFA in 94 patients; TACE in 62 patients; PEI in 6 patients; systemic chemotherapy in 3 patients, radiation therapy in 5 patients and no specific treatment in 14 patients.
Causes of death in the three groups
Forty-three patients (33.3%) in group A, 20 (32.3%) patients in group B and 140 patients (49.3%) in group C died during follow-up. The causes of death in each group were: HCC recurrence (32 patients), liver failure (4 patients) and miscellaneous (7 patients) in group A; HCC recurrence (16 patients), liver failure (4 patients) and miscellaneous (0 patient) in group B; HCC recurrence (95 patients), liver failure (26 patients) and miscellaneous (19 patients) in group C.
Subgroup analyses in patients with LC and without LC
The proportion of patients with LC was significantly lower in group A than that in group B or group C and it is considered to be a major confounder. Hence, we conducted subgroup analyses in patients with LC (n=262) and without LC (n=213).
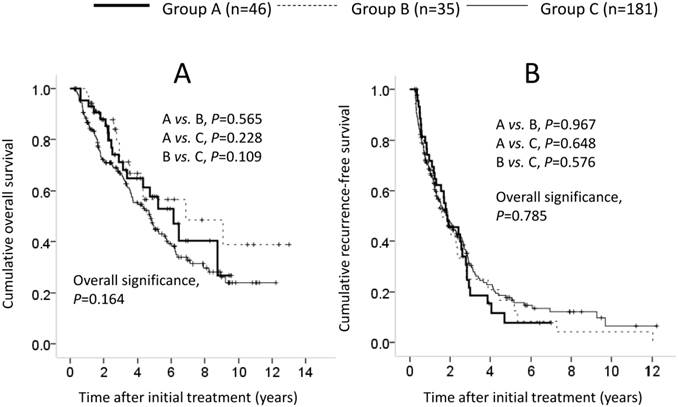
Patients with LC comprised 46 patients in group A, 35 in group B and 181 in group C. In terms of OS and RFS, the overall difference among the three groups failed to reach significance (OS: A vs. B, P=0.565; A vs. C, P=0.228; B vs. C, P=0.109; overall significance, P=0.164; RFS: A vs. B, P=0.967; A vs. C, P=0.648; B vs. C, P=0.576; overall significance, P=0.785). (Figures 3A and 3B). In the univariate analysis of factors contributing to OS, HCC stage (P<0.001), maximum tumor size ≥4 cm (P=0.012), tumor number (P=0.003), serum albumin ≥3.9 g/dL (P=0.010), AFP ≥20 ng/mL (P=0.001), DCP ≥200 mAU/mL (P=0.008) and MVI (P<0.001) were significant predictors (Table 4). In the multivariate analysis involving nine factors with P<0.1 in the univariate analysis, serum albumin ≥3.9 g/dL (P<0.001), platelets ≥13×104/mm3 (P=0.030), AFP ≥20 ng/mL (P=0.002) and MVI (P=0.001) were significant factors associated with OS. The HRs and 95% CIs of these factors are listed in Table 4. In the univariate analysis of factors contributing to RFS, HCC stage (P<0.001), tumor number (P<0.001), GGT ≥80 IU/L (P=0.021), AFP ≥20 ng/mL (P=0.001), DCP ≥200 mAU/mL (P=0.027) and MVI (P<0.001) were significant predictors (Table 4). In the multivariate analysis involving seven factors with P<0.1 in the univariate analysis, BMI ≥23 kg/m2 (P=0.043), AFP ≥20 ng/mL (P=0.003) and MVI (P=0.001) were significant factors associated with RFS. The HRs and 95% CIs of these factors are shown in Table 4.
Univariate and multivariate analysis of factors contributing to overall survival (OS) and recurrence-free survival (RFS) after surgical resection in patients with liver cirrhosis (n=262).
| OS | Multivariate analysis (OS) | RFS | Multivariate analysis (RFS) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | n | P-valuea | HR | 95%CI | P-valueb | P-valuea | HR | 95%CI | P-valueb |
| Age ≥70 years (yes/no) | 117/145 | 0.134 | 0.871 | ||||||
| Gender (male/female) | 170/92 | 0.532 | 0.537 | ||||||
| Cause of liver disease | |||||||||
| Hepatitis B/hepatitis C/nonB nonC | 35/181/46 | 0.164 | 0.785 | ||||||
| HCC stage (I, II/III, IV) | 175/87 | <0.001 | 0.580 | 0.281-1.198 | 0.141 | <0.001 | 0.640 | 0.356-1.151 | 0.126 |
| Maximum tumor size ≥4 cm (yes/no) | 104/158 | 0.012 | 0.760 | 0.513-1.128 | 0.174 | 0.255 | |||
| Tumor number (single/multiple) | 168/94 | 0.003 | 0.976 | 0.479-1.988 | 0.974 | <0.001 | 0.754 | 0.423-1.343 | 0.337 |
| Total bilirubin ≥ 0.8 mg/dl (yes/no) | 159/103 | 0.830 | 0.523 | ||||||
| Serum albumin ≥3.9 g/dl (yes/no) | 100/162 | 0.010 | 2.038 | 1.367-3.039 | <0.001 | 0.932 | |||
| AST ≥50 IU/l (yes/no) | 109/153 | 0.243 | 0.866 | ||||||
| ALT ≥50 IU/l (yes/no) | 88/174 | 0.625 | 0.818 | ||||||
| ALP ≥ 300 IU/l (yes/no) | 158/104 | 0.075 | 0.901 | 0.617-1.315 | 0.589 | 0.664 | |||
| GGT ≥80 IU/l (yes/no) | 108/154 | 0.220 | 0.021 | 0.893 | 0.664-1.201 | 0.454 | |||
| Platelets ≥13×104/mm3 (yes/no) | 71/191 | 0.092 | 0.649 | 0.440-0.959 | 0.030 | 0.379 | |||
| Prothrombin time ≥88 % (yes/no) | 105/157 | 0.151 | 0.885 | ||||||
| Diabetes mellitus (yes/no) | 80/182 | 0.266 | 0.385 | ||||||
| Body mass index ≥ 23 kg/m2 (yes/no) | 126/136 | 0.624 | 0.066 | 0.735 | 0.546-0.990 | 0.043 | |||
| AFP ≥20 ng/ml (yes/no) | 155/107 | 0.001 | 0.566 | 0.391-0.818 | 0.002 | 0.001 | 0.644 | 0.480-0.864 | 0.003 |
| DCP ≥200 mAU/ml (yes/no) | 116/146 | 0.008 | 1.039 | 0.695-1.553 | 0.852 | 0.027 | 0.898 | 0.668-1.209 | 0.479 |
| Microscopic vascular invasion (yes/no) | 98/164 | <0.001 | 0.540 | 0.372-0.785 | 0.001 | <0.001 | 0.610 | 0.450-0.826 | 0.001 |
| Microscopic surgical margin (yes/no) | 71/191 | 0.475 | 0.205 | ||||||
HR; hazard ratio, CI; confidence interval, HCC; hepatocellular carcinoma, AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, AFP; alpha-fetoprotein, DCP; des-γ-carboxy prothrombin, a; Log-rank test, b; Cox proportional hazard model.
Cumulative overall survival (OS) and recurrence-free survival (RFS) in patients with LC (n=262). In terms of OS (A) and RFS (B), the overall difference among the three groups did not reach significance (OS: A vs. B, P=0.565; A vs. C, P=0.228; B vs. C, P=0.109; overall significance, P=0.164; RFS: A vs. B, P=0.967; A vs. C, P=0.648; B vs. C, P=0.576; overall significance, P=0.785).
Cumulative overall survival (OS) and recurrence-free survival (RFS) in patients without LC (n=213). In terms of OS (A) and RFS (B), the difference among the three groups did not reach significance (OS: A vs. B, P=0.750; A vs. C, P=0.938; B vs. C, P=0.857; overall significance, P=0.963; RFS: A vs. B, P=0.958; A vs. C, P=0.448; B vs. C, P=0.658; overall significance, P=0.733).
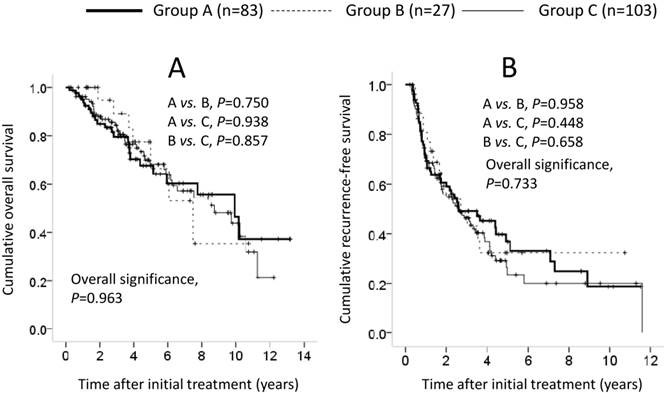
Patients without LC comprised 83 patients in group A, 27 in group B and 103 in group C. In terms of OS and RFS, the difference among the three groups did not reach significance (OS: A vs. B, P=0.750; A vs. C, P=0.938; B vs. C, P=0.857; overall significance, P=0.963; RFS: A vs. B, P=0.958; A vs. C, P=0.448; B vs. C, P=0.658; overall significance, P=0.733) (Figures 4A and 4B). In the univariate analysis of factors contributing to OS, HCC stage (P=0.001), maximum tumor size ≥4 cm (P=0.001), tumor number (P=0.008), serum albumin ≥3.9 g/dL (P=0.010), GGT ≥80 IU/L (P=0.038), AFP ≥20 ng/mL (P=0.028), DCP ≥200 mAU/mL (P=0.011) and MVI (P=0.015) were significant predictors (Table 5). In the multivariate analysis involving seven factors with P<0.1 in the univariate analysis, maximum tumor size ≥4 cm (HR, 0.477; 95% CI, 0.264-0.864; P=0.015) was the only significant predictor associated with OS. In the univariate analysis of factors contributing to RFS, HCC stage (P<0.001), maximum tumor size ≥4 cm (P=0.002), tumor number (P<0.001), ALP ≥300 IU/L (P=0.002), GGT ≥80 IU/l (P<0.001), AFP ≥20 ng/mL (P =0.044), DCP ≥200 mAU/mL (P=0.010) and MVI (P=0.004) were significant predictors (Table 5). In the multivariate analysis involving 11 factors with P<0.1 in the univariate analysis, GGT ≥80 IU/L (HR, 0.642; 95% CI, 0.435-0.949; P=0.026) was the only significant factor associated with RFS (Table 5).
Subgroup analyses in patients who satisfied the Milan criteria
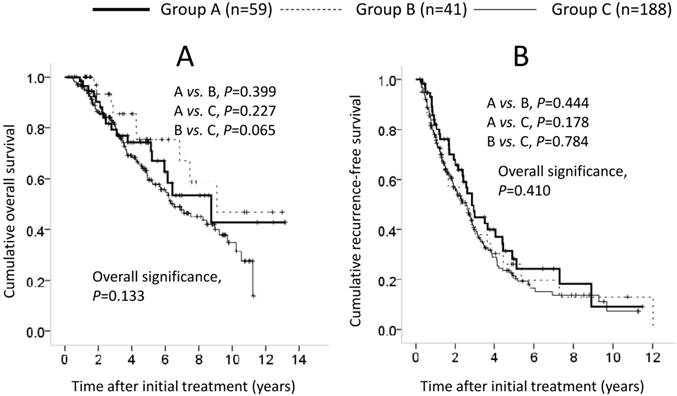
We also carried out subgroup analyses in 288 patients who satisfied the Milan criteria (≤3 cm in size within three nodules or single nodule ≤5 cm in size). They included 59 patients in group A, 41 in group B and 188 in group C. In terms of OS and RFS, the difference among the three groups failed to reach significance (OS: A vs. B, P=0.399; A vs. C, P=0.227; B vs. C, P=0.065; overall significance, P=0.133; RFS: A vs. B, P=0.444; A vs. C, P=0.178; B vs. C, P=0.784; overall significance, P=0.410) (Figures 5A and 5B). In the univariate analysis of factors contributing to OS, LC (P<0.001), serum albumin ≥3.9 g/dL (P=0.002), PT ≥88% (P=0.029), AFP ≥20 ng/mL (P=0.002) and MVI (P<0.001) were significant predictors (Table 6). In the multivariate analysis involving nine factors with P<0.1 in the univariate analysis, LC (P=0.013) and MVI (P<0.001) were significant factors associated with OS. The HRs and 95% CIs for these factors are detailed in Table 6. In the univariate analysis of factors associated with RFS, tumor number (P=0.002), LC (P=0.001), GGT ≥80 IU/L (P=0.001), BMI ≥23 kg/m2 (P=0.008), AFP ≥20 ng/mL (P=0.007) and MVI (P=0.001) were significant predictors (Table 6). In the multivariate analysis involving 12 factors with P<0.1 in the univariate analysis, tumor number (P=0.011), LC (P=0.005), GGT ≥80 IU/l (P=0.005), BMI ≥23 kg/m2 (P=0.008), and MVI (P<0.001) were significant factors associated with RFS. The HRs and 95% CIs for these factors are detailed in Table 6.
Univariate and multivariate analysis of factors contributing to overall survival (OS) and recurrence-free survival (RFS) after surgical resection in patients without liver cirrhosis (n=213).
| OS | Multivariate analysis (OS) | RFS | Multivariate analysis (RFS) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | n | P-valuea | HR | 95%CI | P-valueb | P-valuea | HR | 95%CI | P-valueb |
| Age ≥70 years (yes/no) | 106/107 | 0.527 | 0.939 | ||||||
| Gender (male/female) | 170/43 | 0.548 | 0.091 | 0.650 | 0.397-1.065 | 0.087 | |||
| Cause of liver disease | |||||||||
| Hepatitis B/hepatitis C/nonB nonC | 27/103/83 | 0.963 | 0.733 | ||||||
| HCC stage (I, II/III, IV) | 139/74 | 0.001 | 0.360 | 0.116-1.118 | 0.077 | <0.001 | 0.560 | 0.263-1.194 | 0.133 |
| Maximum tumor size ≥4 cm (yes/no) | 125/88 | 0.001 | 0.477 | 0.264-0.864 | 0.015 | 0.002 | 0.643 | 0.413-1.000 | 0.050 |
| Tumor number (single/multiple) | 146/67 | 0.008 | 1.616 | 0.522-5.006 | 0.405 | <0.001 | 0.979 | 0.462-2.074 | 0.955 |
| Total bilirubin ≥0.8 mg/dl (yes/no) | 77/136 | 0.604 | 0.578 | ||||||
| Serum albumin ≥3.9 g/dl (yes/no) | 155/58 | 0.112 | 0.161 | ||||||
| AST ≥50 IU/l (yes/no) | 105/108 | 0.552 | 0.096 | 1.373 | 0.850-2.216 | 0.195 | |||
| ALT ≥50 IU/l (yes/no) | 94/119 | 0.132 | 0.060 | 0.940 | 0.562-1.572 | 0.813 | |||
| ALP ≥300 IU/l (yes/no) | 79/134 | 0.644 | 0.002 | 0.718 | 0.489-1.053 | 0.090 | |||
| GGT ≥80 IU/l (yes/no) | 99/114 | 0.038 | 0.651 | 0.403-1.053 | 0.080 | <0.001 | 0.642 | 0.435-0.949 | 0.026 |
| Platelets ≥13×104/mm3 (yes/no) | 178/35 | 0.543 | 0.558 | ||||||
| Prothrombin time ≥88 % (yes/no) | 154/59 | 0.444 | 0.115 | ||||||
| Diabetes mellitus (yes/no) | 69/143 | 0.903 | 0.382 | ||||||
| Body mass index ≥23 kg/m2 (yes/no) | 96/117 | 0.981 | 0.210 | ||||||
| AFP ≥20 ng/ml (yes/no) | 82/131 | 0.028 | 0.694 | 0.424-1.135 | 0.146 | 0.044 | 0.753 | 0.514-1.103 | 0.146 |
| DCP ≥200 mAU/ml (yes/no) | 124/89 | 0.011 | 0.977 | 0.518-1.842 | 0.943 | 0.010 | 1.159 | 0.723-1.859 | 0.539 |
| Microscopic vascular invasion (yes/no) | 74/139 | 0.015 | 0.814 | 0.469-1.413 | 0.464 | 0.004 | 0.740 | 0.489-1.122 | 0.156 |
| Microscopic surgical margin (yes/no) | 52/161 | 0.547 | 0.129 | ||||||
HR; hazard ratio, CI; confidence interval, HCC; hepatocellular carcinoma, AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, AFP; alpha-fetoprotein, DCP; des-γ-carboxy prothrombin, a; Log-rank test, b; Cox proportional hazard model.
Cumulative overall survival (OS) and recurrence-free survival (RFS) in patients who satisfied the Milan criteria (n=288). In terms of OS (A) and RFS (B), the difference among the three groups did not reach significance (OS: A vs. B, P=0.399; A vs. C, P=0.227; B vs. C, P=0.065; overall significance, P=0.133; RFS: A vs. B, P=0.444; A vs. C, P=0.178; B vs. C, P=0.784; overall significance, P=0.410).
Univariate and multivariate analysis of factors associated with overall survival (OS) and recurrence-free survival (RFS) after surgical resection in patients who fulfilled Milan Criteria (n=288).
| OS | Multivariate analysis (OS) | RFS | Multivariate analysis (RFS) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | n | P-valuea | HR | 95%CI | P-valueb | P-valuea | HR | 95%CI | P-valueb |
| Age ≥70 years (yes/no) | 130/158 | 0.240 | 0.625 | ||||||
| Gender (male/female) | 198/90 | 0.573 | 0.374 | ||||||
| Cause of liver disease | |||||||||
| Hepatitis B/hepatitis C/nonB nonC | 41/188/59 | 0.133 | 0.410 | ||||||
| Maximum tumor size ≥4 cm (yes/no) | 68/220 | 0.319 | 0.418 | ||||||
| Tumor number (single/multiple) | 245/43 | 0.057 | 0.734 | 0.452-1.191 | 0.211 | 0.002 | 0.602 | 0.408-0.899 | 0.011 |
| LC or non LC | 174/114 | <0.001 | 0.552 | 0.345-0.881 | 0.013 | 0.001 | 0.571 | 0.385-0.847 | 0.005 |
| Total bilirubin ≥0.8 mg/dl (yes/no) | 150/138 | 0.228 | 0.826 | ||||||
| Serum albumin ≥3.9 g/dl (yes/no) | 146/142 | 0.002 | 1.369 | 0.886-2.116 | 0.158 | 0.089 | 0.955 | 0.684-1.334 | 0.788 |
| AST ≥50 IU/l (yes/no) | 136/152 | 0.297 | 0.085 | 1.308 | 0.873-1.960 | 0.193 | |||
| ALT ≥50 IU/l (yes/no) | 111/177 | 0.866 | 0.097 | 1.012 | 0.658-1.555 | 0.957 | |||
| ALP ≥300 IU/l (yes/no) | 129/159 | 0.059 | 0.883 | 0.589-1.324 | 0.547 | 0.121 | |||
| GGT ≥80 IU/l (yes/no) | 98/190 | 0.160 | 0.001 | 0.657 | 0.489-0.882 | 0.005 | |||
| Platelets ≥13×104/mm3 (yes/no) | 133/155 | 0.128 | 0.072 | 0.912 | 0.627-1.328 | 0.631 | |||
| Prothrombin time ≥88 % (yes/no) | 150/138 | 0.029 | 1.100 | 0.723-1.675 | 0.655 | 0.063 | 1.038 | 0.756-1.426 | 0.817 |
| Diabetes mellitus (yes/no) | 81/207 | 0.512 | 0.884 | ||||||
| Body mass index ≥23 kg/m2 (yes/no) | 133/155 | 0.076 | 0.777 | 0.521-1.157 | 0.214 | 0.008 | 0.673 | 0.502-0.904 | 0.008 |
| AFP ≥20 ng/ml (yes/no) | 138/150 | 0.002 | 0.675 | 0.452-1.007 | 0.054 | 0.007 | 0.743 | 0.549-1.005 | 0.054 |
| DCP ≥200 mAU/ml (yes/no) | 102/186 | 0.067 | 0.910 | 0.599-1.381 | 0.656 | 0.080 | 0.910 | 0.666-1.243 | 0.552 |
| Microscopic vascular invasion (yes/no) | 76/212 | <0.001 | 0.455 | 0.295-0.700 | <0.001 | 0.001 | 0.531 | 0.382-0.740 | <0.001 |
| Microscopic surgical margin (yes/no) | 68/220 | 0.587 | 0.460 | ||||||
HR; hazard ratio, CI; confidence interval, LC; liver cirrhosis, AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, AFP; alpha-fetoprotein, DCP; des-γ-carboxy prothrombin, a; Log-rank test, b; Cox proportional hazard model
Discussion
Even though the number of patients with NBNC-HCC in Japan has been increasing gradually, the long-term clinical outcomes of patients with NBNC-HCC have not been examined fully [10]. In general, NBNC-HCC patients have been reported to have poor prognosis, since in these patients, HCCs tend to be diagnosed at an advanced stage due to the absence of surveillance programs for early HCC detection. However, whether NBNC-HCC patients who undergo curative resection have a worse prognosis than hepatitis virus-related HCC patients who undergo curative resection is controversial. Hence, we conducted the comparative study described here.
In our analyses, in terms of OS and RFS, the difference among the three groups did not reach significance. In addition, similar results were obtained in subgroup analyses in patients with LC, those without LC and those who met the Milan criteria. These results suggested that clinical outcomes in NBNC-HCC patients after curative resection were comparable with those in patients with B-HCC or C-HCC after curative resection. This lack of difference in survival after curative resection may be because NBNC-HCC is associated with larger tumors but a better hepatic functional reserve. Conversely, the proportion of liver-unrelated death in group A was the highest among the three groups (7 [16.3%] out of 43 deaths in group A, 0 [0%] out of 20 deaths in group B and 19 [13.6%] out of 140 deaths in group C). The higher frequency of metabolic disorders observed in group A may have accounted for these results.
In their large study, Li et al. reported that females with NBNC-HCC had a poorer prognosis than did males with NBNC-HCC [16]. However, in the present study, in NBNC-HCC patients, no significant difference between males and females was observed in terms of OS (P=0.451) and RFS (P=0.715). Our relatively small patient cohort with NBNC-HCC as compared with that in their study (n=129 in our study vs. n=675 in their study) may be associated with these discrepancies.
The mean tumor size in group A was significantly larger than that in group B or group C. However, in terms of HCC stage, the difference among the three groups did not reach significance. One possible reason for these findings is that (as mentioned above), in NBNC-HCC patients, HCCs are often detected at an advanced stage and are not eligible for curative resection, so such patients were excluded from the current study. Conversely, the proportion of patients with non-LC or metabolic disorders such as DM in group A was significantly higher than that in group B or group C and, with respect to tumor histology, significantly better tumor differentiation was observed in group A. Our results suggested different carcinogenic pathways between hepatitis virus-unrelated liver disease and hepatitis virus-related liver disease. Fibrosis-cirrhosis-hepatocarcinogenesis, which has been clearly recognized as multistep progression in previous studies, may not be the main carcinogenic pathway in patients with hepatitis virus-unrelated liver disease [17-22].
In terms of OS, serum albumin was a significant predictor via multivariate analyses for all cases and, for patients with LC, and presence of LC was an independent factor for all cases and for patients who satisfied the Milan criteria. These results suggest that maintaining liver function after curative resection is essential for optimizing clinical outcomes. In HCC patients with poor hepatic functional reserve who underwent curative resection, therapy with branched-chain amino acids may be effective [23-25]. Conversely, AFP, tumor stage, maximum tumor size and MVI were independent predictors associated with OS. Clinicians should thus be alert to not only liver function-related factors but also tumor-related factors even after curative resection for improving survival.
In terms of RFS, interestingly, the BMI ≥23 kg/m2 was a significant factor via multivariate analyses for all patients, for patients with LC, and for patients who fulfilled the Milan criteria (although the reasons for these findings are unclear). Only 24 patients (5.1%) had a BMI of ≥30 kg/m2, which is defined as obesity according to the WHO classification, so it is difficult to conclude that obesity is related to a worse prognosis in HCC patients who underwent curative resection [26]. Also, GGT was an independent predictor linked to RFS for all patients, for patients without LC and for patients who met the Milan criteria. Several investigators demonstrated that a high level of GGT was related to a higher incidence of HCC development and HCC recurrence [27, 28]. Ju et al. reported that a high GGT level was associated with tumor size and lower serum albumin level [29]. In HCC patients with a high level of GGT before resection, careful follow-up examination after resection is needed. Conversely, it is not surprising that MVI was an independent predictor linked to RFS. In general, HCC has a high tendency to invade the portal and hepatic veins [30]. The predictive value of MVI for survival after curative resection for HCC may be explained by the fact that MVI caused by tumor cells provides an important route for intrahepatic metastasis, and therefore can lead to higher prevalence of HCC recurrence [31].
A limitation of this study is related to its retrospective study design. Only patients who underwent curative resection were included, so there is a potential for bias in the patient population. Thus, our results are not applicable to HCC patients who could not undergo curative therapy. Another limitation is that the sample sizes among the three groups were not well-balanced for survival analyses. These size limitations may have masked further differences in clinical outcomes in each group. Therefore, a well-characterized prospective study will be needed in the future. However, our results demonstrated that NBNC-HCC patients who underwent curative resection have a similar prognosis as compared with that of hepatitis virus-related HCC.
In conclusion, long-term clinical outcomes in NBNC-HCC patients after curative resection were comparable with those in B-HCC and C-HCC patients after curative resection.
Acknowledgements
The authors thank Haruko Takada for help with data collection.
Conflict of Interest
The authors have not received any financial support for this study and have no conflicts of interest to declare.
References
1. Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78:113-124
2. Livraghi T, Mäkisalo H, Line PD. Treatment options in hepatocellular carcinoma today. Scand J Surg. 2011;100:22-29
3. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-73
4. Nishikawa H, Osaki Y, Iguchi E. et al. Radiofrequency ablation for hepatocellular carcinoma: the relationship between a new grading system for the ablative margin and clinical outcomes. J Gastroenterol. 2012 [Epub ahead of print]
5. Zhou WP, Lai EC, Li AJ. et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surgery. 2009;249:195-202
6. Nishikawa H, Osaki Y, Kita R. et al. Transcatheter arterial infusion chemotherapy prior to radiofrequency thermal ablation for single hepatocellular carcinoma reduces the risk of intrahepatic distant recurrence. Int J Oncol. 2012;41:903-9
7. Takuma Y, Nouso K, Makino Y. et al. Outcomes after curative treatment for cryptogenic cirrhosis-associated hepatocellular carcinoma satisfying the Milan criteria. J Gastroenterol Hepatol. 2011;26(9):1417-24
8. Kaibori M, Ishizaki M, Matsui K, Kwon AH. Clinicopathologic characteristics of patients with non-B non-C hepatitis virus hepatocellular carcinoma after hepatectomy. Am J Surg. 2012;204(3):300-7
9. Akahoshi H, Taura N, Ichikawa T. et al. Differences in prognostic factors according to viral status in patients with hepatocellular carcinoma. Oncol Rep. 2010;23(5):1317-23
10. Utsunomiya T, Shimada M. Molecular characteristics of non-cancerous liver tissue in non-B non-C hepatocellular carcinoma. Hepatol Res. 2011;41:711-721
11. Umemura T, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. Hepatol Res. 2007;37(Suppl 2):S95-S100
12. Hatanaka K, Kudo M, Fukunaga T. et al. Clinical characteristics of NonBNonC-HCC: Comparison with HBV and HCV related HCC. Intervirology. 2007;50(1):24-31
13. Yang JD, Harmsen WS, Slettedahl SW. et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol. 2011;9(7):617-23
14. Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236
15. Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer. Jpn J Surg. 1989;19:98-129
16. Li T, Qin LX, Gong X. et al. Hepatitis B virus surface antigen-negative and hepatitis C virus antibody-negative hepatocellular carcinoma: clinical characteristics, outcome, and risk factors for early and late intrahepatic recurrence after resection. Cancer. 2013;119(1):126-35
17. Paradis V, Zalinski S, Chelbi E. et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49(3):851-9
18. Reddy SK, Steel JL, Chen HW. et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55(6):1809-19
19. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51(5):1820-32
20. McGivern DR, Lemon SM. Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene. 2011;30(17):1969-83
21. Farinati F, Cardin R, Bortolami M. et al. Hepatitis C virus: from oxygen free radicals to hepatocellular carcinoma. J Viral Hepat. 2007;14(12):821-9
22. Fung J, Lai CL, Yuen MF. Hepatitis B and C virus-related carcinogenesis. Clin Microbiol Infect. 2009;15(11):964-70
23. a prospective randomized trial. The San-in Group of Liver Surgery. Br J Surg. 1997;84(11):1525-31
24. Nishikawa H, Osaki Y, Iguchi E. et al. The Effect of Long-term Supplementation With Branched-chain Amino Acid Granules in Patients With Hepatitis C Virus-related Hepatocellular Carcinoma After Radiofrequency Thermal Ablation. J Clin Gastroenterol. 2013;47(4):359-66
25. Haruhiko Takeda, Hiroki Nishikawa, Eriko Iguchi. et al. Effect of branched-chain amino acid treatment during sorafenib therapy for unresectable hepatocellular carcinoma. Hepatol Research. 2013 in press
26. WHO. Obesity-preventing and managing the global epidemic Report of WHO consultation on obesity. Geneva: WHO. 1997
27. Yao D, Jiang D, Huang Z. et al. Abnormal expression of hepatoma specific gamma-glutamyl transferase and alteration of gamma-glutamyl transferase gene methylation status in patients with hepatocellular carcinoma. Cancer. 2000;88(4):761-9
28. Zhang JB, Chen Y, Zhang B. et al. Prognostic significance of serum gamma-glutamyl transferase in patients with intermediate hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol. 2011;23(9):787-93
29. Ju MJ, Qiu SJ, Fan J. et al. Preoperative serum gamma-glutamyl transferase to alanine aminotransferase ratio is a convenient prognostic marker for Child-Pugh A hepatocellular carcinoma after operation. J Gastroenterol. 2009;44(6):635-42
30. Nakashima T, Kojiro M. Pathologic characteristics of hepatocellular carcinoma. Semin Liver Dis. 1986;6(3):259-66
31. Lim KC, Chow PK, Allen JC. et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108-113
Author contact
![]() Corresponding author: Hiroki Nishikawa, MD, Department of Gastroenterology and Hepatology, Osaka Red Cross Hospital, 5-30 Fudegasaki-cho, Tennoji-ku, Osaka 543-0027, Japan. Tel: +81-6-6774-5111; Fax: +81-6-6774-5131 E-mail: h-nishikawajrc.or.jp.
Corresponding author: Hiroki Nishikawa, MD, Department of Gastroenterology and Hepatology, Osaka Red Cross Hospital, 5-30 Fudegasaki-cho, Tennoji-ku, Osaka 543-0027, Japan. Tel: +81-6-6774-5111; Fax: +81-6-6774-5131 E-mail: h-nishikawajrc.or.jp.

 Global reach, higher impact
Global reach, higher impact