Impact Factor
ISSN: 1837-9664
J Cancer 2013; 4(7):549-556. doi:10.7150/jca.6888 This issue Cite
Research Paper
Use of ACE Inhibitors and Angiotensin Receptor Blockers and Primary Breast Cancer Outcomes
1. Division of Cancer Medicine,
2. Department of Breast Medical Oncology,
3. Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Received 2013-6-11; Accepted 2013-6-26; Published 2013-8-10
Abstract
BACKGROUND: ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) may have anti-tumor properties. We investigated whether the use of ACEI/ARBs affects the clinical outcomes of primary breast cancer patients receiving taxane and anthracycline-based neoadjuvant chemotherapy.
METHODS: We included 1449 patients with diagnosis of invasive primary breast cancer diagnosed at the MD Anderson Cancer Center between 1995 and 2007 who underwent neoadjuvant chemotherapy. Of them, 160 (11%) patients were identified by review of their medical record, as ACEI/ARBs users. We compared pathologic complete response (pCR) rates, relapse-free survival (RFS), disease-specific survival (DSS) and overall survival (OS) between ACEI/ARB users and non-users. Descriptive statistics and Cox proportional hazards model were used in the analyses.
RESULTS: There was no difference in the pCR rates between ACEI/ARB users and non-users (16% vs 18.1%, p-=0.50). After adjustment for important demographic and clinical characteristics, no significant differences between ACEI/ARB users and nonusers were observed in RFS (HR=0.81; 95% CI=0.54-1.21), DSS (HR=0.83; 95% CI=0.52-1.31), or OS (HR=0.91; 95% CI =0.61-1.37). In a subgroup analysis, the 5-year RFS was 82% in ARB only users versus 71% in ACEI/ARB non-users (P=0.03). In the multivariable analysis, ARB use was also associated with a decreased risk of recurrence (HR=0.35; 95% CI=0.14-0.86). No statistically significant differences in DSS or OS were seen.
CONCLUSION: No differences in pCR and survival outcomes were seen between ACEI/ARB users and non-users among breast cancer patients receiving neoadjuvant chemotherapy. ARB use may be associated with improved RFS. Further research is needed to validate this finding.
Keywords: ACE inhibitor, ARB, breast cancer, neoadjuvant chemotherapy
Introduction
The renin angiotensin system (RAS) is a peptide based homeostatic gatekeeper system known to play a role in blood pressure control and electrolyte balance[1, 2]. ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are the two widely used RAS antagonists. They inhibit the effect of angiotensin II, and they are used in the treatment of hypertension, congestive heart failure, and diabetic nephropathy. There is a growing body of evidence to suggest that ACEIs might have anti-tumor properties[2, 3]. Angiotensin II is now regarded as a tumor growth promoter via angiogenesis from activation of the VEGF pathway[4-6]. In animal studies, ACEI administration has led to decreased neo-vascularization and VEGF levels[7, 8]. ACEIs have also demonstrated anti-inflammatory and pro-apoptotic activity via the NF kappa B and apoptosis pathways, respectively[2, 3]. In addition, although still controversial, epidemiologic studies have found that the use of ACEIs was associated with decreased risk of developing cancers including solid and skin cancers[9-12].
Breast cancer is the second most common cause of cancer-related mortality among women in the US[13]. Breast cancer was shown to express full components of the RAS[14]. Angiotensin II acts as a growth factor both in normal and cancer breast epithelial cells and promotes adhesion and invasion[15, 16]. AGTR1, a gene encoding angiotensin receptor type 1, was found to be markedly expressed in 10-20% of human breast cancer tissues, mutually exclusive with ERBB2 overexpression. Furthermore, the ectopic expression of AGTR1 in primary mammary epithelial cells combined with angiotensin II stimulation induced cell proliferation later attenuated by the ARB[17].
Neoadjuvant systemic therapy (NST) is part of the standard treatment for locally advanced and inflammatory breast cancer, and it is increasingly used in earlier stage disease. NST produces downstaging of the disease leading to better surgical resectability while assessing the efficacy of the chemotherapy regimen. Achieving a pathologic complete response (pCR) is an independent surrogate marker for better long-term outcome[18-22]. The purpose of our study was to determine whether the use of RAS antagonists affected the clinical outcome of primary breast cancer patients receiving NST.
Methods
Patient population
From a prospectively maintained database in the Breast Medical Oncology department at The University of Texas MD Anderson Cancer Center, we identified patients receiving NST. We included a total of 1449 patients with diagnosis of invasive breast cancer between 1995 and 2007 treated with anthracycline and taxane-based NST. We excluded patients with metastatic disease at the time of diagnosis, patients with bilateral disease, or male breast cancer patients. We collected demographic variables, tumor characteristics (histology; grade; lymphovascular invasion [LVI]; estrogen receptor [ER]; progesterone receptor [PR]; and human epidermal growth factor receptor 2 [HER2] status), clinical stage at diagnosis (according to the American Joint Committee on Cancer Criteria, 6th edition[23]), body mass index (BMI), pathological stage, and recurrence and survival information. Patients treated with ACEI/ARBs while on NST were identified by chart and pharmacy medication record review. The type of ACEIs or ARBs and other medications that may affect pCR and relapse (beta-blocker, metformin) were also collected as we previously reported[24-27]. Patients were followed according to current practice guidelines[28]. The institutional review board of our institution approved the retrospective review of the medical records for the purpose of this study.
Pathology
All pathologic specimens were reviewed by dedicated breast pathologists. The histology, grade, pathologic stage, and analysis of ER, PR, and HER2 status were classified as previously described[29]. All surgical breast and axillary lymph node specimens were reviewed to identify the presence of residual disease. pCR was defined as no evidence of invasive carcinoma in the breast and axillary lymph nodes at time of surgery[25].
Treatment
All patients received NST with anthracycline (doxorubicin, epirubicin) and taxane (paclitaxel, docetaxel) based regimen. Detailed chemotherapy regimens are decribed in our early studies[24-26]. At the completion of NST, every patient underwent definitive surgery. Axillary lymph node staging with axillary lymph node dissection or sentinel lymph node biopsy was performed on all patients. Adjuvant hormonal therapy and/or trastuzumab treatment was administered according to standard practice[28]. Trastuzumab was not used in the neoadjuvant setting. Radiation was delivered when patients underwent breast conservation surgery or had locally advanced disease; a primary tumor measuring > 5 cm, or ≥ 4 involved lymph nodes.
Statistical analysis
Patient characteristics were compared according to whether patients received ACEIs/ARBs. Survival analyses were performed to compare the relapse-free survival (RFS), disease-specific survival (DSS) and overall survival (OS) between groups. RFS was defined from the date of diagnosis to the date of first documented local or distant recurrence or last follow-up. Patients who died before experiencing disease recurrence were censored at their date of death. DSS was measured from the date of diagnosis to the date of death from disease or last follow-up. OS was calculated from the date of diagnosis to the date of death from any cause or last follow-up. The Kaplan-Meier method was used to estimate the survival outcomes. A multivariable logistic regression model was fit to examine the relation between the use of ACEI/ARBs and pCR. Variables in the model included age, stage of disease, tumor grade, tumor subtype, LVI, and BMI. We also included the use of metformin and beta-blockers as covariates in the model based on our prior findings[24, 26]. Cox proportional hazards models were fitted to determine the association of ACEi/ARBs use with survival outcomes after adjustment for other patient and clinical characteristics. Subset analyses were carried out within each of the three subtype groups: hormonal receptor positive, HER2 positive and triple receptor negative. Statistical analyses were performed using SAS (version 9.1; SAS Institute Inc, Cary, NC) and S-Plus 7.0 (Insightful Corporation, Seattle, Wash) statistical software. P values<0.05 were considered to be statistically significant, all tests were two sided.
Results
ACE inhibitors and/or ARBs with pCR Rates
Among 1449 breast cancer patients, 160 (11%) used ACEI/ARBs while they were treated with NST and 1289 (89%) patients did not. Among the ACEI/ARB-treated patients, 105 (65%) were on ACEIs and 54 (34%) were on ARBs. Commonly used ACEIs were lisinopril (31.4%), enalapril (19.0%), and benazepril (17.1%); commonly used ARBs were valsartan (37.0%), irbesartan (22.0%), and losartan (22.0%). Patient characteristics are summarized in Table 1. Patients in the ACEI/ARB group were older than those in the non-ACEI/ARB group (P<0.001), and consequently this group had a higher proportion of postmenopausal patients (P<0.001). Fifty-three percent of the patients in the ACEI/ARB group were obese versus 32% in the non-ACEI/ARB group (P<0.001). Twenty-three percent of the black patients versus 14% of the non-black patients took ACEI/ARB (P=0.002). Thirteen percent of the patients in the ACEI/ARB group used metformin versus 2% in the non-ACEI/ARB group (P<0.001). Twenty-nine percent of the patients in the ACEI/ARB group used beta-blockers versus 4.9% in the non-ACEI/ARB group (P<0.001). The other prognostic factors were not significantly different between the two groups.
There was no difference in the estimates of pCR rates between ACEI/ARB and non-ACEI/ARB groups. The proportion of pCR was 16% (95%CI 14%-18.1%) in the non-ACEI/ARB group and 18.1% (95%CI 12.2%-24.1%) in the ACEI/ARB group (P=0.50). The use of ACEI/ARBs was not an independent predictor of pCR (OR= 1.30; 95%CI 0.79-2.13). Table 2 shows the multivariate logistic regression models. When the same analyses were done for ACEI (n=105) and ARB (n=54) users separately, the results were similar.
ACE inhibitors and / or ARBs with Survival Outcomes
Patients stratified by ACE inhibitors/ARBs
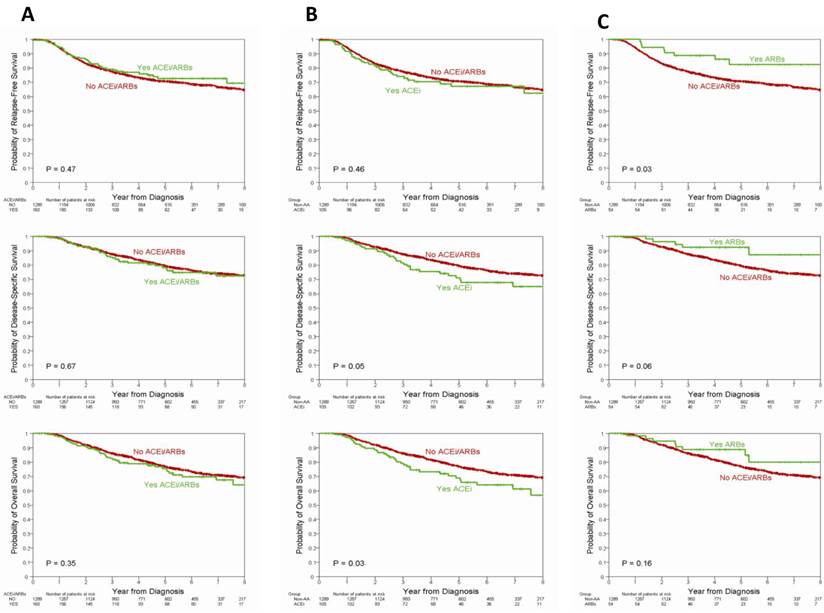
The median follow up was 55 months (range 3-145 months). The survival outcomes according to ACEI/ARB use are listed in Table 3. There were 415 recurrences, 312 disease-specific deaths and 359 deaths. No differences in RFS (P=0.47), DSS (P=0.67), or OS (P=0.35) were observed (Figure 1A). In the multivariable model shown in Table 4 no differences in RFS (HR=0.81; 95%CI 0.54-1.21), DSS (HR=0.83; 95%CI 0.52-1.31), or OS (HR=0.91; 95%CI 0.61-1.37) were seen after adjusting for age, race, BMI, stage, grade, LIV, subtype, metformin and beta-blocker use.
Among patients treated exclusively with ACEIs, the 5-year estimate of RFS and DSS was not statistically different from those not using ACEIs. However, OS was inferior in ACEI users compared with non-users (5 year OS rate 69% vs. 77%, p=0.03) (Table 3, Figure 1B). In the multivariable model there was no significant difference between ACE and non-ACEI/ARB-user group in terms of RFS (HR=1.10; 95%CI=0.71-1.69), DSS (HR=1.07; 95%CI=0.65-1.77), or OS (HR=1.11; 95%CI = 0.70-1.74).
Among patients treated exclusively with ARBs, the 5-year estimate of RFS was 82% while it was 71% in the non-ACEI/ARB group (P=0.03); there was no statistically significant difference in the rate of DDS (P=0.06) or OS (P=0.16) (Table 3, Figure 1C). In the multivariable model, ARBs use was associated with a decreased risk of recurrence (HR=0.35; 95%CI=0.14-0.86). There were no significant differences in terms of DSS (HR=0.41; 95%CI=0.15-1.13) or OS (HR=0.59; 95%CI=0.27-1.27).
In a subset analyses according to tumor subtype ACEI use was associated with a worse 5-year OS rate (77% for ACEI versus 84% for non-ACEI/ARB; P=0.04) in patients with hormone receptor-positive tumors. However, after adjustment for potential confounders, the effect of ACEIs was no longer significant (HR=1.14; 95%CI=0.60-2.19). Similar 5-year OS rates were seen among patients with triple-negative and HER2-positive tumors according to ACE/ARB use.
Patient Characteristics by ACE inhibitors/ARBs
| Non- ACEI/ARB (N=1289) | ACEI/ARB (N=160) | ||||
|---|---|---|---|---|---|
| N | % | N | % | P | |
| Age, Median | 48 | 58 | |||
| Age | |||||
| < 50 | 701 | 54.4 | 27 | 16.9 | |
| ≥ 50 | 588 | 45.6 | 133 | 83.1 | < 0.001 |
| Menopausal Status | |||||
| Pre | 655 | 50.9 | 25 | 15.6 | |
| Post | 631 | 49.1 | 135 | 84.4 | < 0.001 |
| Body Mass Index | |||||
| Normal/underweight | 447 | 35.9 | 25 | 15.8 | |
| Overweight | 404 | 32.4 | 49 | 31.0 | |
| Obese | 394 | 31.6 | 84 | 53.2 | < 0.001 |
| Race | |||||
| White/Other | 1115 | 86.5 | 124 | 77.5 | |
| Black | 174 | 13.5 | 36 | 22.5 | 0.002 |
| Clinical Stage | |||||
| I | 55 | 4.3 | 3 | 1.9 | |
| II | 700 | 54.5 | 86 | 54.1 | |
| III | 530 | 41.2 | 70 | 44.0 | 0.32 |
| Nuclear Grade | |||||
| I | 47 | 3.8 | 4 | 2.6 | |
| II | 417 | 33.3 | 44 | 28.4 | |
| III | 788 | 62.9 | 107 | 69.0 | 0.31 |
| LVI | |||||
| Negative | 850 | 68.4 | 112 | 72.3 | |
| Positive | 393 | 31.6 | 43 | 27.7 | 0.33 |
| Subtype | |||||
| HR- positive | 705 | 55.4 | 86 | 54.1 | |
| HER2 positive | 231 | 18.1 | 27 | 17.0 | 0.79 |
| Triple negative | 337 | 26.5 | 46 | 28.9 | |
| Metformin Use | |||||
| No | 1269 | 98.4 | 140 | 87.5 | |
| Yes | 20 | 1.6 | 20 | 12.5 | < 0.001 |
| Beta-blocker Use | |||||
| No | 1211 | 95.1 | 100 | 71.4 | |
| Yes | 62 | 4.9 | 40 | 28.6 | < 0.001 |
Abbreviations: ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor antagonist; LVI, lymphovascular invasion; HER-2, human epidermal growth factor receptor 2; HR, hormone receptor.
Multivariate Logistic Regression Model for ACE inhibitors/ARBs on pCR among All Patients
| Odds Ratio | 95% CI | P | Adjusted Odds Ratio | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
| ACEI/ARB use: yes vs. no | 1.30 | 0.79 to 2.13 | 0.3 | 1.44 | 0.84 to 2.48 | 0.18 | |
| Age: ≥ 50 vs. < 50 | 0.67 | 0.48 to 0.93 | 0.018 | 0.66 | 0.47 to 0.93 | 0.018 | |
| BMI: overweight vs. normal | 0.68 | 0.45 to 1.01 | 0.022 | 0.69 | 0.46 to 1.04 | 0.021 | |
| BMI: obese vs. normal | 1.04 | 0.71 to 1.52 | 0.16 | 1.10 | 0.75 to 1.63 | 0.1 | |
| Stage: III vs. I/II | 0.69 | 0.49 to 0.95 | 0.025 | 0.70 | 0.5 to 0.98 | 0.036 | |
| Grade: III vs. I/II | 3.69 | 2.31 to 5.89 | <.001 | 3.42 | 2.14 to 5.48 | <.001 | |
| LVI: positive vs. negative | 0.39 | 0.26 to 0.57 | <.001 | 0.37 | 0.25 to 0.56 | <.001 | |
| Subtype: HER2 positive vs. HR positive | 3.06 | 1.99 to 4.69 | <.001 | 3.18 | 2.05 to 4.93 | <.001 | |
| Subtype: Triple negative vs. HR positive | 2.65 | 1.8 to 3.92 | 0.012 | 2.78 | 1.87 to 4.14 | 0.009 | |
| Metformin use: yes v. no | 0.66 | 0.21 to 2.1 | 0.48 | ||||
| Beta-blocker use: yes v. no | 0.84 | 0.43 to 1.62 | 0.59 |
Abbreviations: ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor antagonist; pCR, pathologic complete response; HR: hormonal receptor; LVI, lymphovascular invasion; BMI, body mass index; CI, confidence interval
Five-year Survival Estimates by Patient and Clinical Characteristics among All Patients
| Recurrence-Free Survival | Disease-Specific Survival | Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N Patients | N Events | 5-Year Estimate (95% CI) | P | N Events | 5-Year Estimates (95% CI) | P | N Events | 5-Year Estimates (95% CI) | P | |||
| All | 1449 | 415 | 0.71(0.68, 0.73) | 312 | 0.79(0.77, 0.82) | 359 | 0.77(0.74, 0.79) | |||||
| ACEI/ARB | ||||||||||||
| No | 1289 | 374 | 0.71(0.68, 0.73) | 277 | 0.8(0.77, 0.82) | 316 | 0.77(0.74, 0.8) | |||||
| Yes | 160 | 41 | 0.73(0.64, 0.79) | 0.47 | 35 | 0.79(0.71, 0.85) | 0.67 | 43 | 0.76(0.68, 0.82) | 0.35 | ||
| ACEI | ||||||||||||
| Non-ACEI/ARB | 1289 | 374 | 0.71(0.68, 0.73) | 277 | 0.8(0.77, 0.82) | 316 | 0.77(0.74, 0.8) | |||||
| ACEI | 105 | 33 | 0.67(0.56, 0.76) | 0.46 | 30 | 0.71(0.6, 0.8) | 0.05 | 35 | 0.69(0.58, 0.78) | 0.03 | ||
| ARBs | ||||||||||||
| Non-ACEI/ARB | 1289 | 374 | 0.71(0.68, 0.73) | 277 | 0.8(0.77, 0.82) | 316 | 0.77(0.74, 0.8) | |||||
| ARB | 54 | 8 | 0.82(0.66, 0.91) | 0.03 | 5 | 0.92(0.81, 0.97) | 0.06 | 8 | 0.89(0.77, 0.95) | 0.16 | ||
| Hormone Receptor Positive | 791 | 178 | 0.77(0.73, 0.8) | 126 | 0.86(0.83, 0.88) | 149 | 0.84(0.81, 0.87) | |||||
| Non-ACEI/ARB | 705 | 159 | 0.77(0.73, 0.8) | 111 | 0.86(0.83, 0.89) | 130 | 0.84(0.81, 0.87) | |||||
| ACEI/ARB | 86 | 19 | 0.77(0.65, 0.85) | 0.96 | 15 | 0.84(0.74, 0.91) | 0.47 | 19 | 0.82(0.72, 0.89) | 0.25 | ||
| ACEI | 58 | 16 | 0.73(0.59, 0.83) | 0.34 | 14 | 0.79(0.64, 0.88) | 0.08 | 17 | 0.77(0.63, 0.86) | 0.04 | ||
| ARB | 27 | 3 | 0.83(0.55, 0.94) | 0.20 | 1 | 0.96(0.75, 0.99) | 0.17 | 2 | 0.92(0.73, 0.98) | 0.28 | ||
| HER2 Positive | 258 | 92 | 0.66(0.59, 0.72) | 62 | 0.81(0.75, 0.85) | 70 | 0.78(0.72, 0.83) | |||||
| Non-ACEI/ARB | 231 | 85 | 0.65(0.58, 0.71) | 57 | 0.8(0.74, 0.85) | 63 | 0.78(0.72, 0.83) | |||||
| ACEI/ARB | 27 | 7 | 0.73(0.51, 0.86) | 0.29 | 5 | 0.88(0.68, 0.96) | 0.46 | 7 | 0.81(0.61, 0.92) | 0.80 | ||
| ACEI | 16 | 5 | 0.66(0.37, 0.85) | 0.80 | 4 | 0.79(0.48, 0.93) | 0.91 | 6 | 0.68(0.4, 0.86) | 0.39 | ||
| ARB | 11 | 2 | 0.82(0.45, 0.95) | 0.19 | 1 | 1 | 0.21 | 1 | 1 | 0.18 | ||
| Triple Negative | 383 | 140 | 0.63(0.57, 0.68) | 121 | 0.64(0.59, 0.7) | 136 | 0.61(0.55, 0.66) | |||||
| Non-ACEI/ARB | 337 | 125 | 0.62(0.57, 0.68) | 106 | 0.65(0.59, 0.7) | 119 | 0.61(0.55, 0.67) | |||||
| ACEI/ARB | 46 | 15 | 0.65(0.49, 0.77) | 0.58 | 15 | 0.6(0.41, 0.74) | 0.71 | 17 | 0.58(0.39, 0.73) | 0.65 | ||
| ACEI | 30 | 12 | 0.56(0.35, 0.72) | 0.73 | 12 | 0.5(0.27, 0.69) | 0.23 | 12 | 0.5(0.27, 0.69) | 0.40 | ||
| ARB | 16 | 3 | 0.81(0.52, 0.94) | 0.17 | 3 | 0.81(0.51, 0.93) | 0.34 | 5 | 0.75(0.46, 0.9) | 0.74 | ||
Abbreviations: LVI, lymphovascular invasion; BMI, body mass index; CI, confidence interval; ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor antagonist
Multivariable Cox Proportional Hazards Model by ACE inhibitors/ARBs for All Patients
| Recurrence-Free Survival | Disease-Specific Survival | Overall Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||
| ACEI/ARB use: yes vs. no Age: ≥ 50 vs. < 50 | 0.81 0.92 | 0.54 to 1.21 0.74 to 1.14 | 0.30 0.46 | 0.83 1.03 | 0.52 to 1.31 0.8 to 1.32 | 0.42 0.82 | 0.91 1.16 | 0.61 to 1.37 0.91 to 1.46 | 0.66 0.23 | ||
| Race: black vs. non-black | 1.36 | 1.03 to 1.8 | 0.029 | 1.33 | 0.97 to 1.83 | 0.08 | 1.39 | 1.03 to 1.86 | 0.03 | ||
| BMI: overweight vs. normal | 1.03 | 0.79 to 1.33 | 0.85 | 1.00 | 0.74 to 1.36 | 0.99 | 1.09 | 0.82 to 1.46 | 0.55 | ||
| BMI: obese vs. normal | 1.18 | 0.91 to 1.53 | 0.22 | 1.16 | 0.85 to 1.57 | 0.36 | 1.22 | 0.91 to 1.63 | 0.18 | ||
| Stage: III vs. I/II | 1.81 | 1.46 to 2.24 | <.0001 | 1.95 | 1.51 to 2.51 | <.0001 | 1.89 | 1.5 to 2.4 | <.0001 | ||
| Grade: III vs. I/II | 1.19 | 0.91 to 1.54 | 0.2 | 1.62 | 1.17 to 2.25 | 0.004 | 1.50 | 1.11 to 2.03 | 0.008 | ||
| LVI: positive vs. negative | 1.93 | 1.56 to 2.39 | <.0001 | 1.67 | 1.3 to 2.15 | <.0001 | 1.75 | 1.38 to 2.21 | <.0001 | ||
| Subtype: HER2 positive vs. HR positive | 1.57 | 1.18 to 2.1 | 0.002 | 1.17 | 0.83 to 1.67 | 0.37 | 1.12 | 0.81 to 1.56 | 0.49 | ||
| Subtype: Triple negative vs. HR positive | 2.01 | 1.55 to 2.61 | <.0001 | 2.30 | 1.71 to 3.1 | <.0001 | 2.24 | 1.7 to 2.96 | <.0001 | ||
| Metformin use: yes v. no | 0.98 | 0.5 to 1.95 | 0.96 | 1.36 | 0.65 to 2.84 | 0.41 | 1.07 | 0.51 to 2.22 | 0.86 | ||
| Beta-blocker use: yes v. no | 0.51 | 0.29 to 0.87 | 0.015 | 0.51 | 0.27 to 0.96 | 0.038 | 0.63 | 0.37 to 1.08 | 0.09 | ||
| ACEI vs. non-ACEI/ARB * | 1.10 | 0.71 to 1.69 | 0.68 | 1.07 | 0.65 to 1.77 | 0.79 | 1.11 | 0.7 to 1.74 | 0.66 | ||
| ARB vs. non-ACEI/ARB * | 0.35 | 0.14 to 0.86 | 0.022 | 0.41 | 0.15 to 1.13 | 0.08 | 0.59 | 0.27 to 1.27 | 0.17 | ||
Abbreviations: ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor antagonist; BMI, body mass index; LVI, lymphovascular invasion; HR, hormonal receptor; HR, hazard ratio; CI, confidence interval, * Separate multivariate analysis done as with ACE/ARB.
Recurrence free survival, disease specific survival, and overall survival by the use of ACEI/ARBs (A), ACEI only (B), and ARB only (C) among all patients. Abbreviations: ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor antagonist
Discussion
The purpose of our study was to assess the effect of ACEI/ARB use on pCR rates and subsequent survival outcomes in patients treated with taxane- and anthracycline- based neoadjuvant chemotherapy. We found that the use of ACEI/ARBs at the time of chemotherapy had no effect on either the pCR rates or survival outcome measures including RFS, DSS and OS. Based on previous reports describing AGTR1 overexpression in hormone receptor positive and HER2-negative tumors[1], we explored the effect of ACEI/ARBs according to different tumor subtypes, but no significant differences were observed.
When stratified by ACEIs and ARBs use separately and compared to the non- ACEI/ARB group, we found that ARB use was associated with favorable RFS, there was a trend favoring DSS but there were no differences in OS. Even after controlling for possible confounders, the association remained statistically significant for RFS (HR=0.35, 95%CI=0.14-0.86). ARB use alone has not been previously reported to be associated with decrease risk of breast cancer recurrence. Sensitivity analysis comparing the ACEI group with the non-ACEI group, and the ARB group with the non-ARB group were performed. We found similar results as above indicating that the effect of ARB use may not be from the differences in the control groups (result not shown). The small number of events among ARB users warrants a careful interpretation of this finding.
Previous studies have focused on the association between breast cancer incidence and the use of RAS antagonists rather than cancer progression[9-12]. Two studies have investigated the association between the use of ACEI/ARBs and breast cancer recurrence[30, 31]. We reported that use of ACEI/ARBs was associated with reduced risk of recurrence in a retrospective analysis of 703 stage II and III breast cancer patients (HR=0.49; 95%CI=0.31-0.76)[30]. However, in this study, a similar association was observed in ARB users (HR=0.35; 95%CI=0.14-0.86) and not in ACEI users. On the contrary, the LACE cohort study reported increased risk of recurrence among ACEIs users among early breast cancer patients (HR=1.56; 95%CI=1.02-2.39)[31], but no data on use of ARBs was reported. To our knowledge, no study has explored the association between pCR and the use of RAS antagonists in breast cancer patients receiving NST.
There are many biological models to explain possible anti-tumor or anti-angiogenic effects of RAS antagonists in cell line and animal xenograft models[2-8]. A favorable effect of ARBs in our study may be explained by these properties. However, the consequence of long term suppression of RAS may cause unintended detrimental side effects such as up-regulation of the angiotensin receptors or related downstream pathway molecules. ACEI and ARB may differ in this paradoxical phenomenon. This may, in part, explain the fact that ACEI only use was not favorably associated with any clinical outcomes in our study and also may explain why an increased risk of recurrence with the ACEI use was found in the LACE study. Of note, ARB use was reported to be associated with increase in overall cancer incidence[32], although this has not been validated in subsequently published studies[33-36].
There are some limitations to our study. Although our database was built prospectively, our study is prone to the bias inherent to a retrospective design and we cannot rule out residual confounding by unidentified clinical variables non-randomly distributed between the groups compared. We evaluated the use of ACEI/ARBs prescribed concurrently with NST. We have no data available on the use of these drugs after the definitive breast surgery. We could not evaluate medication compliance. Also, our medication data is solely based on medical records, not verified by outside pharmacy records. It is possible that differences in outcome were diluted by differences in adjuvant treatment including radiation, trastuzumab, and endocrine therapy. However, there is no reason to believe that misclassification, residual confounding or lost-to-follow up occurred in a differential manner between ACEI/ARB and non- ACEI/ARB group.
Our findings are based on one of the largest available breast cancer neoadjuvant chemotherapy databases. Clinical information was collected in a prospective way and all patients were treated in a single institution with relatively homogenous chemotherapeutic regimens and surgical and radiation treatments. We were able to account for most known clinical variables that may affect clinical outcome when assessing the effect of ACEI/ARBs in breast cancer patients receiving NST. We also controlled for metformin and beta-blocker use as we previously demonstrated an association between them and pCR and OS, respectively[24, 26], although other mediation data including aspirin has not been collected[37].
In summary, this study provides the first report that ACEI/ARB use during NST is not associated with pCR rates in breast cancer patients receiving antracyline-and taxane-based NST. However, we found that ARB use, not ACEI, may be linked with favorable RFS. Given the fact that a number of breast cancer survivors with hypertension are on ARBs, this finding harbors vast public health implications and require validation in a larger prospective cohort.
Acknowledgements
This work was supported in part by 2P30 CA016672 and the Nellie B. Connally Breast Cancer Research fund.
Competing Interests
All authors disclose no financial conflicts.
References
1. Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. Journal of managed care pharmacy: JMCP. 2007;13:9-20
2. George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nature reviews Cancer. 2010;10:745-59 doi:10.1038/nrc2945
3. Rosenthal T, Gavras I. Angiotensin inhibition and malignancies: a review. Journal of human hypertension. 2009;23:623-35 doi:10.1038/jhh.2009.21
4. Tamarat R, Silvestre JS, Durie M, Levy BI. Angiotensin II angiogenic effect in vivo involves vascular endothelial growth factor- and inflammation-related pathways. Laboratory investigation, a journal of technical methods and pathology. 2002;82:747-56
5. Suganuma T, Ino K, Shibata K, Kajiyama H, Nagasaka T, Mizutani S. et al. Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11:2686-94 doi:10.1158/1078-0432.CCR-04-1946
6. Fujita M, Hayashi I, Yamashina S, Fukamizu A, Itoman M, Majima M. Angiotensin type 1a receptor signaling-dependent induction of vascular endothelial growth factor in stroma is relevant to tumor-associated angiogenesis and tumor growth. Carcinogenesis. 2005;26:271-9 doi:10.1093/carcin/bgh324
7. Otake AH, Mattar AL, Freitas HC, Machado CM, Nonogaki S, Fujihara CK. et al. Inhibition of angiotensin II receptor 1 limits tumor-associated angiogenesis and attenuates growth of murine melanoma. Cancer chemotherapy and pharmacology. 2010;66:79-87 doi:10.1007/s00280-009-1136-0
8. Ohnuma Y, Toda M, Fujita M, Hosono K, Suzuki T, Ogawa Y. et al. Blockade of an angiotensin type I receptor enhances effects of radiation on tumor growth and tumor-associated angiogenesis by reducing vascular endothelial growth factor expression. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2009;63:136-45 doi:10.1016/j.biopha.2007.11.005
9. Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL. et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352:179-84 doi:10.1016/S0140-6736(98)03228-0
10. Christian JB, Lapane KL, Hume AL, Eaton CB, Weinstock MA, Trial V. Association of ACE inhibitors and angiotensin receptor blockers with keratinocyte cancer prevention in the randomized VATTC trial. Journal of the National Cancer Institute. 2008;100:1223-32 doi:10.1093/jnci/djn262
11. Jick H, Jick S, Derby LE, Vasilakis C, Myers MW, Meier CR. Calcium-channel blockers and risk of cancer. Lancet. 1997;349:525-8 doi:10.1016/S0140-6736(97)80084-0
12. Sjoberg T, Garcia Rodriguez LA, Lindblad M. Angiotensin-converting enzyme inhibitors and risk of esophageal and gastric cancer: a nested case-control study. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2007;5:1160-6 e1. doi:10.1016/j.cgh.2007.08.005
13. Control CfD. Cancer Incidence and Mortality Data. Center for Disease Control Journal for Clinicians, Cancer Statistics 2011. 2011
14. Herr D, Rodewald M, Fraser HM, Hack G, Konrad R, Kreienberg R. et al. Potential role of Renin-Angiotensin-system for tumor angiogenesis in receptor negative breast cancer. Gynecologic oncology. 2008;109:418-25 doi:10.1016/j.ygyno.2008.02.019
15. Greco S, Muscella A, Elia MG, Salvatore P, Storelli C, Mazzotta A. et al. Angiotensin II activates extracellular signal regulated kinases via protein kinase C and epidermal growth factor receptor in breast cancer cells. Journal of cellular physiology. 2003;196:370-7 doi:10.1002/jcp.10313
16. Puddefoot JR, Udeozo UK, Barker S, Vinson GP. The role of angiotensin II in the regulation of breast cancer cell adhesion and invasion. Endocrine-related cancer. 2006;13:895-903 doi:10.1677/erc.1.01136
17. Rhodes DR, Ateeq B, Cao Q, Tomlins SA, Mehra R, Laxman B. et al. AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10284-9 doi:10.1073/pnas.0900351106
18. Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. Journal of the American College of Surgeons. 1995;180:297-306
19. Stearns V, Singh B, Tsangaris T, Crawford JG, Novielli A, Ellis MJ. et al. A prospective randomized pilot study to evaluate predictors of response in serial core biopsies to single agent neoadjuvant doxorubicin or paclitaxel for patients with locally advanced breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:124-33
20. Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V. et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:4414-22 doi:10.1200/JCO.2007.10.6823
21. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. Journal of the National Cancer Institute Monographs. 2001:96-102
22. O'Regan RM, Von Roenn JH, Carlson RW, Malik U, Sparano JA, Staradub V. et al. Final results of a phase II trial of preoperative TAC (docetaxel/doxorubicin/cyclophosphamide) in stage III breast cancer. Clinical breast cancer. 2005;6:163-8 doi:10.3816/CBC.2005.n.019
23. Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI. et al. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. The Surgical clinics of North America. 2003;83:803-19 doi:10.1016/S0039-6109(03)00034-3
24. Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F. et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:2645-52 doi:10.1200/JCO.2010.33.4441
25. Chavez-Macgregor M, Brown E, Lei X, Litton J, Meric-Bernstram F, Mettendorf E. et al. Bisphosphonates and pathologic complete response to taxane- and anthracycline-based neoadjuvant chemotherapy in patients with breast cancer. Cancer. 2012;118:326-32 doi:10.1002/cncr.26144
26. Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM. et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:3297-302 doi:10.1200/JCO.2009.19.6410
27. Bayraktar S, Hernadez-Aya LF, Lei X, Meric-Bernstam F, Litton JK, Hsu L. et al. Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer. 2012;118:1202-11 doi:10.1002/cncr.26439
28. Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB. et al. Breast cancer. Clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network: JNCCN. 2009;7:122-92
29. Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V. et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:1037-44 doi:10.1200/JCO.2005.02.6914
30. Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A. et al. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer investigation. 2011;29:585-93 doi:10.3109/07357907.2011.616252
31. Ganz PA, Habel LA, Weltzien EK, Caan BJ, Cole SW. Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast cancer research and treatment. 2011;129:549-56 doi:10.1007/s10549-011-1505-3
32. Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. The lancet oncology. 2010;11:627-36 doi:10.1016/S1470-2045(10)70106-6
33. Yoon C, Yang HS, Jeon I, Chang Y, Park SM. Use of angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers and cancer risk: a meta-analysis of observational studies. CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne. 2011;183:E1073-84 doi:10.1503/cmaj.101497
34. Chang CH, Lin JW, Wu LC, Lai MS. Angiotensin receptor blockade and risk of cancer in type 2 diabetes mellitus: a nationwide case-control study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:3001-7 doi:10.1200/JCO.2011.35.1908
35. Sugiura R, Ogawa H, Oka T, Koyanagi R, Hagiwara N, Investigators H-C. Candesartan-based therapy and risk of cancer in patients with systemic hypertension (Heart Institute of Japan Candesartan Randomized Trial for Evaluation in Coronary Artery Disease [HIJ-CREATE] substudy). The American journal of cardiology. 2012;109:576-80 doi:10.1016/j.amjcard.2011.09.050
36. Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J. et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. The lancet oncology. 2011;12:65-82 doi:10.1016/S1470-2045(10)70260-6
37. Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:1467-72 doi:10.1200/JCO.2009.22.7918
Author contact
![]() Corresponding author: Mariana Chavez-MacGregor, MD, MSc. Assistant Professor, The University of Texas MD Anderson Cancer Center. 1515 Herman P Pressler CPB 5.3450, Houston, TX 77030. (Tel) 713 792 2817 (Fax) 713 7944385. mchavez1org
Corresponding author: Mariana Chavez-MacGregor, MD, MSc. Assistant Professor, The University of Texas MD Anderson Cancer Center. 1515 Herman P Pressler CPB 5.3450, Houston, TX 77030. (Tel) 713 792 2817 (Fax) 713 7944385. mchavez1org

 Global reach, higher impact
Global reach, higher impact