Impact Factor
ISSN: 1837-9664
J Cancer 2013; 4(8):644-652. doi:10.7150/jca.7093 This issue Cite
Research Paper
A Target-Specific Oral Formulation of Doxorubicin-Protein Nanoparticles: Efficacy and Safety in Hepatocellular Cancer
1. Department Biotechnology and Bioinformatics, University of Hyderabad, and Hyderabad 500046 India;
2. Department of Biochemistry, University of Hyderabad, and Hyderabad 500046 India;
3. Centre for Nanotechnology, University of Hyderabad, and Hyderabad 500046 India.
Received 2013-7-4; Accepted 2013-9-3; Published 2013-9-14
Abstract
Background/Aims: Hepatocellular carcinoma (HCC) also known as malignant hepatoma is a most common liver cancer. Doxorubicin (Doxo) is an anti-cancer drug having activity against a wide spectrum of cancer types. Clinical Utility of doxo has been limited due to its poor bioavailability and toxicity to heart and spleen. Furthermore, cancer chemotherapeutics have limited oral absorption. Transferrin family proteins are highly abundant and plays important role in transport and storage of iron in cells and tissues. Since apotransferrin and lactoferrin receptors are highly expressed on the surface of metabolically active cancer cells, the principal objective of present study is to evaluate efficacy of doxorubicin loaded apotransferrin and lactoferrin nanoparticles (apodoxonano or lactodoxonano) in oral treatment of HCC in rats.
Study Design: HCC was induced in rats by supplementing 100 mg/L of diethylnitrosamine (DENA) in drinking water for 8 weeks. A week after the last day of DENA administration, rats were divided into four groups, each group comprising of five animals. Each group was administered with one of the drug viz., saline, doxorubicin (doxo), apodoxonano and lactodoxonano (4 mg/ kg equivalent of drug). In each case, they received 8 doses of the drug orally with six day interval. One week after the last dose, anticancer activity was evaluated by counting the liver nodules, H & E analysis of tissue sections and expression levels of angiogenic and antitumor markers.
Results: In rats treated with apodoxonano and lactodoxonano, the number of neoplastic nodules was significantly lower than that of rats administered with saline or with doxo. Apodoxonano and lactodoxonano did not exhibit decrease in mean body weight, which was markedly reduced by 22% in the case of doxo administered rats. In rats treated with nanoformulations, the number of liver nodules was found reduced by >93%. Both nanoformulations showed significantly high localization in liver compared to doxo.
Conclusions: Apodoxonano and lactodoxonano showed improved efficacy, bioavailability and safety compared to doxo for treatment of HCC in rats when administered orally.
Keywords: Hepatocellular carcinoma, Oral chemotherapy, doxo, apotransferrin nanoparticles, lactoferrin nanoparticles.
Introduction
Oral administration of drug is the most convenient method of drug delivery due to its simplicity, non-invasiveness and cost effectiveness.1 Especially intravenous infusion of various anticancer drugs often lead to initial rapid increase, followed by sharp decline of concentrations below their therapeutic level leading to increased drug resistance. A prolonged exposure of drug at modest concentrations would be more beneficial than quick initial burst.2 This can be achieved by oral chemotherapy which can maintain optimal concentration levels of drug and provides prolonged exposure of the targeted organ to the drug which in turn will increase the efficacy.3, 4 But the major hindrance for this modality is the poor bioavailability due to the limited absorption of drug into the circulation through the intestinal epithelium and efflux transporters.5 So to achieve the best advantages of oral chemotherapy, the encapsulation of drug in a carrier molecule is necessary to ensure protection against in vivo degradation, reduction of toxic side effects, decrease in repeated bolus injections, favourable pharmacokinetics and sustainable release. Natural biopolymers are the best vehicles for development of such carrier molecules in sustainable drug delivery systems.6
Doxo, an anthracyclin antibiotic, despite of its side-effects, is known for its efficacy and has become an indispensable molecule.7, 8 Since doxo shows very low oral bioavailability (~5%) due to the low permeability, acid hydrolysis in stomach and susceptibility to cytochrome P450,9, 10 it is available in the market as injectables namely Adriamycin, Rubex and Doxil etc. and these intravenous formulations are often fraught with cardiotoxicity. There has been an outstanding progress in the use of novel nanocarriers for the oral application among which biodegradable ones are the most widely employed for the delivery of anticancer drugs since they yield higher therapeutic efficacy with less adverse effects.11 Results of our previous investigation12 using nanoformulations of the doxo with two natural proteins namely, apotransferrin and lactoferrin in the treatment of hepatocellular carcinoma by intravenous administration showed a higher efficacy and pharmacokinetic profile of these nanoformulations with least cardiotoxicity.12 Recently we have evaluated the biocompatibility and absorption profile of the same formulations when delivered orally.13 In the present study we report the efficacy of the nanoformulations against HCC when administered orally in rats. Results showed better performance of apodoxonano and lactodoxonano compared to doxo in terms of efficacy, tissue localization and safety.
Materials and Methods
Materials
Human blood was collected from healthy volunteers as per approval of Institutional Ethics Committee, University of Hyderabad. Apotransferrin was purified from human blood following the method of Cohn et al.14 Doxo was a pharmaceutical preparation by Biochem Pharmaceutical Industries, Pune, India. Lactoferrin was purified from bovine milk as reported earlier.15 All the other reagents, biochemical analysis kits and biochemicals were of analytical and molecular biological grade.
Animals
All the Animal experiments were conducted as per approval of Institutional Animal ethics committee, University of Hyderabad. Wistar rats (Age: 6-8 months, Weight: 0.155-0.175 kg) were maintained in the University animal house and acclimatized for 7 days before the start of the experiment. Rats were randomly divided into four groups, each group comprising five animals.
Methods
Purification of Lactoferrin
In brief, fat in the cow milk was removed through centrifugation at 8000 rpm/ 10 min/ 4°C and diluted with 0.05M Tris-HCl (pH 8.0). CM-Sephadex was added to it (7 g/l) and stirred slowly using a mechanical stirrer for an hour. When the gel got settled, the milk was decanted. The gel was packed into a column and washed with excess of 0.05M Tris-HCl and buffer containing 0.1M NaCl. The lactoferrin was eluted with 0.25M NaCl buffer. Finally the protein was passed through a Sephadex G-100 column (2 x 100 cm) in 0.05M Tris-HCl (pH 8.0) and loaded on to the SDS-PAGE gel.
Protein nanoparticle preparation
Apotransferrin nanoparticles were prepared by sol-oil chemistry as described17 (Indian patent # 1572/CHE/2006).17 Lactoferrin nanoparticles were prepared 12 with some modifications (Indian patent # 4657/CHE/2011 dated 30.12.2011; US/13729214/2012 dt 28 Dec 2012).12
Preparation of Protein nanoparticles
Doxorubicin loaded apotransferrin nanoparticles (Apodoxonano): Apotransferrin nanoparticles were prepared by sol-oil chemistry in the protocol described [Indian patent # 1572/CHE/2006; 17].
Doxorubicin loaded lactoferrin nanoparticles (Lactodoxonano): Lactoferrin nanoparticles were prepared based on the method of Krishna et al., 200917 with some modifications [Indian patent appln # 4657/CHE/2011 dated 30.12.2011; US patent appln. #13729214]. 10 mg of lactoferrin was solubilized in 100 µl of phosphate-buffered saline (pH 7.4) (PBS) and slowly mixed with 4 mg of doxo in 100 µl of double distilled water and the mixture was incubated on ice for 5 min. The mixture of lactoferrin and doxo was slowly added to 15 ml of olive oil at 4 °C with continuous dispersion by gentle manual vortexing. The nanoparticle formation of lactoferrin-doxorubicin hydrochloride (doxo) in oil phase was initiated by sonication at 50-80% power range and the probe used was a solid titanium tip of 0.375 inch diameter (Cat no 0-120-0009). A 2 second pulse was passed with a gap of 2 seconds between successive pulses for a period of 15 min using ultrasonic homogenizer (Model 300V/T of Bioloics Inc., USA). After sonication, the mixture of the olive oil containing lactoferrin-doxorubicin hydrochloride was immediately frozen in liquid nitrogen at -196oC for 10 min. Then it was kept on ice for 4 hours. The Particles formed were separated by centrifugation at 6000 rpm for 10 minutes. The supernatant was decanted and the pellet containing the nanoparticles of doxorubicin loaded lactoferrin was washed twice using 15 ml of ice cold diethyl ether. The pellets were immediately dispersed thoroughly by manual vortexing in 1 ml of phosphate buffered saline and used for experiments. Particles were characterized using scanning electron microscope (SEM), transmission electron microscope (TEM) and atomic force microscope (AFM) as per manufacturer's manual.
Rhodamine123 Conjugation of apotransferrin and lactoferrin
Apotransferrin and lactoferrin were conjugated with Rhodamine123 using the following protocol18. Briefly, 2 mg/ml of Apotransferrin and lactoferrin was dissolved in 0.1M sodium Carbonate buffer of pH 9 separately. Rhodamine123 was added to anhydrous DMSO at 1 mg/ml concentration. To 1ml of protein solution, 50µl of Rhodamine123 solution was added slowly in 5µl aliquots with continuous and gentle stirring. After the addition of Rhodamine123 solution, incubation was carried out in the dark for 8 hrs at 4˚C. 50mM Ammonium chloride was added and incubation was continued for 2 hrs at 4˚C. 0.1% Xylene cyanol and 5% glycerol was added to the mixture and separation of the conjugated apotranferrin and lactoferrin was done by gel filtration using the exclusion limit of 20,000 - 50,000. The eluted conjugates were stored in light proof container. Rhodamine123-conjugated apotransferrin and lactoferrin nanoparticles were directly used for the experiments.
Competition of the lactoferrin receptor antibodies with the lactoferrin-drug Nanoparticles
Sup T1 (T cell lymphoma) cells were used as representative cancer cells. One million cells were incubated in serum free media for 30 min in a 12 well plate. Rhodamine123 labeled apodoxonano or lactodoxonano (equivalent to 50 µg protein) were added to the cells in the presence and absence of anti-intestinal lactoferrin receptor polyclonal antibody (2 µg/ millions of cells) and incubated for one hour. After incubation, the cells were washed thrice with PBS and observed under Leica laser confocal microscope and fluorescently labelled cells were counted using Partec Flow Cytometer.
Anticancer activity of nanodoxo and doxo in DENA treated rats
Male Wistar rats (4 - 6 months old; Weight: 0.155-0.175 kg) were housed in cages and kept on a 12 h light/dark cycle. After acclimatization, Hepatocellular carcinoma (HCC) was induced in rats by supplementation of 100 mg/L of diethylnitrosamine (DENA) in drinking water for 8 weeks19. The rats (6 - 8 months old) were randomly divided into 4 groups (5 animals per group) and maintained for one week. After one week of DENA induction, a dose of 4 mg/kg of doxo or doxo equivalent of protein nanoparticles were administered orally with oral canula on 1, 7, 14, 21, 28, 35,42 and 49 days. During the treatment, weight of animals was monitored and the animals were sacrificed at the end of study under sodium pentabarbitrate anaesthesia. Tissues (liver, kidneys, heart and spleen) were collected and blood was drawn by puncturing the heart. Tumour nodules >3 mm in diameter (measured with a digital caliper) were counted on the surface of each lobe of the liver and the variation in the nodule numbers amongst four groups was statistically evaluated. To make neoplastic nodules more evident, further evaluation of tumour growth was performed by fixing the lobes in 10% formalin. The upper and lower surfaces of each fixed lobe, together with a millimeter graded bar were photographed. To avoid uncertainty in the result, the lumps smaller than 2 mm were excluded. The antitumor effect of the formulations was estimated by comparing the number of animals with more than 40 tumour masses in each of the four experimental groups. Formalin fixed lobes were embedded in paraffin and routinely stained with hematoxylin and eosin (H&E).
RNA isolation and semi-quantitative RT-PCR for tumour associated genes20
Total RNA was isolated from the frozen liver tissues using Trizol Reagent (Invitrogen, USA) and cDNAs were synthesized from total RNA using oligo-(dT)12-18 as per the manufacturer's protocol (Superscript III First strand synthesis kit, Invitrogen). Now the semi-quantitative polymerase chain reaction was optimized using the primer sets of tumour associated genes. Data was normalized to the expression of the GAPDH which is used as internal control. The following primers were used: p53: sense CCATGAGCGTTGCTCTGATG, antisense TTATCCGGGTGGAAGGAAATC; p21: sense CCTGTTCCACACAGGAGCAA, antisense GATTGCGATGCGCTCATG; VEGFR1: sense: CGTACCCGCAACGGAGAA, antisense: GCGTCCTCGGCAGTTACATC. RNA isolated from liver of healthy rat was used as a control.
Safety analysis of nanoformulations
Safety analysis was carried out to test the toxicity induced in the corresponding organs with biochemical analysis kits manufactured by Qualigens (India) for SGOT, SGPT, BUN (Blood Urea Nitrogen) and creatinine (for liver and kidneys). Cardiotoxicity was measured by lactate dehydrogenase (LDH) using the following protocol.
Lactate dehydrogenase assay21
The reaction rate was determined by a decrease in absorbance at 340 nm resulting from the oxidation of NADH (Adams et al., 1973). One unit of LDH causes the oxidation of one micromole of NADH per minute at 250C and pH 7.3, under the specified conditions. After Initialization and equilibration, blank rate was established. 300 ml of serum was added to 2.5 ml of 0.2M Tris_HCl (pH 7.3), 0.1 ml of 6.6mM NADH and 0.1 ml of 30mM sodium pyruvate to record A340/min from initial linear portion.
Statistical Analysis
All studies in vitro were carried out in triplicate for each of the experimental groups. Results are presented in terms of mean and standard deviation. The significance of differences between treatments was analyzed by one-way ANOVA with age and treatment as factors using Sigma Stat. The level of statistical significance (P) was set at P < 0.05.
Results
Receptor mediated entry of protein nanoparticles
The entry of doxo loaded nanoparticles through receptor mediated endocytosis was analysed. First the cells were pretreated with apotransferrin and lactoferrin receptor antibodies. Then the cells were mixed with nanoformulations of both proteins where apotransferrin and lactoferrin were tagged with rhodamine123 which emits green fluorescence, in contrast to doxo which emits intrinsic red fluorescence. Flow Cytometry analysis showed an increase in the number of fluorescent cells when treated with nanoformulations alone, while it got reduced when the cells were preincubated with receptor antibodies. The result was confirmed using confocal microscopy (Supplementary Figure 1).
Treatment of hepatocellular carcinoma
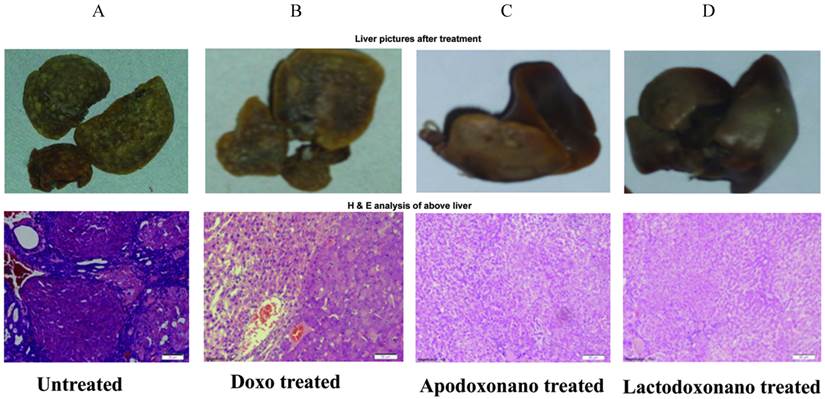
HCC was induced by oral administration of DENA and was found evident through the formation of nodules on liver tissue and also from the cancerous cellular growth profile in liver histochemical sections (Fig 1A). In the case of rats treated with doxo, the nodule formation was found relatively reduced though some cancerous growth was still evident in liver sections (Fig 1B, 2B). In contrast, in the group treated with apodoxonano or lactodoxonano the nodules in the liver were significantly reduced suggesting highest inhibition of HCC when doxo loaded nanoformulations were employed (Fig 2B). This is further confirmed by histochemical analysis, where the liver tissue sections showed normal cellular morphology (Fig 1C, 1D). The above observations suggest that the protein nanoparticle formulations of apotransferrin and lactoferrin are highly effective when compared to soluble doxo.
Efficacy of nanoformulations
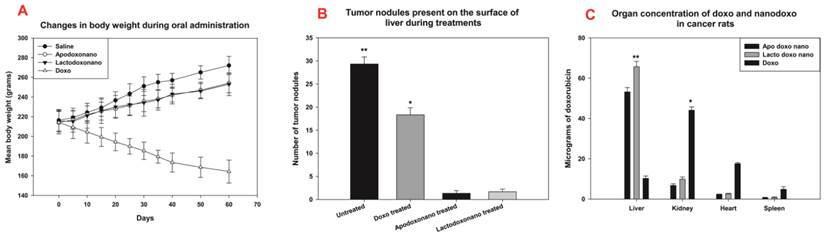
HCC bearing rat showed an increase in mean body weight (MBW), which gets drastically reduced when treated with doxo indicating the severe side effects exerted by the drug (Fig 2A). Whereas in the case of treatment with apodoxonano and lactodoxonano, the MBW of animals was significantly restored (Fig 2A) with a slight decrease in weight due to cancer inhibition. Thus the nanoparticle encapsulation has reduced the doxo-treatment associated weight loss demonstrating decreased side effects
Concentration of doxo during the treatment was estimated in the tissue homogenate prepared from liver, kidney, heart, spleen of treated rat. The results shown in Fig 2C indicate that high levels of doxo are present in liver, while the levels were significantly lower in kidney, heart and spleen when administered through apodoxonano and lactodoxonano. Since the liver is the site of action, the presence of higher drug levels in it when administered through apodoxonano and lactodoxonano would help in enhancing the efficacy of treatment.
Semi-quantitative RT-PCR analysis of Tumor associated genes
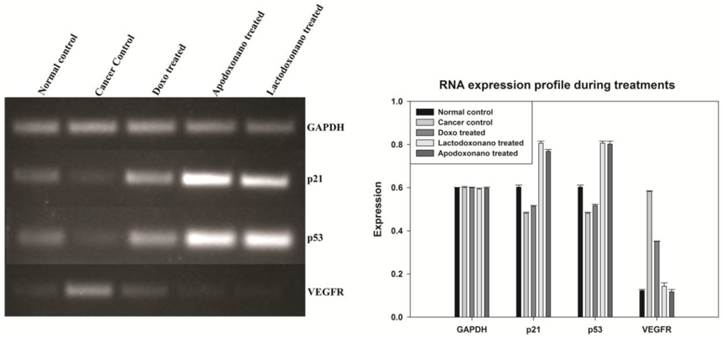
The major proteins that play a vital role in controlling the cancer namely, p53 and p21 are over expressed under tumor suppressing conditions. The levels of p53 and p21 were analysed in treated rats and the results shown in Fig 3 indicate that though the levels are significantly elevated in rats treated with doxo, the rats treated with apodoxonano and lactodoxonano showed highest up regulation. To confirm the results, vascular development around the cancer tissue was analyzed by monitoring VEGFR expression. VEGFR levels enhanced with progression of cancer and thus serve as strong indicator of disease progression. Results show that VEGFR levels got significantly down regulated in apodoxonano and lactodoxonano treated rats and this reduction is higher when compared to doxo treatment (Fig 3). These results point out that apodoxonano and lactodoxonano enhance tumour suppressing milieu and efficiently block vasculature around the cancer tissue efficiently through targeted localization of drug.
Expression profile of tumour associated and apoptotic markers
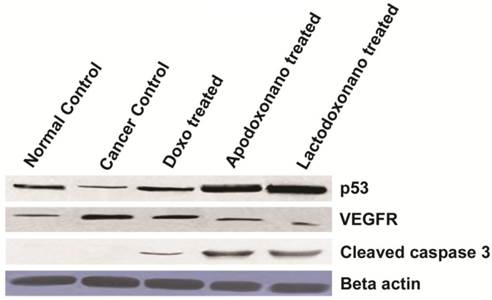
The protein levels of p53 and VEGFR exhibited profiles very similar to that of their RNA levels, thus corroborating the efficacy of nanoformulations used. This is further confirmed through the observation of an increased expression of an apoptotic marker, cleaved caspase-3 during the treatment with nanoparticles indicating the highest rate of apoptosis when treatment is carried out with nanoformulations (Fig 4).
Safety profile
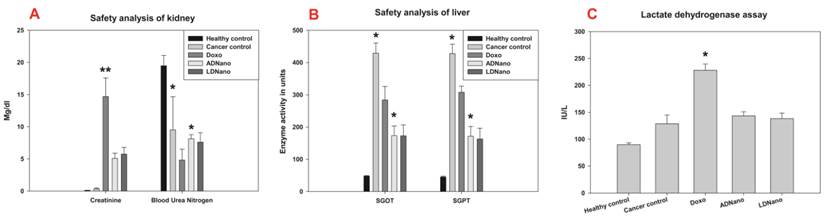
Non-specific toxicities of nanoformulations were analysed in tissue homogenate of treated rat using specific-markers. After absorption from the GI tract the carrier molecule enters the liver and gets excreted through kidney. Here the safety analysis for the kidney was done by estimating the parmeters Creatinine and Blood Urea Nitrogen (BUN). Significant increase in Creatinine levels and poor elimination of BUN indicates the kidney damage which in the present study is evident in doxo treated rats (Fig 5A). But in the case of the rats treated with nanoformulations the safety parameters clearly indicated a significant reduction in the extent of damage. Liver damage was analysed by estimating the levels of SGOT and SGPT which were found increased in case of doxo treatment and reduced substantially, when treated with nanoformulations (Fig 5B). Heart toxicity which is a major complication in doxo treatment was measured by LDH level. LDH levels were found significantly elevated in doxo treated group, whereas these levels remained minimal in the animals treated with nanoformulations similar to that of the group treated with saline (Fig 5C).
Induction and treatment of HCC: HCC was induced by adding 100 mg/L of DENA in drinking water for eight weeks. The animals received 4 mg/kg of drug equivalent of apodoxonano, lactodoxonano and Doxo through oral administration on 1, 7, 14, 21, 28, 35, 42, 49 day. Panel A shows untreated liver with maximum number of nodules which were reduced to some extent when treated with doxo (panel B). HCC was significantly reduced when treated with Apodoxonano (Panel C) and Lactodoxonano (Panel D), which is indicated by the decrease in the number of nodules. Lower panel shows H & E analysis of liver nodules along with treatments.
Efficacy of nanoformulations: Panel -A shows that the body weights of rats were decreased significantly when treated with doxo and gets increased in saline injected ones. When treated with nanoformulations, a slight increase in body weight was observed. Panel-B shows tumour nodules on the surface of liver were reduced to a large extent when treated with apodoxonano and lactodoxonano, as against Doxo and untreated rats (control). Panel C: After treatment, animals were sacrificed to collect the organs for the estimation of doxo in them. Here the highest levels were found only in the liver of rats treated with nanoformulations due to their targeted delivery.
Expression profile of tumor associated genes: Semiquantitative analysis of markers indicated the tumour burden was greatly reduced during lactodoxonano and apodoxonano treatment when compared to doxo. The over expression of the major tumour suppressor genes namely p53 and p21 and the reduced vasculature due to decreased levels of VEGFR during apodoxonano and lactodoxonano treatment corroborates the efficacy of nanoformulations.
Expression levels of tumour associated and apoptotic markers: Increased Protein levels of p53 and decreased protein levels of VEGFR during the treatment with nanofrmulations showed significant correlation with their RNA counterparts confirming the results of efficacy. The protein level of Cleaved caspase-3, which is an indispensable marker for apoptosis also showed highest levels during the treatment with nanoformulations indicating the maximum rate of apoptosis in these cells. In this study, Beta actin is used as an internal control.
Safety analysis in cancer treated rats: After the oral administration of lactodoxonano, apodoxonano and doxo, Heart toxicity was measured in terms of LDH profile, which showed minimal expression when treated with nanoformulatios similar to the control but gets increased during doxo treatment. Kidney toxicity was evaluated by the levels of Creatinine, Blood Urea Nitrogen, whereas the Liver toxicity was estimated in terms of SGOT and SGPT levels. The level of these 4 markers also followed the same pattern as that of LDH where they showed minimal levels when treated with nanoformulations as opposed to doxo.
Discussion
For treatment of a wide spectrum of cancers, oral delivery is the most preferred route of drug administration but becomes the toughest because of various barriers encountered by the drug in reaching its target organ. Secondly, since the doxo is the substrate of P-gp efflux pump, various inhibitors like Myricetin, Quercetin and Cyclosporin A22, 23 were generally employed to escape from the drug efflux pathways in the intestine, kidney and liver. But major limiting factors in the usage of these inhibitors are the adverse effects like the suppression of immune system which prevent their usage in combination with the anti-cancer drugs.24 So nanoparticle formulation has now become the most preferred strategy to increase the bioavailability of drugs when delivered orally. Studies on the polystyrene latex has shown that maximum uptake of the particles is possible when their diameter is in between 50-100 nm.25 Several formulations including hydrogels, dendrimers, liposomes etc, which were made up of various biodegradable polymers like PEG, PLGA, PAMAM, PAA etc., were tried to improve oral bioavailbility of doxo.26-29
Among the biodegradable polymers, natural ones are the best candidates for the sustainable delivery systems.30 Apotransferrin and lactoferrin were reported as delivery vehicles since the receptors for these proteins were known to be over expressed on various types of cancers.19, 31 In the recent past, proteins have become indispensable for the diagnosis and treatment of many diseases due to their nontoxic, non-immunogenic, biocompatible and biodegradable nature. They provide good pharmacokinetics and better cancer tissue accumulation. Protein nanoparticulate systems are gaining importance due to their modifiable functionalities and potential applications.32-40
We previously reported the efficacy, bioavailability and pharmacokinetic profile of the nanoformulations of doxo with the above two proteins when delivered intravenously.12 Recently it was also shown that these nanoformulations were absorbed more efficiently through the small intestine when delivered orally and the stability of these formulations was reported to be better in the presence of simulated fluids.13 More over these formulations appear to be safer and do not lead to any toxic side effects.
In the present study, the efficacy and safety of the apodoxonano and lactodoxonano was shown where 4mg/kg of doxo was used against the 2 mg/kg of intravenous formulation that was employed previously.12 To maintain the efficacy, the number of doses were increased to eight. Thus, the efficacy of the oral formulations were maintained on par with the intravenous formulation as is evident from the number of nodules observed on the treated liver samples and also the mean body weight. The highest amount of doxo was found to be present in the liver when delivered through these nanoformulations, which points out to the efficient delivery of drug to the target tissue. The safety profile of the formulations in the treated rats was found to be similar to that of untreated rats. The efficacy of drug in nanoformulation was confirmed by the semi-quantitative analysis of molecular markers which showed significant enhancement in the expression of the tumor suppressor genes p53 and p21 indicating the rate of tumor suppression. This is further supported by the observed down regulation of VEGFR expression which is an indicator of decreased vasculature around the tumor. While the results of the studies on small animals like rats are encouraging, further studies need to be undertaken particularly in determining dosages in human studies. One of the major concerns is the oral absorption of the drug when delivered with nanoparticle carriers in humans. Mouse, rat, sheep etc can absorb nanoparticles efficiently but the relevance of these studies and extrapolation of the data to humans remains unaddressed.41 Secondly, the dosage used in the present formulations is very less compared to the various formulations which range from 7.5- 12.5mg/kg body weight as reported in the literature. Furthermore, the extrapolation of the dose to humans is not possible because small animals eliminate drugs much faster than large mammals.42-44
Conclusions
The data indicates that the apodoxonano and lactodoxonano increased the anti-neoplastic efficacy of doxo in HCC rats. Delivery of doxo through apotransferrin and lactoferrin nanoformulation enhances the efficacy and bioavailability of doxo in a target-specific manner, and hence it is an effective and safer oral drug delivery approach.
Abbreviations
HCC: hepatocellular carcinoma; DENA: diethylnitrosamine; apodoxonano: doxorubicin loaded apotransferrin nanoparticles; lactodoxonano:doxorubicin loaded lactoferrin nanoparticles; doxo: doxorubicin; Tris:trisaminomethane; HCl: Hydrochloride; CM: Carboxymethyl; NaCl: Sodium chloride; SDS-PAGE: sodium dodecylsulphate - polyacrylamide gel electrophoresis; RT-PCR: real time polymerase chain reaction; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; p53: tumor suppressor protein 53; p21: Cyclin-dependent kinase inhibitor 1A; VEGFR1:Vascular endothelial growth factor receptor 1; SGOT: Serum glutamic oxaloacetic transaminase; SGPT: serum glutamic pyruvic transaminase; BUN: blood urea nitrogen; LDH: lactate dehydrogenase; NADH: Nicotinamide adenine dinucleotide; ANOVA: Analysis of variance; MBW: mean body weight; GI: gastrointestinal; PEG:Polyethylene glycol; PLGA: Poly(lactic-co-glycolic acid); PAMAM: Poly(amido amine); PAA: poly(acrylic acid).
Supplementary Material
Supplementary Figure 1Acknowledgements
We thank M. Nalini, Confocal Microscope facility, UoH for performing Confocal Microscopy.
Financial support
This work is supported by the research projects funding by Department of Biotechnology and Department of Science and Technology, Government of India. GK is a CSIR SRF.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Varma MVS, Khandavilli S, Ashokraj Y. et al. Biopharmaceutic classification system: a scientific framework for pharmacokinetic optimization in drug research. Curr. Drug Metab. 2004;5(5):375-388
2. Feng SS, Chien S. Chemotherapeutic engineering: application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem. Eng. Sci. 2003;58:4087-4114
3. Zhang Z, Feng SS. Nanoparticles of poly (lactide)/vitamin E TPGS copolymer for cancer chemotherapy: synthesis, formulation, characterization and in vitro drug release. Biomaterials. 2006;27:262-270
4. Dong Y, Feng SS. Poly (D,L-lactide-co-glycolide)/montmorillonite nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26:6068-6076
5. Bellamy WT. P-glycoproteins and multidrug resistance. Annual Rev. Pharmacol. Toxicol. 1996;36:161-183
6. Oh JK, Lee DI, Park JM. Biopolymer-based microgels/nanogels for drug delivery applications. Progress in Polymer Science. 2009;34:1261-1282
7. Ryberg M, Nielsen D, Skovsgaard T. et al. Pirubicin cardiotoxicity: an analysis of 469 patients with metastatic breast cancer. J. Clin. Oncol. 1998;16:3502-3508
8. Gonsette RE. A comparison of the benefits of mitoxantrone and other recent therapeutic approaches in multiple sclerosis. Expert Opin. Pharmacother. 2004;5:747-765
9. Beijnen JH, Van der Houwen O, Underberg WJM. Aspects of the degradation kinetics of doxorubicin in aqueous solution. Int. J. Pharm. 1986;32:123-131
10. Jain AK, Swarnakar NK, Das M. et al. Augmented anticancer efficacy of doxorubicin loaded polymeric nanoparticles after oral administration in breast cancer induced animal model. Mol. Pharmaceutics. 2011;8(4):1140-1151
11. Italia JL, Bhatt DK, Bhardwaj V. et al. PLGA nanoparticles for oral delivery of cyclosporine: nephrotoxicity and pharmacokinetic studies in comparison to Sandimmune Neoral®. J. Control. Release. 2007;119:197-206
12. Golla K, Cherukuvada B, Ahmed F. et al. Efficacy, safety and anticancer activity of protein nanoparticle-based delivery of doxorubicin through intravenous administration in rats. PLoS One. 2012;7(12):e51960
13. Golla K, Reddy PS, Bhaskar C. et al. Biocompatibility, absorption and safety of protein nanoparticle-based delivery of doxorubicin through oral administration in rats. Drug Deliv. 2013;20(3-4):156-67
14. Cohn EJ, Strong LE, Hughes WL. et al. Preparation and Properties of Serum and Plasma Proteins. IV. A System for the Separation into Fractions of the Protein and Lipoprotein Components of Biological Tissues and Fluids. J. Am. Chem. Soc. 1964;68:459-475
15. Sharma AK, Paramasivam M, Srinivasan A. et al. Three-dimensional structure of mare diferriclactoferrin at 2.6Aresolution. J. Mol. Biol. 1999;289:303-317
16. Reddy LH, Meda N, Murthy RR. Rapid and sensitive HPLC method for the estimation of doxorubicin in dog blood-the silver nitrate artifact. Acta Pharm. 2005;55:81-91
17. Krishna ADS, Mandraju RK, Kishore G. et al. An Efficient Targeted Drug Delivery through Apotransferrin Loaded Nanoparticles. PLoS ONE. 2009;4(10):e7240
18. McDonagh PF, Williams SK. The preparation and use of fluorescent-protein conjugates for microvascular research. Microvasc Res. 1984;27(1):14-27
19. Fiume L, Bolondi L, Busi C, Chieco P, Kratz F. et al. Doxorubicin coupled to lactosaminated albumin inhibits the growth of hepatocellularcarcinomas induced in rats by diethylnitrosamine. J Hepatol. 2005;43:645-52
20. Borbath I, Leclercq IA, Sempoux C. et al. Efficacy of lanreotide in preventing the occurrence of chemically induced hepatocellular carcinoma in rats. ChemBiol Interact. 2010;183:238-48
21. Adams M, Buehner M, Chandrasekhar K. et al. Structure-Function Relationships in Lactate Dehydrogenase. ProcNatlAcadSci. 1973;70:1968
22. Choi SJ, Shin SC, Choi JS. Effects of myricetin on the bioavailability of doxorubicin for oral drug delivery in rats: possible role of CYP3A4 and P-glycoprotein inhibition by myricetin. Arch Pharm Res. 2011;34(2):309-15
23. Ke W, Zhao Y, Huang R. et al. Enhanced oral bioavailability of doxorubicin in a dendrimer drug delivery system. J.Pharm. Sci. 2008;97(6):2208-2216
24. van Zuylen L, Nooter K, Sparreboom A. et al. Development of multidrug-resistance convertors: sense or nonsense? Invest New Drugs. 2000;18(3):205-20
25. Jani P, Halbert GW, Langridge J. et al. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol. 1990;42(12):821-6
26. Batrakova EV, Dorodnych TY, Klinskii EY, et al.Anthracycline antibiotics non- ovalently incorporated into the block copolymer micelles. in vivo evaluation of anti-cancer activity. BrJCancer. 1996;74:1545-52
27. Nakanishi T, Fukushima S, Okamoto K. et al. Development of the polymer micelle carrier system for doxorubicin. JControl Release. 2001;74:295-302
28. Kim TY, Kim DW, Chung JY. et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10:3708-16
29. Kukowska LJF, Candido KA, Cao Z. et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317-24
30. Oh JK, Lee DI, Park JM. Biopolymer-based microgels/nanogels for drug delivery applications. Progress in Polymer Science. 2009;34:1261-1282
31. Schneider Y, Abarca J, Pirak AE. et al. Drug targeting in human cancerchemotherapy. Receptor-mediated targeting of drugs. NATO ASI series A: life sciences. 1984:1-25
32. Yewale C, Baradia D, Vhora I. et al. Proteins: emerging carrier for delivery of cancer therapeutics. Expert Opin Drug Deliv. 2013 [PMID: 23789923]
33. Elzoghby AO, Saad NI, Helmy MW. et al. Ionically-crosslinked milk protein nanoparticles as flutamide carriers for effective anticancer activity in prostate cancer-bearing rats. Eur J Pharm Biopharm. doi: 10.1016/j.ejpb.2013.07.003
34. Zhen X, Wang X, Xie C. et al. Cellular uptake, antitumor response and tumor penetration of cisplatin-loaded milkprotein nanoparticles. Biomaterials. 2013;34:1372-82
35. Yang Y, Burkhard P. Encapsulation of gold nanoparticles into self-assembling protein nanoparticles. J Nanobiotechnology. 2012;10:42
36. Li X, Qiu L, Zhu P. et al. Epidermal growth factor-ferritin H-chain protein nanoparticles for tumor active targeting. Small. 2012;8(16):2505-14
37. Gong G, Xu Y, Zhou Y. et al. Molecular switch for the assembly of lipophilic drug incorporated plasma protein nanoparticles and in vivo image. Biomacromolecules. 2012;13(1):23-8
38. Kim W, Xiao J, Chaikof EL. Recombinant amphiphilic protein micelles for drug delivery. Langmuir. 2011;27(23):14329-34
39. Pokorski JK, Hovlid ML, Finn MG. Cell targeting with hybrid Qβ virus-like particles displaying epidermal growth factor. Chembiochem. 2011;12(16):2441-7
40. Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60(8):876-85
41. Thanosa C, Sandora M, Jonga Y. et al. Inter-species uptake of polymeric particles. Mater. Res. Proc. 1998;550:65-70
42. Chiannilkulchai N, Driouich Z, Benoit JP. et al. Doxorubicin-loaded nanoparticles: increased efficiency in murine hepatic metastases. Sel Cancer Ther. 1989;5:1-11
43. Chiannilkulchai N, Ammoury N, Caillou B. et al. Hepatic tissue distribution of doxorubicin-loaded nanoparticles after i.v. administration in reticulosarcoma M 5076 metastasis-bearing mice. Cancer Chemother Pharmacol. 1990;26:122-126
44. Verdun C, Brasseur F, Vranckx H. et al. Tissue distribution of doxorubicin associated with polyisohexylcyanoacrylate nanoparticles. Cancer Chemother Pharmacol. 1990;26:13-18
Author contact
![]() Corresponding author: Anand K. Kondapi, Ph.D. Professor of Biotechnology, Department of Biotechnology and Bioinformatics, University of Hyderabad, Hyderabad 500046 India. Ph: (O) 91-40-23134571 ® 91-40-23000654 Cell: 91-9246212654 Fax: 91-40-23010145 Email: akkslernet.in, akondapicom.
Corresponding author: Anand K. Kondapi, Ph.D. Professor of Biotechnology, Department of Biotechnology and Bioinformatics, University of Hyderabad, Hyderabad 500046 India. Ph: (O) 91-40-23134571 ® 91-40-23000654 Cell: 91-9246212654 Fax: 91-40-23010145 Email: akkslernet.in, akondapicom.

 Global reach, higher impact
Global reach, higher impact