Impact Factor
ISSN: 1837-9664
J Cancer 2014; 5(7):499-509. doi:10.7150/jca.9257 This issue Cite
Research Paper
Sorafenib Therapy for BCLC Stage B/C Hepatocellular Carcinoma; Clinical Outcome and Safety in Aged Patients: A Multicenter Study in Japan
1. Department of Gastroenterology and Hepatology, Osaka Red Cross Hospital;
2. Department of Gastroenterology and Hepatology, Musashino Red Cross Hospital;
3. Center for Liver-Biliary-Pancreatic Diseases, Matsuyama Red Cross Hospital;
4. Department of Gastroenterology, Takamatsu Red Cross Hospital;
5. Department of Gastroenterology, Japanese Red Cross Medical Center;
6. Department of Gastroenterology and Hepatology Nagoya Daini Red Cross Hospital;
7. Department of Gastroenterology, Matsue Red Cross Hospital.
* Hiroki Nishikawa and Haruhiko Takeda equally contributed to this work.
Received 2014-3-31; Accepted 2014-5-11; Published 2014-6-7
Abstract
Background and aims: We aimed to compare clinical outcomes and safety after sorafenib therapy between patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma (HCC) aged ≥75 years (aged group, n=179) and those with BCLC stage B or C HCC aged <75 years (control group, n=279).
Patients and methods: We compared overall survival (OS), progression free survival (PFS), best treatment response and sorafenib related serious adverse events (SAEs) of grade 3 or more in the two groups. Furthermore, for reducing the selection bias, we compared clinical outcome of these two groups using propensity score matching analysis.
Results: The median OS and PFS intervals were 9.7 and 3.8 months in the aged group and 8.2 and 3.3 months in the control group (P=0.641 for OS and P=0.068 for PFS). Disease control rates were 49.2% (88/179) in the aged group and 49.1% (137/279) in the control group (P>0.999). Objective response rates were 15.1% (27/179) in the aged group and 14.3% (40/279) in the control group (P=0.892). Treatment related SAEs of grade 3 or more were observed in 51 patients (28.5%) in the aged group and in 69 patients (24.7%) in the control group (P=0.385). In the propensity score matched cohort (132 pairs), no significant difference in the two groups was observed in terms of OS (P=0.898) and PFS (P=0.407).
Conclusion: In BCLC stage B or C HCC patients treated with sorafenib, life expectancy, disease progression, treatment efficacy and SAEs are unaffected by age over 75 years.
Keywords: Hepatocellular carcinoma, Sorafenib, Aged patients, Clinical outcome, Safety.
Introduction
Hepatocellular carcinoma (HCC) ranks fifth among the most prevalent deadly cancers in the world, and is the third most common cause of cancer related death. [1-4] The incidence of cancer has been reported to increase markedly with age, with >60% of all cancers developing in patients aged 65 years or more. [5] The risk of HCC development is known to be age dependent. [6] Thus, there will be an increasing number of elderly HCC patients in the coming years owing to the increased longevity of the population. The current established HCC therapy includes surgical resection, liver transplantation, transcatheter arterial chemoembolization (TACE), ablative therapies such as radiofrequency ablation (RFA) or percutaneous ethanol injection (PEI) and molecular targeted drug (sorafenib). [3]
Sorafenib, a multi-kinase inhibitor that blocks tumor growth and cell proliferation, was the first systemic chemotherapeutic drug found to improve the survival time of patients with advanced HCC both in the SHARP trial and the Asian Pacific trial. [7-9] Sorafenib has thus opened a novel era for the treatment of advanced HCC. In general, sorafenib therapy is indicated for patients with Barcelona Clinic Liver Cancer (BCLC) stage B HCC who are refractory to or had contraindications to locoregional therapies or for patients with BCLC stage C HCC. [7, 9]
As compared with younger patients, elderly patients generally have more comorbid diseases. Furthermore, in the management of elderly patients with advanced cancer, systemic chemotherapy is frequently either modified or withheld for fear of potential toxicities to chemotherapy. [10] To our knowledge, there have been few reports on clinical outcomes and safety in elderly HCC patients treated with sorafenib, although there have been several reports regarding patients treated with other therapies such as surgical resection, RFA, PEI and TACE. [11-32] Thus, there is urgent need for investigation of clinical outcomes and safety in elderly patients with HCC treated with sorafenib and this is a relevant topic for clinicians.
We have conducted a multicenter study of sorafenib therapy for HCC in Japanese Red Cross Liver Study Group. The aims of the present study were to evaluate clinical outcomes and safety after sorafenib therapy in BCLC stage B or C HCC patients aged ≥75 years as compared with BCLC stage B or C HCC patients aged <75 years. Furthermore, for reducing the selection bias, we compared clinical outcome of these two groups using propensity score matching analysis.
Patients and Methods
Patients
A total of 465 patients with unresectable HCC in the Japanese Red Cross Liver Study Group were treated with sorafenib between June 2008 and August 2013.
Our indications for sorafenib therapy were as follows: (1) Child-Pugh classification of A or B, (2) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, 1 or 2 (3) unresectable HCC determined by dynamic computed tomography (CT) scan and/or magnetic resonance imaging (MRI), (4) presence of extrahepatic metastases, (5) refractory to previous HCC therapies such as TACE and other locoregional therapies, (6) unsuitability for TACE for anatomical reasons, or (7) with vascular invasion such as portal vein tumor thrombus. However, in cases with Child-Pugh C in whom no other effective therapy could be adoptable, sorafenib was given according to decision by each attending physician after full explanation for sorafenib therapy. Of 465 patients, 7 patients had BCLC stage A or D. Thus, a total of 458 patients were analysed in the current analysis. They included 179 patients aged 75 years old or more (the aged group) and 279 patients aged less than 75 years old (the control group). We chose the cut off age of 75 years considering the aging population of HCC patients in our country. [12, 15-19, 29-32] In addition, in our country, patients aged ≥75 years are covered by a health insurance system which is different from that for patients aged <75 years. All patients analysed had at least one dose of sorafenib.
We compared the clinical outcomes and safety including overall survival (OS), progression-free survival (PFS), best response rate during follow-up period and SAEs of grade 3 or more between these two groups after sorafenib therapy. Prior to sorafenib therapy, written informed consent was obtained from all patients. The current study comprised a retrospective analysis of patient records and all treatments were conducted in an open-label manner. The ethics committees of all facilities that participated in this study approved the current study protocol and this study protocol complied with all of the provisions of the Declaration of Helsinki.
HCC diagnosis
HCC was diagnosed using abdominal ultrasound, dynamic computed tomography (CT) scans (hyperattenuation during the arterial phase in all or some part of the tumor and hypoattenuation in the portal-venous phase) and/or MRI. [33] In some patients, CT hepatic angiography (CTHA) and CT arterio-portography (CTAP) were performed to confirm HCC diagnosis. All patients were confirmed ineligible for surgery or locoregional therapies radiologically. In 90 patients (19.7%) out of 458 patients analysed in this study, percutaneous tumor biopsy was performed. They included Edmondson grade I (Ed. I) HCC in 29 patients, Ed. II HCC in 37, Ed. III HCC in 23 and mixed type HCC in one.
Sorafenib therapy
The recommended initial dose of sorafenib for HCC is 400 mg twice a day. [7-9] Nevertheless, according to the phase-I study by Miller et al., patients with insufficient liver function or renal function (serum total bilirubin >1.5 mg/dL, serum albumin <2.5 g/dL and/or creatinine clearance <40 mg/mL) are recommended to receive a reduced dose of sorafenib. [34] Studies in several countries have reported SAEs in several individuals with advanced HCC given an initial dose of sorafenib of 800 mg/day, which led to treatment discontinuation. Moreover, in Japan, the proportion of the population with a body mass index (BMI) of ≥30 kg/m2 has been reported to be lower than that in western countries and the recommended initial dose of sorafenib in elderly patients with advanced HCC is not well established because of paucity of available data. [35-37] Taking this information into consideration, the initial dose of sorafenib was determined according to factors such as body weight (BW), BMI, age, comorbid diseases, PS and liver function. Hence, the initial sorafenib dose in this study ranged from 200-800 mg/day. In cases of sorafenib related SAEs of grade 3 or more as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), sorafenib dose was reduced by 200 to 400 mg/day at the discretion of attending physicians. Temporary treatment interruptions were also allowed. Sorafenib treatment continued until disease progression, unacceptable drug-related toxicity, or the patient's wish to discontinue treatment. Even if sorafenib therapy was discontinued, in patients who were potentially tolerable for other anti-cancer therapies, post-sorafenib therapies such as other systemic chemotherapies, TACE, transcathether arterial infusion chemotherapy and several ongoing clinical trials as second-line chemotherapy were considered.
Assessments of treatment efficacy and follow up
Best treatment efficacy of sorafenib during treatment was assessed in accordance with the modified Response Evaluation Criteria in Solid Tumors for Hepatocellular Carcinoma (mRECIST) criteria and/or tumor marker levels. [38] The treatment efficacy was classified as: (1) complete response (CR), (2) partial response (PR), (3) stable disease (SD) and progressive disease (PD). CR was defined as disappearance of any arterial enhancement within all target tumors. PR was defined as 30 % or greater decrease in tumor size as determined by evaluation of the sum of the diameters of the target tumors, whose size was estimated using unidirectional measurement. PD was defined as 20 % or greater increase in tumor size as determined by evaluation of the sum of the maximal dimensions of the target tumors. SD was defined as the absence of either PR or PD. [38] The objective response rate (ORR) was defined as the percentage of patients who had a best response rate of CR and PR. The disease control rate (DCR) was defined as the percentage of patients who had a best response rate of CR, PR and SD. Follow-up consisted of weekly or bi-weekly blood test analyses and physical examination at each visit.
Statistical analysis
Data were expressed as the median value (range) or the mean ± standard deviation (SD). Differences between the two groups were analyzed using the unpaired t test for continuous variables, and categorical variables were analyzed using Fisher's exact test. Data were analyzed using univariate and multivariate analysis. OS was defined as the interval between the date of sorafenib and the date of death from any cause or the last follow-up date. PFS was defined as the interval between the date of sorafenib and the date of disease progression or the last follow-up date. For analysis of OS, follow-up ended at the time of death from any cause, censoring the remaining patients at the last follow-up visit. For analysis of PFS, follow-up was terminated at the time of first radiologically confirmed tumor progression; other patients were censored at their last follow-up visit and at the time of death from any cause without tumor progression. The treatment duration of sorafenib was calculated from the date of treatment commencement until treatment termination or last follow-up date, including times of interruptions. The cumulative OS and PFS rates between the two groups were calculated using the Kaplan-Meier method, and tested using the log-rank test. The Cox proportional hazard model was used for multivariate analysis of factors with P<0.1 in univariate analysis. Values of P<0.05 were considered to be statistically significant.
Propensity score analysis
To overcome biases due to the different distribution of covariates between aged group and control group, a one-to-one match was created using propensity score analysis. [39] Clinical variables entered in the propensity model were gender, C-P score, cause of liver disease, BCLC stage, ECOG PS, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and initial dose of sorafenib. Subsequently, a one-to-one match between these two groups was obtained by using the nearest-neighbor matching method. [39]
Results
Clinical characteristics
The baseline clinical characteristics of the two groups (the elderly group [n=179] and the control group [n=279]) are shown in table 1. There were male and Child-Pugh A predominance in both groups. There was a significantly lower positivity rate for hepatitis B surface antigen, poorer PS, lower BW, lower hemoglobin level, higher serum creatinine level and lower levels of hepatobiliary enzymes such as alkaline phosphatase (ALP) and gamma glutamyl transpeptidase (GGT) in the aged group. In terms of previous therapies for HCC, TACE was most commonly performed procedure in both groups. Initial sorafenib dose of 800 mg/day was administered in 51 patients (28.5%) in the aged group and 132 patients (47.3%) in the control group (P<0.001), whereas initial dose of sorafenib based on BW tended to be lower in the aged group than in the control group (P=0.057). Regarding BCLC stage, no significant difference in the two groups was observed (P=0.921). Sixty six patients (36.9%) in the aged group and 129 patients (46.2%) in the control group had extrahepatic metastases with the most common sites being bone and lung.
Comparison of OS and PFS rates in the two groups
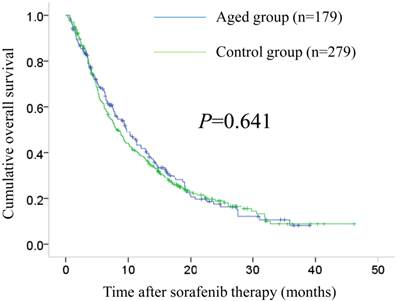
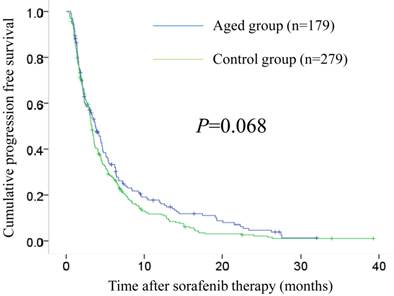
The median follow-up periods after sorafenib therapy were 7.5 months (range, 0.7-39.1 months) in the aged group and 7.6 months (range, 0.3-46.2 months) in the control group. The median OS intervals were 9.7 months (95% confidence interval [CI], 7.5-12.0 months) in the aged group and 8.2 months (95% CI, 6.9-9.6 months) in the control group (P=0.641). (Fig. 1) The median PFS intervals were 3.8 months (95% CI, 2.9-4.6 months) in the aged group and 3.3 months (95% CI, 3.0-3.6 months) in the control group (P=0.068). (Fig. 2)
Baseline characteristics between the aged group and the control group.
| Variables | Aged group (n=179) | Control group (n=279) | P value |
|---|---|---|---|
| Age (years) | 79.4 ± 3.3 | 64.1 ± 7.6 | <0.001a |
| Gender, male/female | 136 / 43 | 233 / 46 | 0.053b |
| Body weight (kg) | 56.6 ± 11.0 | 60.4 ± 12.0 | 0.001a |
| Child-Pugh A / B | 152 / 27 | 222 / 57 | 0.174b |
| Causes of liver disease | |||
| B/C/non B and non C/B and C | 6 / 124 / 47 / 2 | 62 /140 / 74 / 3 | <0.001b |
| BCLC stage B/C | 63/116 | 100/179 | 0.921b |
| ECOG PS, 0/1/2 | 117/54/8 | 229/44/6 | <0.001b |
| Portal vein invasion, yes/no | 36/143 | 71/208 | 0.214b |
| Extrahepatic metastasis, yes/no | 66/113 | 129/150 | 0.053b |
| Previous therapies for HCC, yes/no | |||
| Transarterial chemoembolization | 162/17 | 223/56 | 0.003b |
| Ablative therapies (RFA or PEI) | 107/72 | 122/157 | 0.001b |
| Surgical resection | 31/148 | 59/220 | 0.337b |
| AST (IU/L) | 64.6 ± 57.1 | 70.3 ± 69.1 | 0.356a |
| ALT (IU/L) | 43.9 ± 34.1 | 51.3 ± 43.6 | 0.054a |
| Total bilirubin (mg/dL) | 0.92 ± 0.44 | 1.04 ± 1.87 | 0.386a |
| Albumin (g/dL) | 3.48 ± 0.46 | 3.53 ± 0.53 | 0.309a |
| ALP (IU/L)c | 434.1 ± 228.3 | 539.3 ± 445.9 | 0.004a |
| GGT (IU/L)d | 103.7 ± 120.3 | 171.0 ± 194.8 | <0.001a |
| LDHe | 251.6 ± 75.9 | 254.8 ± 105.8 | 0.735a |
| Serum creatinine (mg/dL)f | 0.97 ± 0.47 | 0.86 ± 0.64 | 0.036a |
| Prothrombin time (%)g | 85.6 ± 19.4 | 86.8 ± 17.8 | 0.506a |
| Hemoglobin (g/dL)h | 11.7 ± 1.9 | 12.4 ± 2.4 | 0.002a |
| Platelets (×104/mm3)i | 12.8 ± 5.7 | 13.5 ± 6.0 | 0.183a |
| AFP (ng/mL)j | 14679 ± 111897 | 12459 ± 56756 | 0.773a |
| DCP (mAU/mL)k | 21678 ± 142635 | 18709 ± 63674 | 0.796a |
| Initial dose of sorafenib (mg/day) | |||
| 800mg/600mg/400mg/200mg | 51 / 0 / 120 / 8 | 132 / 2 / 134 / 11 | <0.001b |
| Initial dose of sorafenib based on BW (mg/kg) | 9.2 ± 3.9 | 9.9 ± 3.9 | 0.057a |
Data are expressed as number or mean ± standard deviation. BCLC; Barcelona Clinic Liver Cancer, ECOG PS; Eastern Cooperative Oncology Group Performance Status, HCC; hepatocellular carcinoma, RFA; radiofrequency ablation, PEI; percutaneous ethanol injection, AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, LDH; lactose dehyrogenase, AFP; alpha-fetoprotein, DCP; des-γ-carboxy prothrombin, BW; body weight, a; unpaired t test, b; Fisher's exact test, c; missing values, n=9, d; missing values, n=8, e; missing values, n=19, f; missing values, n=1, g; missing values, n=5, h; missing values, n=1, i; missing values, n=1, j; missing values, n=9, k; missing values, n=17
Cumulative overall survival (OS) in the aged group (n=179) and the control group (n=279). The median OS intervals were 9.7 months (95% confidence interval [CI], 7.5-12.0 months) in the aged group and 8.2 months (95% CI, 6.9-9.6 months) in the control group (P=0.641).
Cumulative progression free survival (PFS) in the aged group (n=179) and the control group (n=279). The median PFS intervals were 3.8 months (95% CI, 2.9-4.6 months) in the aged group and 3.3 months (95% CI, 3.0-3.6 months) in the control group (P=0.068).
Treatment duration, treatment discontinuation rate and dose reduction rate in the two groups
In patients with initial dose of sorafenib of 800 mg/day (n=51 in the aged group and n=132 in the control group), the median treatment durations were 3.1 months (range, 0.1-30.0 months) in the aged group and 3.2 months (range, 0.2-40.4 months) in the control group (P=0.629). Treatment discontinuation rates were 90.2% (46/51) in the aged group and 92.4% (122/132) in the control group (P=0.764). Dose reduction rates were 62.7% (32/51) in the aged group and 57.6% (76/132) in the control group (P=0.616).
In patients with reduced initial dose of sorafenib (n=128 in the aged group and n=147 in the control group), the median treatment durations were 3.3 months (range, 0.1-32.1 months) in the aged group and 3.8 months (range, 0.1-29.0 months) in the control group (P=0.381). Treatment discontinuation rates were 89.8% (115/128) in the aged group and 89.1% (131/147) in the control group (P>0.999). Dose reduction rates were 42.2% (54/128) in the aged group and 29.9% (44/147) in the control group (P=0.043), suggesting that aged group patients with reduced initial dose of sorafenib had significantly higher dose reduction rate than control group patients.
Treatment tumor response rate
The best treatment tumor response rates during follow-up period were: CR in 4 patients, PR in 23, SD in 61, PD in 50 and not evaluated (NE) in 41, respectively, in the aged group; CR in 2 patients, PR in 38, SD in 97, PD in 98 and NE in 44, respectively, in the control group. The objective response rates (ORRs) were 15.1% (27 out of 179 patients) in the aged group and 14.3% (40 out of 279 patients) in the control group (P=0.892). The disease control rates (DCRs) were 49.2% (88 out of 179 patients) in the aged group and 49.1% (137 out of 279 patients) in the control group (P>0.999). (Table 2)
Best treatment response rate in the aged group and the control group.
| Aged group | Control group | P valuea | |
|---|---|---|---|
| Complete response | 4 (2.2%) | 2 (0.7%) | |
| Partial response | 23 (12.8%) | 38 (13.6%) | |
| Stable disease | 61 (34.1%) | 97 (34.8%) | |
| Progressive disease | 50 (27.9%) | 98 (35.1%) | |
| Unavailable response | 41 (22.9%) | 44 (15.8%) | |
| Disease control rate | 88/179 (49.2%) | 137/279 (49.1%) | >0.999 |
| Objective response rate | 27/179 (15.1%) | 40/279 (14.3%) | 0.892 |
a; Fisher's exact test
Treatment response according to Edmondson grade
In HCC patients with Edmondson grade I (n=29; n=16 in the aged group and n=13 in the control group), the ORRs were 18.8% (3/16) in the aged group and 30.8% (4/13) in the control group (P=0.667), while the DCRs were 62.5% (10/16) in the aged group and 76.9% (10/13) in the control group (P=0.454). In HCC patients with Edmondson grade II (n=37; n=13 in the aged group and n=24 in the control group), the ORRs were 23.1% (3/13) in the aged group and 4.2% (1/24) in the control group (P=0.115), while the DCRs were 46.2% (6/13) in the aged group and 29.2% (7/24) in the control group (P=0.472). In HCC patients with Edmondson grade III (n=23; n=4 in the aged group and n=19 in the control group), the ORRs were 25.0% (1/4) in the aged group and 5.3% (1/19) in the control group (P=0.324), while the DCRs were 25.0% (1/4) in the aged group and 36.8% (7/19) in the control group (P>0.999).
Univariate and multivariate analyses of factors contributing to OS
In the univariate analysis, Child-Pugh classification (P<0.001), BCLC stage (P<0.001), portal vein invasion (P<0.001), extrahepatic spread (P<0.001), EOCG PS (P=0.001), AST ≥50 IU/L (P<0.001), ALP ≥400 IU/L (P<0.001), GGT ≥90 IU/L (P<0.001), lactose dehydrogenase (LDH) ≥240 IU/L (P<0.001), alpha-fetoprotein (AFP) ≥200 ng/mL (P<0.001) and des-γ-carboxy prothrombin (DCP) ≥700 mAU/mL (P<0.001) were significant factors contributing to OS. (Table 3) In the multivariate analysis involving 12 factors with P<0.1 in the univariate analyses, Child-Pugh classification (P=0.005), causes of liver disease (viral) (P=0.001), portal vein invasion (P=0.007), extrahepatic spread (P=0.002), GGT ≥90 IU/L (P<0.001), LDH ≥240 IU/L (P<0.001), AFP ≥200 ng/mL (P<0.001) and DCP ≥700 mAU/mL (P=0.002) were significant factors contributing to OS. The hazard ratios (HRs) and 95% CIs for these factors are detailed in table 4.
Univariate and multivariate analyses of factors contributing to PFS
In the univariate analysis, Child-Pugh classification (P=0.002), BCLC stage (P=0.023), portal vein invasion (P=0.005), AST ≥50 IU/L (P=0.002), ALP ≥400 IU/L (P=0.001), GGT ≥90 IU/L (P<0.001), LDH ≥240 IU/L (P<0.001), AFP ≥200 ng/mL (P<0.001) and DCP ≥700 mAU/mL (P<0.001) were significant factors associated with PFS. (Table 3) In the multivariate analysis involving 10 factors with P<0.1 the univariate analysis, Child-Pugh classification (P=0.031), GGT ≥90 IU/L (P=0.008), LDH ≥240 IU/L (P=0.043), AFP ≥200 ng/mL (P=0.009) and DCP ≥700 mAU/mL (P=0.009) were significant factors linked to PFS. The HRs and 95% CIs for these factors are detailed in table 4.
Causes of death in the two groups
One hundred and twenty seven patients (70.9%) in the aged group and 215 (77.1%) patients in the control group died during the follow-up period. The causes of death in the aged group were as follows: HCC progression (90 patients); liver failure (19 patients); miscellaneous (15 patients); and unknown causes (3 patients). In the control group the causes of death were: HCC progression (178 patients); liver failure (13 patients); miscellaneous (17 patients); and unknown causes (7 patients).
Univariate analyses of factors contributing to overall survival (OS) and progression free survival (PFS).
| OS | PFS | ||
|---|---|---|---|
| Variables | n | P valuea | P valuea |
| Age (≥75 years), yes/no | 179/279 | 0.641 | 0.068 |
| Gender (male), yes/no | 369/89 | 0.353 | 0.828 |
| Child-Pugh classification, A/B | 374/84 | <0.001 | 0.002 |
| BCLC stage, B/C | 163/295 | <0.001 | 0.023 |
| Causes of liver disease (viral), yes/no | 337/121 | 0.054 | 0.134 |
| Portal vein invasion, yes/no | 107/351 | <0.001 | 0.005 |
| Extrahepatic spread, yes/no | 195/263 | <0.001 | 0.394 |
| EOCG PS 0, yes/no | 346/112 | 0.001 | 0.291 |
| AST (≥50 IU/L), yes/no | 251/207 | <0.001 | 0.002 |
| ALT (≥50 IU/L), yes/no | 206/252 | 0.270 | 0.346 |
| ALP (≥400 IU/L), yes/nob | 220/229 | <0.001 | 0.001 |
| GGT (≥90 IU/L), yes/noc | 209/241 | <0.001 | <0.001 |
| LDH (≥240 IU/L), yes/nod | 202/237 | <0.001 | <0.001 |
| Platelets (≥12×104/mm3), yes/noe | 224/233 | 0.259 | 0.658 |
| AFP (≥200 ng/mL), yes/nof | 211/238 | <0.001 | <0.001 |
| DCP (≥700 mAU/mL), yes/nog | 217/224 | <0.001 | <0.001 |
| Initial dose of sorafenib (800 mg/day), yes/no | 183/275 | 0.950 | 0.788 |
| Initial dose of sorafenib based on BW ≥8.4 mg/kg/day, yes/no | 222/236 | 0.470 | 0.187 |
BCLC; Barcelona Clinic Liver Cancer, ECOG PS; Eastern Cooperative Oncology Group Performance Status, AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, LDH; lactose dehyrogenase, AFP; alpha-fetoprotein, DCP; des-γ-carboxy prothrombin, BW; body weight, a, log-rank test, b; missing values, n=9, c; missing values, n=8, d; missing values, n=19, e; missing values, n=1, f; missing values, n=9, g; missing values, n=17
Multivariate analyses of factors contributing to overall survival (OS) and progression free survival (PFS).
| OS | PFS | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P valuea | HR | 95% CI | P valuea |
| Age (≥75 years) | 0.926 | 0.746-1.151 | 0.490 | |||
| Child-Pugh classification, A/B | 0.658 | 0.491-0.882 | 0.005 | 0.741 | 0.564-0.972 | 0.031 |
| BCLC stage, B/C | 0.952 | 0.632-1.434 | 0.815 | 0.840 | 0.660-1.070 | 0.158 |
| Causes of liver disease (viral) | 0.628 | 0.472-0.836 | 0.001 | |||
| Portal vein invasion | 0.657 | 0.485-0.891 | 0.007 | 0.947 | 0.719-1.248 | 0.699 |
| Extrahepatic spread | 0.599 | 0.433-0.828 | 0.002 | |||
| EOCG PS, 0/1,2 | 0.785 | 0.581-1.060 | 0.115 | |||
| AST (≥50 IU/L) | 1.140 | 0.858-1.514 | 0.368 | 1.025 | 0.809-1.298 | 0.840 |
| ALP (≥400 IU/L) | 0.960 | 0.740-1.246 | 0.760 | 1.008 | 0.799-1.271 | 0.946 |
| GGT (≥90 IU/L) | 0.609 | 0.472-0.786 | <0.001 | 0.729 | 0.578-0.921 | 0.008 |
| LDH (≥240 IU/L) | 0.558 | 0.434-0.719 | <0.001 | 0.794 | 0.635-0.992 | 0.043 |
| AFP (≥200 ng/mL) | 0.601 | 0.474-0.763 | <0.001 | 0.749 | 0.604-0.930 | 0.009 |
| DCP (≥700 mAU/mL) | 0.676 | 0.529-0.863 | 0.002 | 0.766 | 0.616-0.952 | 0.016 |
HR; hazard ratio, CI; confidence interval, BCLC; Barcelona Clinic Liver Cancer, ECOG PS; Eastern Cooperative Oncology Group Performance Status, AST; aspartate aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, LDH; lactose dehyrogenase, AFP; alpha-fetoprotein, DCP; des-γ-carboxy prothrombin, a, Cox proportional hazard model.
Serious adverse events (SAEs)
Grade 3 or more SAEs as defined by CTCAE were observed in 51 patients (28.5%) in the elderly group and 69 patients (24.7%) in the control group (P=0.385): rash (5.7% [10/175] vs. 2.2% [6/274], P=0.066), hand-foot syndrome (6.9% [12/175] vs. 4.4% [12/275], P=0.285), diarrhea (2.3% [4/174] vs. 2.2% [6/277], P>0.999), fever (1.1% [2/177] vs. 1.4% [4/278], P>0.999), fatigue (4.0% [7/175] vs. 2.5% [7/276], P=0.412), hypertension (0.6% [1/175] vs. 1.8% [5/276], P=0.412), gastrointestinal bleeding (1.7% [3/175] vs. 1.4% [4/276], P>0.999), liver damage (9.7% [17/175] vs. 13.0% [36/276], P=0.299) and lung injury (4.0% [7/174] vs. 0% [0/276], P=0.001). (Table 5)
Treatment related serious adverse events of grade 3 or more in the aged group and the control group.
| Adverse events | Aged group | Control group | |
|---|---|---|---|
| Grade 3 or more SAEs | Grade 3 or more SAEs | P valuea | |
| Overall | 51/179 (28.5%) | 69/279 (24.7%) | 0.385 |
| Rashb | 10/175 (5.7%) | 6/274 (2.2%) | 0.066 |
| Hand foot syndromec | 12/175 (6.9%) | 12/275 (4.4%) | 0.285 |
| Diarrhead | 4/174 (2.3%) | 6/277 (2.2%) | >0.999 |
| Fevere | 2/177 (1.1%) | 4/278 (1.4%) | >0.999 |
| Fatiguef | 7/175 (4.0%) | 7/276 (2.5%) | 0.412 |
| Hypertensiong | 1/175 (0.6%) | 5/276 (1.8%) | 0.412 |
| Gastrointestinal bleedingh | 3/175 (1.7%) | 4/276 (1.4%) | >0.999 |
| Liver damagei | 17/175 (9.7%) | 36/276 (13.0%) | 0.299 |
| Lung injuryj | 7/174 (4.0%) | 0/276 (0%) | 0.001 |
SAEs; serious adverse events, a; Fisher's exact test, b; missing values, n=9, c; missing values, n=8, d; missing values, n=7, e; missing values, n=3, f; missing values, n=7, g; missing values, n=7, h; missing values, n=7, i; missing values, n=7, j; missing values, n=8
Subgroup analyses according to Child-Pugh classification
In patients with Child-Pugh A (n=152 in the aged group and n=222 in the control group), the median OS intervals were 11.3 months (95% CI, 9.0-13.6 months) in the aged group and 9.3 months (95% CI, 7.0-11.7 months) in the control group (P=0.690). The median PFS intervals were 4.2 months (95% CI, 3.5-5.0 months) in the aged group and 3.3 months (95% CI, 3.0-3.6 months) in the control group (P=0.047), suggesting that the aged group patients with Child-Pugh A had significantly higher PFS rate compared with the control group. In patients with Child-Pugh B (n=27 in the aged group and n=57 in the control group), the median OS intervals were 4.9 months (95% CI, 2.8-7.0 months) in the aged group and 4.4 months (95% CI, 3.3-5.4 months) in the control group (P=0.704). The median PFS intervals were 1.6 months (95% CI, 0.2-3.0 months) in the aged group and 2.6 months (95% CI, 1.3-3.8 months) in the control group (P=0.554).
Subgroup analyses according to BCLC stage
In patients with BCLC stage B (n=63 in the aged group and n=100 in the control group), the median OS intervals were 14.6 months (95% CI, 9.6-19.7 months) in the aged group and 15.0 months (95% CI, 11.9-18.0 months) in the control group (P=0.530). The median PFS intervals were 3.8 months (95% CI, 2.6-5.1 months) in the aged group and 4.2 months (95% CI, 3.2-5.3 months) in the control group (P=0.768). In patients with BCLC stage C (n=116 in the aged group and n=179 in the control group), the median OS intervals were 7.9 months (95% CI, 5.4-10.3 months) in the aged group and 6.1 months (95% CI, 5.0-7.2 months) in the control group (P=0.269). The median PFS intervals were 3.6 months (95% CI, 2.4-4.7 months) in the aged group and 2.9 months (95% CI, 2.4-3.3 months) in the control group (P=0.046), indicating that the aged group patients with BCLC stage C had significantly higher PFS rate than the control group patients.
Subgroup analyses according to initial dose of sorafenib
We further analysed clinical outcomes according to initial dose of sorafenib since the proportion of patients with initial dose of sorafenib of 800 mg/day in the aged group was significantly lower than that in the control group. In patients with initial dose of sorafenib of 800 mg/day (n=51 in the aged group and n=132 in the control group), the median OS intervals were 12.0 months (95% CI, 7.8-16.3 months) in the aged group and 7.1 months (95% CI, 5.3-8.9 months) in the control group (P=0.332). The median PFS intervals were 4.2 months (95% CI, 3.3-5.1 months) in the aged group and 3.2 months (95% CI, 2.9-3.5 months) in the control group (P=0.079). In patients with reduced initial dose of sorafenib (n=128 in the aged group and n=147 in the control group), the median OS intervals were 9.3 months (95% CI, 7.1-11.6 months) in the aged group and 9.2 months (95% CI, 6.9-11.6 months) in the control group (P=0.850). The median PFS intervals were 3.6 months (95% CI, 2.7-4.5 months) in the aged group and 3.4 months (95% CI, 2.9-3.9 months) in the control group (P=0.253).
Baseline characteristics and clinical outcomes in the aged and control groups after propensity score matching
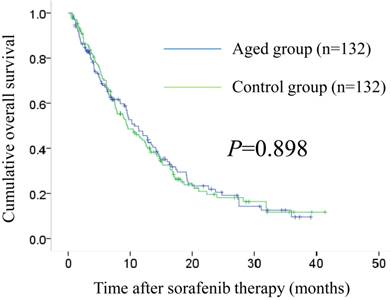
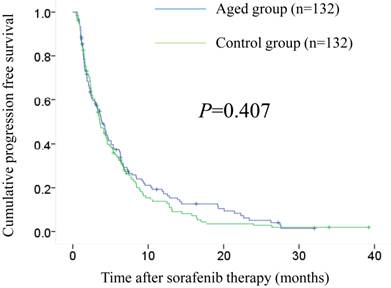
Baseline characteristics in the two groups (aged group: n=132, control group: n=132) after propensity score matching are demonstrated in Table 6. In all analysed variables, no significant differences were observed. The median OS intervals were 10.7 months (95% CI, 8.0-13.4 months) in the aged group and 9.5 months (95% CI, 6.6-12.4 months) in the control group (P=0.898). (Fig. 3) The median PFS intervals were 3.8 months (95% CI, 2.6-5.1 months) in the aged group and 3.8 months (95% CI, 2.9-4.8 months) in the control group (P=0.407). (Fig. 4)
Clinical outcome in the two groups according to different cut-off age
When using cut-off age of 80 years (n=81 in patients aged ≥80 years and n=377 in patients aged <80 years), the median OS intervals were 9.3 months (95% CI, 7.4-11.3 months) in the aged group and 8.8 months (95% CI, 7.5-10.1 months) in the control group (P=0.827), while the median PFS intervals were 3.8 months (95% CI, 2.2-5.4 months) in the aged group and 3.4 months (95% CI, 3.1-3.7 months) in the control group (P=0.668). When using cut-off age of 70 years (n=249 in patients aged ≥70 years and n=209 in patients aged <70 years), the median OS intervals were 10.1 months (95% CI, 8.5-11.8 months) in the aged group and 7.7 months (95% CI, 6.2-9.2 months) in the control group (P=0.950), whereas the median PFS intervals were 3.7 months (95% CI, 3.1-4.4 months) in the aged group and 3.1 months (95% CI, 2.8-3.4 months) in the control group (P=0.046).
Cumulative overall survival (OS) in the aged group (n=132) and the control group (n=132) after propensity score matching. The median OS intervals were 10.7 months (95% CI, 8.0-13.4 months) in the aged group and 9.5 months (95% CI, 6.6-12.4 months) in the control group (P=0.898).
Cumulative progression free survival (PFS) in the aged group (n=132) and the control group (n=132) after propensity score matching. The median PFS intervals were 3.8 months (95% CI, 2.9-4.8 months) in the aged group and 3.6 months (95% CI, 2.9-4.3 months) in the control group (P=0.407).
Baseline characteristics between the aged group and the control group after propensity score matching.
| Variables | Aged group (n=132) | Control group (n=132) | P value |
|---|---|---|---|
| Age (years) | 79.4 ± 3.3 | 64.1 ± 6.2 | <0.001a |
| Gender, male/female | 101 / 31 | 108 / 24 | 0.363b |
| Child-Pugh A / B | 110 / 22 | 115 / 17 | 0.488b |
| Causes of liver disease | |||
| B/C/non B and non C/B and C | 6 / 85 / 41 / 0 | 7 / 85 / 40 / 0 | >0.999b |
| BCLC stage B/C | 48/84 | 42/90 | 0.516b |
| ECOG PS, 0/1/2 | 94/35/3 | 95/34/3 | >0.999b |
| AST (IU/L) | 65.7 ± 64.6 | 60.8 ± 35.6 | 0.296a |
| ALT (IU/L) | 45.8 ± 38.1 | 48.0 ± 35.2 | 0.622a |
| Total bilirubin (mg/dL) | 0.93 ± 0.45 | 0.89 ± 0.47 | 0.422a |
| Albumin (g/dL) | 3.50 ± 0.46 | 3.54 ± 0.50 | 0.458a |
| ALP (IU/L)c | 443.9 ± 238.0 | 469.3 ± 360.6 | 0.505a |
| GGT (IU/L)d | 112.6 ± 129.0 | 143.0 ± 164.6 | 0.101a |
| LDHe | 248.0 ± 76.1 | 252.4 ± 101.5 | 0.696a |
| Prothrombin time (%) | 85.7 ± 18.2 | 88.1 ± 16.7 | 0.261a |
| Platelets (×104/mm3)f | 13.1 ± 6.0 | 13.9 ± 6.3 | 0.285a |
| AFP (ng/mL)g | 6779 ± 26576 | 15102 ± 67697 | 0.191a |
| DCP (mAU/mL)h | 13873 ± 75599 | 21164 ± 80945 | 0.457a |
| Initial dose of sorafenib (mg/day) | |||
| 800mg/600mg/400mg/200mg | 44 / 0 / 82 / 6 | 48 / 2 / 76 / 6 | 0.593b |
Data are expressed as number or mean ± standard deviation. BCLC; Barcelona Clinic Liver Cancer, ECOG PS; Eastern Cooperative Oncology Group Performance Status, AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, LDH; lactose dehyrogenase, AFP; alpha-fetoprotein, DCP; des-γ-carboxy prothrombin, a; unpaired t test, b; Fisher's exact test, c; missing values, n=7, d; missing values, n=6, e; missing values, n=11, f; missing values, n=1, g; missing values, n=2, h; missing values, n=8
Discussion
To the best of our knowledge, this is the largest study comparing clinical outcomes and safety between aged and non-aged HCC patients treated with sorafenib. [29-32] Current guidelines for the management of HCC do not satisfy strategies according to age. [2, 3] Few studies assessed the clinical outcomes in HCC patients treated with sorafenib based on age. [29, 30, 32] With the aging population, HCC in the elderly represents a significant health burden. In Japan, the proportion of elderly patients with HCC and their average age is increasing. These trends have led to a rising demand in our country for investigations related to the outcome of sorafenib therapy in elderly HCC patients: hence the reasons for the current comparative study.
In our results, the aged group patients had comparable OS rate, PFS rate, DCR and ORR as compared with the control group patients. The difference in the two groups in terms of sorafenib related SAEs of grade 3 or more did not reach significance except for the development of lung injury. In subgroup analyses, in patients with Child-Pugh A and in those with BCLC-C, the median PFS intervals in the aged group were significantly longer than those in the control group and in all other subgroup analyses, no significant difference in the two groups was observed in terms of OS and PFS. Furthermore, in the propensity score matched cohorts, no significant difference in the two groups was found in terms of OS and PFS and when using different cut-off age (80 years or 70 years), and similar results were obtained. Systemic anticancer therapy in aged patients with malignancies tends to be viewed with skepticism owing to the greater frequency of treatment related SAEs in aged than in younger patients. However, our results suggest that aged HCC patients treated with sorafenib had comparable prognosis and well tolerable drug related toxicity compared with younger HCC patients treated with sorafenib, which are in line with results reported by Jo, et al. [40] Since our study regarding effect of sorafenib on clinical outcome stratified by age is the largest that has been published so far and includes unselected cases by fourteen centers scattered throughout in Japan, our study results faithfully reflect what actually occurs in clinical practice.
The prevalence of aged subjects in our population was higher than in other previous reports. [11-26] This was possibly due to a lower proportion of patients with HBV infection who often develop HCC in younger age as compared with those with HCV infection and the shift towards older ages of HCC occurrence in Japan. The proportion of male patients in the aged group was almost significantly lower than that in the control group (P=0.053). This may have been associated with a larger female elderly population because of their longer life expectancy. Furthermore, the observations of significantly lower hemoglobin level, lower BW and higher serum creatinine levels in the aged group of this study may well reflect the actual situations in aged HCC patients in clinical practice.
In aged group, the difference in patients with initial dose of sorafenib of 800 mg/day and those with reduced dose of sorafenib did not reach significance in terms of OS (P=0.445) and PFS (P=0.691). Iavarone M, et al reported that the effectiveness of half-dosed sorafenib may have implications for tailored therapy in HCC patients. [41] Since in aged HCC patients, high frequency of sorafenib related SAEs were expected when given an initial dose of sorafenib of 800 mg/day, leading to treatment discontinuation or interruptions, reduced initial dose of sorafenib can be considered in elderly patients for avoiding SAEs although further examination is needed to confirm these results.
As described earlier, in patients with Child-Pugh A and in those with BCLC-C, PFS intervals in the aged group were significantly longer than that in the control group. These findings might be associated with the slower cancer growth in aged patients or to a higher susceptibility of vasculature to antiangiogenic agents in aged patients. [42] On the other hand, it is of interest that GGT level was the significant predictor linked to both OS and PFS in our multivariate analysis. Several studies reported that a high level of GGT was related to a higher incidence of HCC progression, which are in line with our results. [43, 44] As for other significant predictors observed in our multivariate analyses, our study results were consistent with previous reports. [7, 9, 29, 30, 32, 38]
It is noteworthy that sorafenib related lung injury of grade 3 or more occurred in 7 aged patients, whereas no such lung injury was observed in the control group and 2 out of 7 died due to respiratory failure. The reasons for these results are unclear, however, during sorafenib therapy, caution should be exercised for lung injury especially in aged patients.
Although several studies have examined the predictive factors linked to the response to sorafenib in advanced HCC patients, the factors predicting a favorable response remained unclear. [45] However, recent studies demonstrated that polymorphisms of VEGF and its receptor genes may regulate angiogenesis and tumor growth and they may influence OS and PFS in HCC patients undergoing sorafeinb therapy. [46, 47] In addition, Lee, et al. reported that differences in the incidence of sorafenib-related hand foot skin reaction in HCC patients treated with sorafenib may have been caused by ethnic differences in genetic polymorphisms of the tumor necrosis factor-alpha, VEGF, and uridine diphosphate glucuronosyltransferase 1 family-polypeptide A9 genes. [48] Although such polymorphisms were not tested in the current analyses, these may be associated with clinical outcome in elderly HCC patients treated with sorafenib and in this regard, further investigations will be required.
This study included several limitations. First, our study is a retrospective observational study, although the major strength of our study is a large sample size. Second, the initial sorafenib dose varied among individual patients, leading to bias. Third, various therapies were performed after discontinuation of sorafenib in some patients, also potentially leading to bias in concerning their OS. Lastly, our study cohort is limited to Japanese patients with relatively low BW in contrast to patients in Western countries. Hence, our results cannot be extended to patients with other racial cohorts and caution should be exercised when interpreting these results. Thus, further prospective studies will be necessary. However, our results indicated that in HCC patients treated with sorafenib, life expectancy, disease progression, treatment efficacy and SAEs are unaffected by age over 75 years. In conclusion, aged HCC patients treated with sorafenib had comparable clinical outcomes compared with younger HCC patients treated with sorafenib. Sorafenib therapy for HCC should not be determined solely based on age.
Acknowledgements
The authors would like to thank all the staff in the Japanese Red Cross Liver Study group for their valuable support.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
1. Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78:113-124
2. Livraghi T, Mäkisalo H, Line PD. Treatment options in hepatocellular carcinoma today. Scand J Surg. 2011;100:22-29
3. de Lope CR, Tremosini S, Forner A. et al. Management of HCC. J Hepatol. 2012;56(Suppl 1):S75-S87
4. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273
5. Hankey BF, Ries LA, Kosary CL. et al. Partitioning linear trends in age-adjusted rates. Cancer Causes Control. 2000;11:31-35
6. Cho SJ, Yoon JH, Hwang SS, Lee HS. Do young hepatocellular carcinoma patients with relatively good liver function have poorer outcomes than elderly patients? J Gastroenterol Hepatol. 2007;22:1226-1231
7. Llovet JM, Ricci S, Mazzaferro V. et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390
8. Abou-Alfa GK, Schwartz L, Ricci S. et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293-4300
9. Cheng AL, Kang YK, Chen Z. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34
10. Fentiman IS, Tirelli U, Monfardini S. et al. Cancer in the elderly: why so badly treated? Lancet. 1990;335(8696):1020-2
11. Takahashi H, Mizuta T, Kawazoe S. et al. Efficacy and safety of radiofrequency ablation for elderly hepatocellular carcinoma patients. Hepatol Res. 2010;40:997-1005
12. Bove A, Bongarzoni G, Di Renzo RM, Marsili L, Chiarini S, Corbellini L. Efficacy and safety of ablative techniques in elderly HCC patients. Ann Ital Chir. 2011;82:457-463
13. Massarweh NN, Park JO, Yeung RS, Flum DR. Comparative assessment of the safety and effectiveness of radiofrequency ablation among elderly medicare beneficiaries with hepatocellular carcinoma. Ann Surg Oncol. 2012;19:1058-1065
14. Hiraoka A, Michitaka K, Horiike N. et al. Radiofrequency ablation therapy for hepatocellular carcinoma in elderly patients. J Gastroenterol Hepatol. 2010;25:403-407
15. Kondo K, Chijiiwa K, Funagayama M, Kai M, Otani K, Ohuchida J. Hepatic resection is justified for elderly patients with hepatocellular carcinoma. World J Surg. 2008;32:2223-2229
16. Oishi K, Itamoto T, Kobayashi T. et al. Hepatectomy for hepatocellular carcinoma in elderly patients aged 75 years or more. J Gastrointest Surg. 2009;13:695-701
17. Huang J, Li BK, Chen GH. et al. Long-term outcomes and prognostic factors of elderly patients with hepatocellular carcinoma undergoing hepatectomy. J Gastrointest Surg. 2009;13:1627-1635
18. Yau T, Yao TJ, Chan P. et al. The outcomes of elderly patients with hepatocellular carcinoma treated with transarterial chemoembolization. Cancer. 2009;115:5507-5515
19. Mirici-Cappa F, Gramenzi A, Santi V. et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: a 20-year multicentre experience. Gut. 2010;59:387-396
20. Yanaga K, Kanematsu T, Takenaka K, Matsumata T, Yoshida Y, Sugimachi K. Hepatic resection for hepatocellular carcinoma in elderly patients. Am J Surg. 1988;155:238-241
21. Fortner JG, Lincer RM. Hepatic resection in the elderly. Ann Surg. 1990;211:141-145
22. Yamamoto K, Takenaka K, Matsumata T. et al. Right hepatic lobectomy in elderly patients with hepatocellular carcinoma. Hepatogastroenterology. 1997;44:514-518
23. Ezaki T, Yukaya H, Ogawa Y. Evaluation of hepatic resection for hepatocellular carcinoma in the elderly. Br J Surg. 1987;74:471-473
24. Nagasue N, Chang YC, Takemoto Y, Taniura H, Kohno H, Nakamura T. Liver resection in the aged (seventy years or older) with hepatocellular carcinoma. Surgery. 1993;113:148-154
25. Takenaka K, Shimada M, Higashi H. et al. Liver resection for hepatocellular carcinoma in the elderly. Arch Surg. 1994;129:846-850
26. Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589-594
27. Nishikawa H, Osaki Y, Iguchi E. et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma: clinical outcome and safety in elderly patients. J Gastrointestin Liver Dis. 2012;21(4):397-405
28. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. Surgical resection for hepatocellular carcinoma: clinical outcomes and safety in elderly patients. Eur J Gastroenterol Hepatol. 2013;25(8):912-9
29. Wong H, Tang YF, Yao TJ. et al. The outcomes and safety of single-agent sorafenib in the treatment of elderly patients with advanced hepatocellular carcinoma (HCC). Oncologist. 2011;16(12):1721-8
30. Di Costanzo GG, Tortora R, De Luca M. et al. Impact of age on toxicity and efficacy of sorafenib-targeted therapy in cirrhotic patients with hepatocellular carcinoma. Med Oncol. 2013;30(1):446
31. Nishikawa H, Kimura T, Kita R, Osaki Y. Treatment for Hepatocellular Carcinoma in Elderly Patients: A Literature Review. J Cancer. 2013;4(8):635-643
32. Morimoto M, Numata K, Kondo M. et al. Higher discontinuation and lower survival rates are likely in elderly Japanese patients with advanced hepatocellular carcinoma receiving sorafenib. Hepatol Res. 2011;41(4):296-302
33. Kudo M, Izumi N, Kokudo N, et al; HCC Expert Panel of Japan Society of Hepatology. Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364
34. Miller AA, Murry DJ, Owzar K. et al. Phase I and Pharmacokinetic Study of Sorafenib in Patients with Hepatic or Renal Dysfunction. J Clin Oncol. 2009;27:1800-5
35. McCurry J. Japan battles with obesity. Lancet. 2007;369:451-52
36. Examination Committee of Criteria for 'Obesity Disease' in Japan; Japan Society for the Study of Obesity. New criteria for 'obesity disease' in Japan. Circ J. 2002;66:987-992
37. Shiwaku K, Anuurad E, Enkhmaa B, Kitajima K, Yamane Y. Appropriate BMI for Asian populations. Lancet. 2004;363:1077
38. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52-60
39. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17: 2265-2281. 1998
40. Jo M, Yasui K, Kirishima T. et al. Efficacy and safety of sorafenib in very elderly patients aged 80 years and older with advanced hepatocellular carcinoma. Hepatol Res. 2014 [Epub ahead of print]
41. Iavarone M, Cabibbo G, Piscaglia F, et al; SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54(6):2055-63
42. Pili R, Guo Y, Chang J, Nakanishi H, Martin GR, Passaniti A. Altered angiogenesis under lying age-dependent changes in tumor growth. J Natl Cancer Inst. 1994;86:1303-14
43. Yao D, Jiang D, Huang Z. et al. Abnormal expression of hepatoma specific gamma-glutamyl transferase and alteration of gamma-glutamyl transferase gene methylation status in patients with hepatocellular carcinoma. Cancer. 2000;88(4):761-9
44. Zhang JB, Chen Y, Zhang B. et al. Prognostic significance of serum gamma-glutamyl transferase in patients with intermediate hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol. 2011;23(9):787-93
45. Shao YY, Hsu CH, Cheng AL. Predictive biomarkers of antiangiogenic therapy for advanced hepatocellular carcinoma: where are we? Liver Cancer. 2013;2:93-107
46. Sampat KR, O'Neil B. Antiangiogenic therapies for advanced hepatocellular carcinoma. Oncologist. 2013;18(4):430-8
47. Scartozzi M, Faloppi L, Svegliati Baroni G. et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: The ALICE-1 study. Int J Cancer. 2014 [Epub ahead of print]
48. Lee JH, Chung YH, Kim JA. et al. Genetic predisposition of hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer. 2013;119(1):136-42
Author contact
![]() Corresponding author: Hiroki Nishikawa, Department of Gastroenterology and Hepatology, Osaka Red Cross Hospital, 5-30 Fudegasaki-cho, Tennoji-ku, Osaka 543-0027, Japan. Tel: +81-6-6774-5111; Fax: +81-6-6774-5131 E-mail: h-nishikawajrc.or.jp.
Corresponding author: Hiroki Nishikawa, Department of Gastroenterology and Hepatology, Osaka Red Cross Hospital, 5-30 Fudegasaki-cho, Tennoji-ku, Osaka 543-0027, Japan. Tel: +81-6-6774-5111; Fax: +81-6-6774-5131 E-mail: h-nishikawajrc.or.jp.

 Global reach, higher impact
Global reach, higher impact