Impact Factor
ISSN: 1837-9664
J Cancer 2015; 6(5):412-419. doi:10.7150/jca.11242 This issue Cite
Research Paper
Prognostic Significance of Lymphovascular Space Invasion in Epithelial Ovarian Cancer
1. Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
2. Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Received 2014-12-5; Accepted 2015-1-19; Published 2015-2-27
Abstract
Object: To assess the effects of lymphovascular space invasion (LVSI) on cancer recurrence and survival in patients with primary epithelial ovarian cancer.
Methods: A retrospective study was conducted of patients with stage I-IV primary epithelial ovarian cancer who underwent cytoreductive surgery. LVSI is defined as the presence of tumor cells within an endothelium-lined space, and the patients' pathologic slides were reevaluated by gynecological pathologists. Survival analysis was performed to compare risk factors.
Results: A total of 492 patients were included in the analysis. The incidence of LVSI was 58.5% in our cohort (288 cases), and it was significantly associated with advanced stage, high-grade serous histology, high grade, and lymph node metastasis (P<0.001). Kaplan-Meier analysis demonstrated that LVSI was only correlated with decreased PFS (5-year rate, 39% vs. 66%, P<0.001) and OS (5-year rate, 44% vs. 78%, P<0.001) in patients at early stage but not at advanced stage (5-year rate, PFS: 14% vs. 11%, P<0.001; OS: 29% vs. 29%, P=0.141). Multivariate analysis showed that LVSI remained a significant variable with PFS and OS in early-stage ovarian cancer (PFS: HR 2.29, 95% CI 1.45-3.57; OS: HR 2.20, 95% CI 1.59-3.44, both P<0.001).
Conclusion: LVSI is an independent predictor of progression and survival in patients with primary epithelial ovarian cancer at early stage but not at advanced stage.
Keywords: Lymphovascular space invasion, Ovarian carcinoma, Prognosis, Survival rate.
Introduction
Ovarian cancer is the leading cause of death from gynecologic cancer in the United States and is the country' fifth most common cause of cancer mortality in women.1 Cytoreductive surgery followed by combination platinum-based cytotoxic chemotherapy has long been considered to be an appropriate treatment, but disease-related mortality rates for advanced ovarian cancer remain considerably high.2,3 Prognostic factors predominantly include FIGO (the International Federation of Gynecology and Obstetrics) stage, residual tumor volume after cytoreductive surgery, BRCA mutation status and platinum sensitivity.4-7 However, the optimal treatment for this type of cancer has not yet been satisfactorily established because of the poor understanding of its natural history, histopathological characteristics and treatment response. More specific elucidation of the high-risk pathologic features of ovarian cancer is required to aid in the further stratification of patients into risk groups and to enable the additional refinement of adjuvant treatment recommendations.
Lymphovascular space invasion (LVSI) is defined as the presence of tumor cells inside of the capillary lumens of either the lymphatic or microvascular drainage systems within the primary tumor.8 Recently, it has been identified as an important risk factor in the progression of many neoplasms, especially in endometrial, cervical and lung cancers.9-12 In each of these cancer types, the presence of LVSI in tumors is associated with the increased risk of disease spread (especially nodal metastases), increased chance of disease recurrence, and decreased survival time. In ovarian cancer, several studies also suggested the presence of LVSI had linked to worse survival.13-17 But the relevant literatures regarding LVSI in ovarian cancer were limited and the reported incidence of LVSI covered a wide range from 17.5% to 83.5%.15-17 Moreover, unlike endometrial or cervical cancer, most patients with ovarian cancer present with advanced disease but LVSI is a histopathologic evidence of early tumor spread. Therefore, whether LVSI could be a prognostic factor in ovarian cancer is still in doubt. Previous investigators have suggested that the role of LVSI might be different stratified by lymph node status in urothelial carcinoma.18 But the relationship of LVSI and lymph node metastasis in ovarian cancer is still unclear. In the present study, we aimed to assess the prevalence of LVSI in our population with stage I-IV primary ovarian cancer after complete lymphadenectomy as well as its prognostic value based on lymph node status. In addition, we also examined the risk factors that are associated with the presence of LVSI.
Materials and methods
Study group
This study received the University Institutional Review Board approval from Peking Union Medical College. We retrospectively reviewed the medical charts of patients with epithelial ovarian cancer who were treated and received complete surgical staging or cytoreductive surgery between January 2004 and December 2010 at the Division of Gynecological Oncology within the Department of Obstetrics and Gynecology at Peking Union Medical College Hospital, Peking Union Medical College, Beijing, China. All patients with stage I and II tumors received complete staging surgeries. Women with advanced (stage III and IV) cancer underwent optimal cytoreduction, with the exception of those with unresectable tumors who received suboptimal operations and were left with macroscopic residual disease of greater than 1.0 cm in maximal diameter. The staging was made on the basis of final pathological findings according to the 2014 Federation of Obstetrics and Gynecology (FIGO) classification. Clinical and pathological variables included patient age, surgical procedure, and final pathological analysis (histology type and grade). Written informed consents had been achieved from all participants.
Exclusion criteria include: patients with a diagnosis of another primary invasive cancer within 2 years of their diagnosis of ovarian cancer; patients who had no follow-up after surgery, or had less than 6 months of follow up; patients who didn't have complete adjuvant chemotherapy when indicated or recommended. Patients died within 30 days of surgery due to severe operative complications were also excluded.
Histopathologic diagnosis of histotypes, grades and LVSI
All archived histopathology sides for hematoxylin and eosin (H&E) stain, of the cases that met inclusion criteria were pulled and examined by two gynecologic pathologists. The pathologists who evaluated the slides in this cohort were completely blinded to the clinical information.
The histotypes of epithelial cancer were defined by the 2014 WHO classification.19 For serous histology, we grouped them into high-grade serous carcinoma and low-grade serous carcinoma based on the MDACC two-tier grading system, in order to divided them into two histotypes.20,21 The histology grading was made into three categories according to conventional FIGO grading system.22 Clear cell carcinoma and undifferentiated carcinoma were not routinely graded.23 In the serous histology, the high-grade serous carcinoma using the two-tier system were grouped as FIGO grade 2 and 3 serous carcinomas, whereas low-grade serous carcinoma were grouped as grade 1 tumors, which was consistent to the previous literature.20
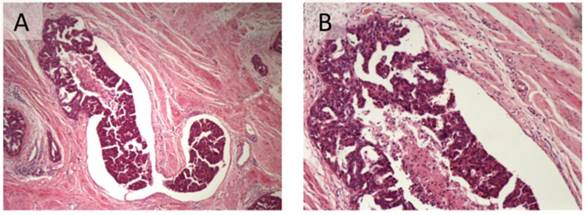
For the evaluation of tumoral LVSI, all the archived H&E stain sides were all re-examined. LVSI was diagnosed when viable tumor nests were observed within endothelial-lined spaces with or without intraluminal red cells or lymphocytes (Figure 1).24 No attempts were made to differentiate between lymphatic and vascular vessels because of the difficulty and lack of reproducibility that is associated with routine light microscopy.25 In addition, because the extent of LVSI was not shown to impact the survival outcome of epithelial ovarian cancer patients based on Matsuo's previous study and a dichotomized fashion was easier for clinical application,15 we only determined LVSI to be positive (present) or negative (absent). The percentage of inter-observer agreement was 91.9% with a kappa statistic of 0.832. A third reviewer evaluated the discordances.
Treatment and follow-up
Neoadjuvant chemotherapy was applied for one to three cycles before surgery with the aim of shrinking the cancer and making it easier to remove all of the cancer. After the completion of surgery, women with Grade 1, stage IA disease were observed without adjuvant chemotherapy; all other women with G2 or G3 or greater than stage IA disease received adjuvant chemotherapy containing platinum and taxane according to the 2014 National Comprehensive Cancer Network (NCCN) ovarian cancer guidelines.26 Carboplatin was calculated as the area under the curve = 6, and cisplatin was administered at 75 mg/m2. Paclitaxel was administered at 175 mg/m2. Patients who relapse within 6 months of completing first-line therapy have been classified as being “platinum resistant”.27
The median follow-up time was 43.5 months (range, 7 - 87 months). The patients were examined every 3 months for the first 2 years, every 6 months for the next 3 years and yearly thereafter. The date of recurrence was determined by clinical examination, imaging studies, and CA 125 levels. Progression-free status was defined as the time interval from the date of primary surgery to the date of documented first recurrence of disease. Overall survival (OS) was defined as the number of months from the date of primary surgery to the date of death. Survival was censored by the closeout date (May 1, 2014).
Statistical analyses
The chi-square test was used to compare the relevant risk factors of LVSI. A survival curve was constructed using the Kaplan-Meier method. As recurrence is a time-dependent event, cumulative risks for recurrence and death were also evaluated in survival analysis by life tables. The prognostic relevance of the clinicopathological parameters (age, stage, histotype, tumor grade, residual disease and LVSI) for PFS and OS were evaluated using the multivariate (Cox proportional hazard regression test) analysis with conditional forward method as appropriate expressed with hazard ratio (HR) and 95% confidence intervals (CIs). Statistical analyses were performed with SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). All the test were done by two-tailed analysis and results with P values <0.05 were considered statistically significant.
Results
Basic characteristics of patients
A total of 826 patients were enrolled during the study period. Of these 128 were excluded due to lost of follow-up, 68 due to another primary cancer within 2 years, 54 due to abjuration of adjuvant treatment or death in one month after operation, and 84 due to inadequate lymphadenectomy (the number of bilateral pelvic lymph nodes less than ten). Finally 492 patients were enrolled into our study.
Association of lymphovascular invasion with clinicopathologic characteristics in patients with epithelial ovarian cancer.
| Variables | Total | LVSI positive (N=288) | LVSI negative (N=204) | P |
|---|---|---|---|---|
| No. of cases (%) | No. of cases (%) | No. of cases (%) | ||
| Age (y) | 0.800 | |||
| <50 | 150 (30.5%) | 86 (57.3%) | 64 (42.7%) | |
| ≥50 | 342 (69.5%) | 202 (59.1%) | 140 (40.9%) | |
| Stage | <0.001 | |||
| I | 58 (11.8%) | 10 (17.2%) | 48 (82.8%) | |
| II | 96 (19.5%) | 34 (35.4%) | 62 (64.6%) | |
| III | 308 (62.6%) | 221 (71.8%) | 87 (28.2%) | |
| IV | 30 (6.1%) | 23 (76.7%) | 7 (23.3%) | |
| Histotype | <0.001 | |||
| High-grade serous | 334 (67.9%) | 224 (67.1%) | 110 (32.9%) | |
| Low-grade serous | 22 (4.5%) | 6 (27.3%) | 16 (72.7%) | |
| Clear cell | 54 (11.0%) | 38 (70.4%) | 16 (29.6%) | |
| Endometrioid | 48 (9.8%) | 10 (20.8%) | 38 (79.2%) | |
| Mucinous | 14 (2.9%) | 1 (7.1%) | 13 (92.9%) | |
| Squamous | 6 (1.2%) | 4 (66.7%) | 2 (33.3%) | |
| Undifferentiated | 14 (2.8%) | 6 (42.9%) | 8 (57.1%) | |
| Grade* | <0.001 | |||
| 1 | 38 (7.7%) | 8 (21.1%) | 30 (78.9%) | |
| 2 | 26 (5.3%) | 8 (30.8%) | 18 (69.2%) | |
| 3 | 360 (73.2%) | 246 (68.3%) | 114 (31.7%) | |
| Lymph node metastasis | <0.001 | |||
| Negative | 154 (31.3%) | 42 (27.3%) | 112 (72.7%) | |
| Positive | 248 (50.4%) | 177 (71.4%) | 71 (28.6%) | |
| Lymphadenectomy not performed | 90 (18.3%) | 69 (76.7%) | 21 (23.3%) | |
| Neo-adjuvant chemotherapy | 0.015 | |||
| Yes | 132 (26.8%) | 94 (71.2%) | 38 (28.8%) | |
| No | 360 (73.2%) | 194 (53.9%) | 166 (46.1%) | |
| Response to adjuvant chemotherapy | 0.125 | |||
| Platinum resistant | 40 (8.1%) | 28 (70.0%) | 12 (30.0%) | |
| Platinum sensitive | 452 (91.9%) | 260 (57.5%) | 192 (42.5%) | |
| Recurrence | <0.001 | |||
| Yes | 348 (70.7%) | 236 (67.8%) | 112 (32.2%) | |
| No | 144 (29.3%) | 52 (36.1%) | 92 (63.9%) |
Bold values indicate statistically significant differences.
*No grading for the histotypes of clear cell (n=27) and undifferentiated carcinoma (n=7).
LVSI: lymphovascular space invasion.
All the patients were Chinese. Patient characteristics are shown in Table 1. The median age of the study patients was 61 years old (range, 23-86 years). The most common histology was high-grade serous carcinoma (67.9%, 334/492). The majority of patients had advanced-stage disease (FIGO stages III-IV, 68.7%). The optimal cytoreduction rate was 85.4% (420/492). Four hundred and two patients underwent complete pelvic lymphadenectomy, of whom 296 (73.6%) underwent para-aortic lymphadenectomy at the same time. The median numbers of pelvic and para-aortic lymph nodes were 18 and 4 respectively. Ninety-four patients did not undergo lymphadenectomy due to the presence of advanced-stage disease (stage III-IV). The positive rate of lymph node metastasis was 50.4% in the whole cohort. One hundred and thirty-two patients (26.8%) received neoadjuvant chemotherapy. With the exception of 12 patients with stage IA disease, the other 480 patients had received adjuvant chemotherapy after operation.
The incidence of LVSI was 58.5% in our cohort (288 cases). It was significantly associated with advanced stage, high-grade serous histology, high grade, and lymph node metastasis (P<0.001, Table 1). The prevalence of LVSI in patients with neoadjuvant chemotherapy was higher since these patients were in advanced stage. Patients with LVSI-positive tumors were more likely to receive neoadjuvant chemotherapy (32.6% vs. 18.6%, P<0.001) than those without lymphovascular space invasion. No significant relationship was observed between LVSI and platinum sensitivity of adjuvant chemotherapy (32.6% vs. 18.6%, P<0.001). A total of 81.9% (236/288) of the patients who presented with LVSI experienced disease recurrence, while recurrence only occurred in 54.9% (112/204) of those without LVSI (P<0.001).
Association of LVSI and stage with clinical outcomes
During the follow-up period, which ranged from 7 to 116 months (median, 48 months), 71.5% (352/492) of the patients relapsed, and 51.0% (251/492) of them died of the disease.
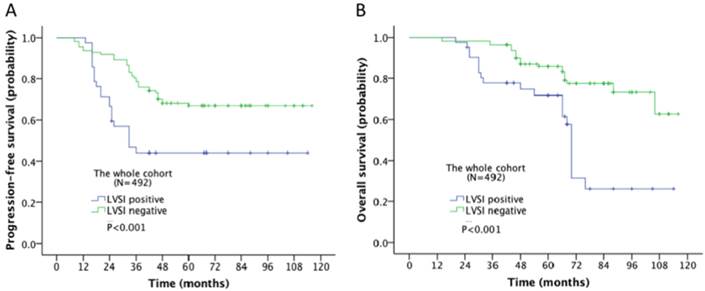
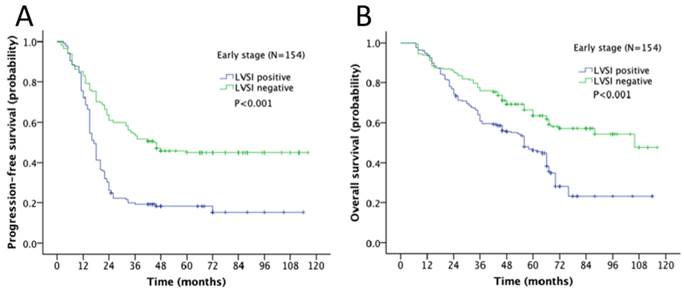
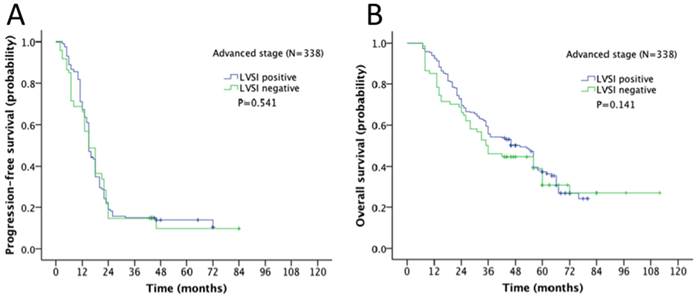
Survival outcome of the association of LVSI and different stages in ovarian cancer was examined. The 5-year progression-free survival (PFS) and overall survival (OS) rate for the whole cohort was 28% and 43% respectively. In Kaplan-Meier analysis, LVSI was significantly associated with decreased PFS (5-year rate, 18% vs. 44%, P<0.001; Fig. 2A) and decreased OS (31% vs. 58%, P<0.001; Fig. 2B). When stratified by FIGO stages, LVSI was correlated with decreased PFS (5-year rate, 39% vs. 66%, P<0.001; Fig. 3A) and OS (5-year rate, 44% vs. 78%, P<0.001; Fig. 3B) in patients at early stage. There was no significant association of LVSI status and PFS or OS in patients with advanced disease (5-year rate, PFS: 14% vs. 11%, P=0.541; OS: 29% vs. 29%, P=0.141; Fig. 4A-B).
Lymphovascular space invasion in epithelial ovarian cancer (A: H&E 40x; B: H&E 100x).
Kaplan-Meier survival curve for patients with epithelial ovarian cancer (A: progression-free survival curve; B: overall survival curve).
Kaplan-Meier survival curve for patients with early stage epithelial ovarian cancer (A: progression-free survival curve; B: overall survival curve).
Kaplan-Meier survival curve for patients with advanced stage epithelial ovarian cancer (A: progression-free survival curve; B: overall survival curve).
Multivariate analyses predicting survival in the whole cohort (N=492).
| Risk factor | PFS | OS | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age (y) | 0.351 | 0.391 | ||
| <50 | 1 | 1 | ||
| ≥50 | 1.13(0.77-1.72) | 1.234(0.75-1.86) | ||
| Stage | <0.001 | <0.001 | ||
| I- II | 1 | 1 | ||
| III-IV | 2.450(1.22-4.91) | 2.007(1.58-4.76) | ||
| Histotype | 0.635 | 0.874 | ||
| Not high-grade serous | 1 | 1 | ||
| High-grade serous | 1.48 (0.58 -2.50) | 1.23 (0.51-1.69) | ||
| Grade | 0.125 | 0.272 | ||
| 1 | 1 | 1 | ||
| 2 | 1.31 (0.75-2.04) | 1.05 (0.53-1.54) | ||
| 3 | 2.13 (0.86-3.72) | 1.39 (0.82-2.19) | ||
| Residual | <0.001 | <0.001 | ||
| ≤1cm | 1 | 1 | ||
| >1cm | 2.59(1.61-4.73) | 2.29 (1.53-3.67) | ||
| LVSI | 0.011 | 0.129 | ||
| Negative | 1 | 1 | ||
| Positive | 1.50 (1.22-1.96) | 1.38 (1.16-2.09) | ||
Bold values indicate statistically significant differences.
PFS: progression-free survival; OS: overall survival; LVSI: lymphovascular space invasion; HR: hazard ratio; CI: confidence interval.
We further performed the multivariate analysis to investigate the relevant prognostic factors of ovarian cancer (Table 2). In the whole cohort, the presence of LVSI remained as a prognostic indicator that was associated with worse PFS (HR 1.50, 95% CI 1.22-1.96, P=0.011) but not OS (HR 1.38, 95% CI 1.16-2.09, P=0.129). Moreover, the Cox regression analysis revealed that advanced stage and residual disease >1 cm after surgery were important prognostic factors associated with both PFS and OS (P<0.001).
Multivariate analysis showed the prognostic value of LVSI was different according to the stage of disease. In the 154 patients with disease at stage I-II, LVSI was correlated with PFS and OS (PFS: HR 2.29, 95% CI 1.45-3.57; OS: HR 2.20, 95% CI 1.59-3.44, both P<0.001; Table 3). Tumor stage (stage I vs. II) was also an independent factor influencing survival (PFS: HR 2.68, 95% CI 1.90-3.20; OS: HR 2.43, 95% CI 1.32-4.53, P=0.015; Table 3). Age, histotype and tumor grade had no significant impacts on survival. Because all 154 patients underwent optimal cytoreductive surgery, we did not include residual disease in the Cox models.
However, among the 338 patients at advanced stage, LVSI was not an independent prognostic factor in a multivariate analysis (PFS: HR 1.193, 95% CI 0.628-1.471, P=0.824; OS: HR 1.295, 95% CI 0.697-1.414, P=0.189; Table 4). FIGO stage and residual disease after operation continued to be significant predictors for both progression-free and overall survival (FIGO stage: PFS: HR 1.44, P=0.043; OS: HR 1.46, P=0.020; residual disease: PFS: HR 2.43, P=0.010; OS: HR 2.56, P=0.026). Age, histotype and tumor grade had no significant impacts on survival.
Multivariate analyses predicting survival in patients with epithelial ovarian cancer at early stage (N=154).
| Risk factor | PFS | OS | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age (y) | 0.059 | 0.069 | ||
| <50 | 1 | 1 | ||
| ≥50 | 1.26 (0.93-3.03) | 2.48 (0.93-6.57) | ||
| Stage | 0.028 | 0.015 | ||
| I | 1 | 1 | ||
| II | 2.68 (1.90-3.20) | 2.43 (1.32-4.53) | ||
| Histotype | 0.268 | 0.566 | ||
| Not high-grade serous | 1 | 1 | ||
| High-grade serous | 1.12 (0.81-1.48) | 1.23 (0.61-2.48) | ||
| Grade | 0.517 | 0.551 | ||
| 1 | 1 | 1 | ||
| 2 | 1.31 (0.62-1.90) | 1.21 (0.33-2.18) | ||
| 3 | 1.62 (0.78-2.04) | 1.62 (0.75-2.89) | ||
| LVSI | <0.001 | <0.001 | ||
| Negative | 1 | 1 | ||
| Positive | 2.29 (1.45-3.57) | 2.20 (1.59-3.44) | ||
Bold values indicate statistically significant differences.
PFS: progression-free survival; OS: overall survival; LVSI: lymphovascular space invasion; HR: hazard ratio; CI: confidence interval.
In order to confirm the effects of LVSI on lymph node metastasis in early stage ovarian cancer, we further investigated the upstage rate in patients with “apparent stage I disease” in the whole cohort. Seventy-two patients were diagnosed with tumors confined to one or two ovaries based on both pre-operative and intraoperative evaluations. Following the evaluation of final pathology results, the LVSI-positive rate was 25.0% (18/72) and 14 patients (19.4%) were found to have upstaged disease. Of the patients who were upstaged, six patients had lymph node metastasis and were upstaged to stage IIIA1. One patient had a positive lymph node and microscopic peritoneal implants outside the pelvis and was upstaged to stage IIIA2. In these 7 patients with lymph node metastasis, six cases were found to have LVSI-positive tumor. Therefore, in “apparent stage I disease”, patients with LVSI-positive epithelial ovarian cancer showed a significantly higher lymph node metastasis than those with LVSI-negative tumor (P<0.001). The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the presence of LVSI as a predictor of nodal metastases were 85.7%, 81.5%, 33.3%, 98.1%, and 67.2%, respectively.
Multivariate analyses predicting survival in patients with epithelial ovarian cancer at advanced stage (N=338).
| Risk factor | PFS | OS | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age (y) | 0.541 | 0.927 | ||
| <50 | 1 | 1 | ||
| ≥50 | 1.14 (0.75-1.74) | 1.02 (0.66-1.59) | ||
| Stage | 0.043 | 0.020 | ||
| III | 1 | |||
| IV | 1.44 (1.19-2.04) | 1.46 (1.21-2.74) | ||
| Histotype | 0.896 | 0.778 | ||
| Not high-grade serous | 1 | 1 | ||
| High-grade serous | 1.17 (0.65-1.86) | 0.938(0.60-1.46) | ||
| Grade | 0.366 | 0.594 | ||
| 1 | 1 | 1 | ||
| 2 | 1.05 (0.43-1.57) | 1.21 (0.60-1.44) | ||
| 3 | 1.63 (0.72-2.45) | 1.45 (0.76-2.01) | ||
| Residual disease | 0.010 | 0.026 | ||
| ≤1cm | 1 | 1 | ||
| >1cm | 2.43 (1.23-4.78) | 2.56 (1.12-5.85) | ||
| LVSI | 0.824 | 0.189 | ||
| Negative | 1 | 1 | ||
| Positive | 1.19 (0.63-1.47) | 1.29 (0.70-1.41) | ||
Bold values indicate statistically significant differences.
PFS: progression-free survival; OS: overall survival; LVSI: lymphovascular space invasion; HR: hazard ratio; CI; confidence interval.
Discussion
Ovarian cancer is the most common cause of death among gynecologic malignancies; thus, the identification of associated histological risk factors facilitates the selection of effective surgeries and adjuvant therapies by clinicians. The key findings in our study are that in epithelial ovarian cancer, (i) tumoral LVSI was correlated with biologically aggressive features, including advanced stage, high-grade serous carcinoma, high-grade disease and lymph node metastasis; (ii) tumoral LVSI was an independent predictor of progression-free and overall survival in patients at early stage, but not in those with advanced disease.
The infiltration of vascular and/or lymphatic structures by tumor cells is an important step in tumor dissemination, which probably links to the estrogen and vascular endothelial growth factor (VEGF) pathways; these pathways enable the tumor cells to gain access to distant organs.28-30 Therefore, the presence of LVSI likely correlates with a relatively higher tumor burden and more aggressive behavior.31 Our study showed that tumoral LVSI was significantly associated with advanced stage, high-grade disease and lymphatic metastasis, in accordance with the literature.15,32 Among the histotypes, high-grade serous carcinoma had the highest prevelance of LVSI positivity, followed by clear cell carcinoma. Though LVSI was reported to be related to chemotherapy response in breast cancer,33 we didn't observe any significantly different distribution of LVSI in platinum-resistant and -sensitive ovarian cancers (P=0.125).
LVSI is known to be a poor prognostic indicator of tumor progression and metastasis in many tumors including lung cancer, breast cancer, endometrial and cervical cancer, which usually present at an early stage.34-39 However, most of the ovarian cancers progressed fast and reached advanced stage when diagnosed. Thus the prognostic value of LVSI in ovarian cancer might not be similar to that in other neoplasms. Our data showed that LVSI was correlated with PFS (HR 1.504, 95% CI 1.218-1.957, P=0.011) but not OS. Consistent with our result, Matsuo et al. found that the presence of LVSI was not correlated with the overall survival either in the training set or validation cohort, but a worsened progression-free survival was observed in the training set group (HR 2.06, 95% CI 1.01-4.24, P=0.048).15 Qian et al. found that LVSI was associated with both progression-free survival and overall survival in Chinese patients, with a small sample size of 66 cases.32
However, none of the studies about LVSI in ovarian cancer had evaluated the prognostic value of LVSI according to the FIGO stage. Our multivariate analysis stratified by stage demonstrated that LVSI was only correlated with decreased PFS (5-year rate, 39% vs. 66%) and OS (5-year rate, 44% vs. 78%) in ovarian cancer patients at early stage but not in patients with advanced disease. Possible explanation is that LVSI is only a predictor in occult small-volume metastatic disease. When tumor is still confined to local region, LVSI in the primary tumor could indicate that tumor cells already invaded the surrounding tissues and that they are already circulating through the systemic pathway, which predicts a high risk of recurrence. However, after these disseminated cells develop into overt lymph node, omental or systematic metastases, LVSI is no longer predictive for progression. In agreement with our study, Kikuchi et al. also reported that in 1453 patients with upper urinary tract urothelial carcinoma, the inclusion of LVSI in the multivariate analysis improved the predictive accuracies for both disease recurrence and survival in patients with negative lymph nodes (PFS: HR 1.85, P <0.001; OS: HR 1.53, P=0.006) but not in node-positive patients.18 In contrast, LVSI is a risk factor in early-stage ovarian cancer. Matsuo evaluated 434 patients with stage I epithelial ovarian cancer. They showed that LVSI was associated with recurrence and patients with LVSI, stage I ovarian cancer who received six or more cycles of postoperative chemotherapy had better survival outcomes than those who received less than six cycles (PFS: P=0.052, OS: P=0.059).16 Although LVSI has not yet been mentioned as a risk factor of ovarian cancer in the NCCN guidelines, its importance deserves further evaluation.
Strength of our study is that the all specimens were reevaluated by two senior gynecological pathologists according to the diagnostic criteria. Second, our sample size was large and patients were enrolled in recent years (2004-2010); thus, these treatments could be representative of current practice patterns. The limitations of our study included the retrospective design and the single-center experience. In addition, we did not use immunohistochemical staining to identify the vessels because its use is controversial and not clinically practical.15,37 In our experience, the diagnosis of LVSI is possible with sufficient reliability when using hematoxylin and eosin.
In conclusion, tumoral LVSI invasion is an important histologic feature associated with more aggressive behavior in epithelial ovarian cancer. LVSI is a significant predictor of progression-free survival and overall survival in patients with ovarian cancer at early stage but not in those with advanced disease. The routine evaluation of tumoral LVSI in ovarian cancer is highly recommended in daily practice.
Acknowledgements
We thank the members of the Department of Pathology at Peking Union Medical College for their help with the pathologic review that was performed in this study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90
2. Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371-1382
3. Morrison J, Haldar K, Kehoe S, Lawrie TA. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2012;8:CD005343
4. Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70:209-262
5. Elattar A, Bryant A, Winter-Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev. 2011;10:CD007565
6. Alsop K, Fereday S, Meldrum C. et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654-2663
7. Rutherford T, Orr J Jr, Grendys E Jr. et al. A prospective study evaluating the clinical relevance of a chemoresponse assay for treatment of patients with persistent or recurrent ovarian cancer. Gynecol Oncol. 2013;131:362-367
8. Sakuragi N, Takeda N, Hareyama H. et al. A multivariate analysis of blood vessel and lymph vessel invasion as predictors of ovarian and lymph node metastases in patients with cervical carcinoma. Cancer. 2000;88:2578-2583
9. Zaorsky NG, Patil N, Freedman GM, Tuluc M. Differentiating lymphovascular invasion from retraction artifact on histological specimen of breast carcinoma and their implications on prognosis. J Breast Cancer. 2012;15:478-480
10. Cheng L, Jones TD, Lin H. et al. Lymphovascular invasion is an independent prognostic factor in prostatic adenocarcinoma. J Urol. 2005;174:2181-2185
11. Bendifallah S, Canlorbe G, Raimond E. et al. A clue towards improving the European Society of Medical Oncology risk group classification in apparent early stage endometrial cancer? Impact of lymphovascular space invasion. Br J Cancer. 2014;110:2640-2646
12. Song S, Song C, Kim HJ. et al. 20 year experience of postoperative radiotherapy in IB-IIA cervical cancer patients with intermediate risk factors: impact of treatment period and concurrent chemotherapy. Gynecol Oncol. 2012;124:63-67
13. O'Hanlan KA, Kargas S, Schreiber M. et al. Ovarian carcinoma metastases to gastrointestinal tract appear to spread like colon carcinoma: implications for surgical resection. Gynecol Oncol. 1995;59:200-206
14. Fujimoto T, Sakuragi N, Okuyama K. et al. Histopathological prognostic factors of adult granulosa cell tumors of the ovary. Acta Obstet Gynecol Scand. 2001;80:1069-1074
15. Matsuo K, Sheridan TB, Yoshino K. et al. Significance of lymphovascular space invasion in epithelial ovarian cancer. Cancer Med Oct. 2012;1:156-164
16. Matsuo K, Yoshino K, Hiramatsu K. et al. Effect of lymphovascular space invasion on survival of stage I epithelial ovarian cancer. Obstet Gynecol. 2014;123:957-965
17. Matsuo K, Sheridan TB, Mabuchi S. et al. Estrogen receptor expression and increased risk of lymphovascular space invasion in high-grade serous ovarian carcinoma. Gynecol Oncol. 2014;133:473-479
18. Kikuchi E, Margulis V, Karakiewicz PI. et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol. 2009;27:612-618
19. Robert J. Kurman, Maria Lusia Carcangiu, C. Simon Herrington, Robert H.Young. WHO classification of tumours of ovary. In: (ed.) Robert J. Kurman, Maria Lusia Carcangiu, C. Simon Herrington. et al. WHO classification of tumours of female reproductive organs (4th edition). Switzerland: WHO press. 2014:12
20. Malpica A, Deavers MT, Lu K. et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496-504
21. Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460:237-249
22. International Federation of Gynecology and Obstetrics. Classification and staging of malignant tumours in the female pelvis. Acta Obstet Gynecol Scand. 1971;50:1-7
23. Malpica A. Grading of ovarian cancer: a histotype-specific approach. Int J Gynecol Pathol. 2008;27:175-181
24. Honoré LH, Hanson J. Statistical analysis of pathologic risk factors for intramyometrial lymphvascular space involvement in myoinvasive endometrial carcinoma. Int J Gynecol Cancer. 2006;16:1330-1335
25. Salomao DR, Graham SD, Bostwick DG. Microvascular invasion in prostate cancer correlates with pathologic stage. Arch Pathol Lab Med. 1995;119:1050-1054
26. NCCN Clinical Practice Guidelines in Oncology; Ovarian Cancer (Version 3). National comprehensive cancer network group. http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
27. Davis A, Tinker AV, Friedlander M. "Platinum resistant" ovarian cancer: what is it, who to treat and how to measure benefit? Gynecol Oncol. 2014;133:624-631
28. Shariat SF, Kattan MW, Song W. et al. Early postoperative peripheral blood reverse transcription PCR assay for prostate-specific antigen is associated with prostate cancer progression in patients undergoing radical prostatectomy. Cancer Res. 2003;63:5874-5878
29. Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol. 2008;5:194-204
30. Huang CY, Ho CM, Chen YL, You SL, Chen CA, Cheng WF. Impact of lymphadenectomy in uterine endometrioid carcinoma. Eur J Surg Oncol. 2013;39:350-357
31. Liotta LA, Kohn E. Cancer invasion and metastases. JAMA. 1990;263:1123-1126
32. Qian X, Xi X, Jin Y. The grading of lymphovascular space invasion in epithelial ovarian carcinoma. Int J Gynecol Cancer. 2010;20:895-899
33. Sullivan PS, Apple SK. Should histologic type be taken into account when considering neoadjuvant chemotherapy in breast carcinoma? Breast J. 2009;15:146-154
34. Al-Alao BS, Gately K, Nicholson S, McGovern E, Young VK, O'Byrne KJ. Prognostic impact of vascular and lymphovascular invasion in early lung cancer. Asian Cardiovasc Thorac Ann. 2014;22:55-64
35. Woodward WA, Strom EA, Tucker SL. et al. Locoregional recurrence after doxorubicin-based chemotherapy and postmastectomy: Implications for breast cancer patients with early-stage disease and predictors for recurrence after postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2003;57:336-344
36. Narayan K, Khaw P, Bernshaw D, Mileshkin L, Kondalsamy-Chennakesavan S. Prognostic significance of lymphovascular space invasion and nodal involvement in intermediate- and high-risk endometrial cancer patients treated with curative intent using surgery and adjuvant radiotherapy. Int J Gynecol Cancer. 2012;22:260-266
37. Lee KB, Ki KD, Lee JM. et al. The risk of lymph node metastasis based on myometrial invasion and tumor grade in endometrioid uterine cancers: a multicenter, retrospective Korean study. Ann Surg Oncol. 2009;16:2882-2887
38. Murakami I, Fujii T, Kameyama K. et al. Tumor volume and lymphovascular space invasion as a prognostic factor in early invasive adenocarcinoma of the cervix. J Gynecol Oncol. 2012;23:153-158
39. Rouzier R, Preti M, Haddad B, Martin M, Micheletti L, Paniel BJ. Development and validation of a nomogram for predicting outcome of patients with vulvar cancer. Obstet Gynecol. 2006;107:672-677
Author contact
![]() Corresponding author: Lingya Pan, MD. Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 1 Shuai Fu Yuan, Wang Fu Jing Street, Beijing 100730, China. TEL: 86-10-65296218; FAX: 86-10-65124875 E-mail: panlycn.
Corresponding author: Lingya Pan, MD. Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 1 Shuai Fu Yuan, Wang Fu Jing Street, Beijing 100730, China. TEL: 86-10-65296218; FAX: 86-10-65124875 E-mail: panlycn.

 Global reach, higher impact
Global reach, higher impact