3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2015; 6(5):420-429. doi:10.7150/jca.11228 This issue Cite
Research Paper
Increased Expression of GOLPH3 is Associated with the Proliferation of Prostate Cancer
1. Department of Urology, Linyi People's Hospital Affiliated to Shandong University, Shandong, China
2. Department of Urology, Ninth People's Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China
3. Department of Urology, HuaShan Hospital, Fudan University, Shanghai, China
* These authors contributed equally to this work.
Received 2014-12-3; Accepted 2015-1-19; Published 2015-2-27
Abstract
Background: Golgi phosphoprotein 3 (GOLPH3) is a metastasis-associated gene, however its role in cell proliferation of prostate cancer (PCa) has not yet been elucidated.
Methods: The level of expression of GOLPH3 and other genes was examined by quantitative real-time PCR (QPCR) and western blot analysis. Furthermore, we performed a comprehensive analysis of the expression of GOLPH3 in PCa using a tissue microarray (TMA) and correlated our findings with pathological parameters of PCa. RNA interference (RNAi) was used to silence the expression of GOLPH3 in PC-3 cells and to measure the effects on proliferation and cell cycle using the CCK-8 assay and flow cytometry. Western blots were also employed to assess AKT-mTOR and cell cycle-related proteins.
Results: We showed that the expression of GOLPH3 was located at the trans-Golgi membranes in PCa cells. We found that GOLPH3 was expressed in all PCa cells and was significantly higher in two androgen-independent cell lines, DU145 and PC-3. TMA immunohistochemistry showed that GOLPH3 was positive in 64% of cancer tissue samples compared with 20% in normal and 30% in benign samples (P<0.05). In vitro, silencing GOLPH3 expression inhibited cell proliferation and arrested the cell cycle at the G2/M phase. Silencing GOLPH3 also activated P21 expression but suppressed the expression of CDK1/2 and cyclinB1 protein together with the phosphorylation of AKT and mTOR.
Conclusions: The expression of the GOLPH3 protein was significantly elevated in PCa. GOLPH3 can promote cell proliferation by enhancing the activity of AKT-mTOR signaling. Altogether, these findings suggest that GOLPH3 play important roles in proliferation and cell cycle regulation in PCa and might serve as promising biomarkers for PCa progression as well as potential therapeutic targets.
Keywords: GOLPH3, AKT, mTOR, proliferation, cell cycle, prostate cancer (PCa).
Introduction
With the rapid development of gene technology and proteomics, novel Golgi proteins and Golgi-associated proteins have been identified. Golgi phosphoprotein 3 (GOLPH3, also designated as GMx33, GPP34, MIDAS or yeast Vps74p) is considered to be the most promising marker of cancer biology. GOLPH3 is a highly conserved Golgi membrane protein that was originally identified by proteomic analyses of the Golgi apparatus [1, 2]. GOLPH3 has been shown to be a mitochondrial protein that is involved in the regulation of mitochondrial lipids [3]. Indeed, GOLPH3 is highly modified post-translationally and dynamically associated with the trans surface of the Golgi membranes. Similarly to many other Golgi matrix proteins, GOLPH3 presents in a large cytosolic pool. However, GOLPH3 is an atypical Golgi matrix protein that is relatively small (molecular weight of 34 kDa) and lacks a long coiled-coil domain [4]. The biological functions of the GOLPH3 protein are still unknown. Previous studies have demonstrated that the association of GOLPH3 with the Golgi is modulated in a GTP-dependent manner [4]. GOLPH3 protein is an effector of Golgi phosphatidylinositol (PtdIns) 4-kinases, which are regarded as key regulators of the Golgi structure and function [5, 6]. GOLPH3 binds to PtdIns 4-phosphate-rich trans-Golgi membranes, and myosin 18A conveys the tension that keeps the Golgi structure intact in its stretched shape to maintain its function [7]. This phenomenon reveals the potential role of GOLPH3 in protein trafficking and maintaining Golgi function.
Recently, GOLPH3 has emerged as a potent oncoprotein that participates in the regulation of protein glycosylation, a common form of post-translational modification and a hallmark feature of cancers [8, 9]. The gene encoding GOLPH3 resides on human chromosome band 5p13, which is frequently amplified in a variety of human solid tumors, including lung, prostate, ovary, breast, pancreas, and gastric cancer, and melanoma [10-15]. This amplification of the GOLPH3 locus in malignancies is involved in oncogenic transformation, suggesting that GOLPH3 may originate from cancerous cells and play an important role in carcinogenesis. GOLPH3 is a regulator of protein synthesis and can act as a proto-oncogene. Thus, the activity of GOLPH3 may be associated with cancer cell growth and survival. These observations support the notion of GOLPH3 as a matrix protein with potent oncogenic activity at the Golgi, although very little is known regarding its role in the development and treatment of PCa.
In this study, we investigated the expression of GOLPH3 in human PCa and its effect on cell proliferation. We found that the expression of the GOLPH3 protein was significantly elevated in each pathological grade and stage of PCa. However, a significant association could not be established between GOLPH3 expression and the pathological parameters of PCa. On the other hand, reduced expression of GOLPH3 by shRNA inhibited the growth of PC-3 cancer cells, and silencing of GOLPH3 in PCa cells led to G2/M cell-cycle arrest. Using these loss-of-function approaches, we showed that down regulation of GOLPH3 inhibited the proliferation of PCa cells via downregulation of the expression of cyclinB1 and CDK1/2 accompanied with phosphorylation of AKT and mammalian target of rapamycin (mTOR). The study of the expression profiles of GOLPH3 and its effect on cell proliferation could lay a foundation for the prevention of PCa. Overall, the present study enriches our understanding of biological characteristics of GOLPH3 in PCa.
Materials and Methods
Cell culture
Four human PCa cell lines, 22Rv1, LNCaP, DU145, and PC-3 purchased from the cell bank of Chinese Academy of Sciences (Shanghai, China), were routinely cultured in RPMI-1640 media (Hyclone, Logan, UT). All cultures were supplemented with 10% fetal bovine serum (GIBCO, Grand Island, NY) and incubated at 37°C in a 5% CO2 incubator.
Immunofluorescence
The localization of GOLPH3 in the PCa cell line DU145 was assessed using laser confocal immunofluorescence microscopy. Briefly, cells were cultured on cover slips and fixed for 15 minutes in 4% paraformaldehyde. After permeabilization with 0.3% Triton X-100 (Sigma, St. Louis, MO) for 15 minutes, the samples were blocked with 0.05% BSA for 1 h. The samples were incubated with primary antibodies (1:100) and an anti-Golgi marker TGN46 antibody (1:100) overnight at 4°C, washed, and incubated with the corresponding goat anti-mouse and anti-rabbit IgG secondary antibodies for 1 h. The nuclei were labeled with 4,6-diamidino-2-phenylindole (DAPI), and the cells were visualized using confocal microscopy.
mRNA-expression analysis
For each cell line, the total RNA was extracted using RNAiso (Takara, Dalian, China), and reverse transcription was performed using RevertAid Reverse Transcriptase (Fermentas, Glen Burnie, MD), according to the manufacturer's instructions. The cDNA was used to amplify the GOLPH3 gene using PCR with the following primers: GOLPH3 forward, 5'-ACATCCCCTCACCAATAACAAC-3', and GOLPH3 reverse, 5'-TAGCCAAATCATACTGCTCGTC-3'. The QPCR reactions were performed in triplicate in a 15 μl reaction volume containing 7.5 μl of 2×SYBR mix (Takara), 0.2 μl of each primer (10 μM), and 0.3 μl cDNA. The conditions were as follows: 94°C for 2 min, followed by 40 cycles at 95°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec. The expression values of GOLPH3 were normalized to the endogenous reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Western blot analysis
Briefly, PCa cell lines were lysed with 150 μl lysis buffer supplemented with protease and phosphatase inhibitors. The cell lysates were collected by scraping and centrifugation at 12,000 rpm for 15 min. The protein concentrations were measured using the BCA protein assay and adjusted to equivalent amounts. Total protein (20 μg) from each sample was electrophoresed in 10% SDS-PAGE gels and transferred onto nitrocellulose membranes (Millipore, Billerica, MA). The membranes were first blocked in 5% non-fat milk in TBS containing 0.1% Tween 20 (TBST) for 1 h at room temperature and then incubated with specific antibodies (Abcam, Cambridge, UK) for different western blot analyses for 2 h at room temperature. After washing and incubating with a horseradish peroxidase-conjugated secondary antibody, the protein bands were detected using ECL reagents (Millipore).
Tissue microarrays and immunohistochemical analysis
Prostate adenocarcinoma TMAs were obtained from Biomax, Inc. (Rockville, MD). Immunohistochemistry was performed using the EliVision plus system according to the manufacturer's instructions (Maixin Biological, Fuzhou, China). Briefly, the array slides were deparaffinized in xylene, rinsed with phosphate-buffered saline (PBS), and subjected to epitope retrieval treatment by boiling in 0.01 M citrate buffer solution (pH 6.0) for 2 min. The slides were cooled at room temperature and further rinsed with PBS three times for 5 min each. The slides were then incubated with 3% hydrogen peroxide at room temperature for 10 min to quench the endogenous peroxidase activity and rinsed three more times for 5 min each. The tissue arrays were subsequently incubated with the anti-GOLPH3 primary antibody (Epitomics Inc., Burlingame, CA) at a 1:25 dilution overnight at 4°C. The arrays were then treated with a polymer accentuator at room temperature for 20 min and incubated with horseradish peroxidase-labeled goat anti-mouse/rabbit IgG polymer at room temperature for 30 min. The sections were rinsed thoroughly with PBS containing 0.1% Tween 20 for 5 min each. The color reaction was performed using diaminobenzidine (DAB) solution for 5 min. Finally, the slides were washed, counterstained with hematoxylin, dehydrated, and mounted. The normal rabbit IgG isotype served as the negative control.
Expression of transfected GOLPH3 shRNA
Knockdown of GOLPH3 gene expression in PC-3 cells was carried out using GOLPH3 shRNA expressing lentiviral vectors. Briefly, recombinant lentiviral particles containing non-target and human GOLPH3 targeted shRNA were prepared using transfection of 293T cells. Cells were cultured in DMEM (GIBCO) with 10% FBS. Exponentially proliferating cells were seeded into 24-well culture plates and incubated overnight, allowing cells to adhere. A multiplicity of infection (MOI) of 20 was adopted. To each well, 40 µl of Lenti-GOLPH3 or negative control (NC) was added. After five days of incubation, the cells were collected. RNA and protein were extracted to analyze the expression of GOLPH3 in PC-3 cells. PC-3 cells were stably transfected with GOLPH3 shRNA (pUEGH-GOLPH3shRNA), or pUEGH using TARGET TransIn transfection reagent (Tri-D Biopharm, Shanghai, China) according to the manufacturer's instructions.
To select stably transfected PC3 cells, the cell cultures were incubated with 200 µg/ml hygromycin for one week before individual clones were isolated, then established and maintained in the medium containing 50 µg/ml of hygromycin. The stable GOLPH3-underexpression cells were seeded into well plates, and CCK-8 and flow cytometry were used to assess cell viability, and cell cycle status. Knockdown of GOLPH3 expression was determined by detecting the mRNA level and protein level using QPCR and western blot analysis, respectively. All experiments were performed in at least triplicate cultures and repeated 3 times.
Cell proliferation assay
To investigate the effects of GOLPH3 on the rate of cell proliferation, we used Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan). Cells (3×103 cells/well) were first seeded into 96-well flat-bottom plates for 8 h to adhere. After 24 h, 10 μl of solution from CCK-8 was added to each well. These plates were incubated for 45 minutes in a humidified CO2 incubator at 37°C. Finally, the absorbance of sample taken from each well was measured at 450 nm, on the basis of which the percentage of surviving cells in each treatment group was plotted relative to the untreated one.
Cell cycle analysis
The cell cycle of PC-3 cells was analyzed by flow cytometry using BD FACSAria Cell Sorter (Becton Dickinson, Franklin Lakes, NJ). PC-3 stable transfectant cells were seeded onto 20-mm2 dishes for 24 h. After incubation, the cells were trypsinized and collected by centrifugation and fixed with 70% ethanol. Samples were treated with 5 μL RNase A (10 μg/mL) for 1 h at room temperature, stained with 10 μL propidium iodide (10 μg/mL) for 30 min at 4°C and analyzed using the FACSCalibur flow cytometer.
Measurement of AKT-mTOR signaling and cell cycle-related proteins
The expression of AKT-mTOR proteins (AKT, mTOR, phospho-AKT, phospho-mTOR, p70S6K, and phospho-p70S6K) and cell cycle-related proteins (P21, cyclinB1, and CDK1/2) were analyzed by western blots. Western blot was applied as described above to detect GOLPH3 protein expression in the four PCa cell lines. Primary antibodies AKT, mTOR, phospho-AKT, phospho-mTOR, p70S6K, phospho-p70S6K, P21, cyclinB1, and CDK1/2 were purchased from Cell Signaling Technology (CST, Beverly, MA) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and used at a concentration of 1: 1000, 1: 500 and 1: 200, respectively.
Statistical analysis
The statistical significance of the correlation between GOLPH3 expression in PCa and pathological variables was analyzed by the chi-square test. QPCR, western blot data and cell growth data were analyzed by the Student's t-test. The level of statistical significance was set at P <0.05.
Results
Localization of GOLPH3 in PCa cells
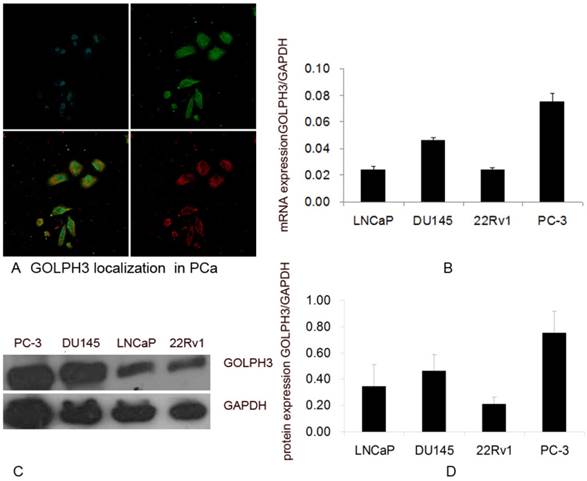
To study the role of GOLPH3 in PCa, we first attempted to define the subcellular localization of GOLPH3 in the PCa cell lines by immunofluorescence using the trans-Golgi marker TGN46. We observed that GOLPH3 localized in Golgi complex and co-existed with TGN46 (Figure 1A). These findings suggest that GOLPH3 and TGN46 co-localize to the Golgi complex in PCa cells. GOLPH3 targeting to the Golgi may serve a structural role to the Golgi apparatus.
Expression of GOLPH3 mRNA and protein in PCa cells
To verify the expression of the GOLPH3 gene in PCa, we assessed the relative abundance of GOLPH3 in four PCa cell lines (22Rv1, LNCaP, DU145, and PC-3) using QPCR. The LNCaP cells are androgen-dependent human PCa cell line, whereas the 22Rv1, DU145 and PC-3 cells are considered androgen-independent human PCa cell lines. GOLPH3 mRNA was expressed in all PCa cell lines (Figure 1B). The QPCR results indicated that the expression of GOLPH3 was higher in the androgen-independent cell lines DU145 and PC-3 than the androgen-dependent cell line LNCaP. The expression of GOLPH3 in both DU145 and PC-3 cells was at least double those in LNCaP cells (Figure 1B).
Based on the QPCR results, we evaluated the expression of GOLPH3 protein in the four prostate cell lines (22Rv1, LNCaP, DU145, and PC-3) using western blot analysis. Clear and specific bands of the predicted molecular weight (34 kDa) corresponding to the predicted size of the GOLPH3 protein were detected (Figure 1C). Consistent with the QPCR results, the expression of the GOLPH3 protein was also higher in DU145 and in PC-3 than in LNCaP cells (Figure 1D). The levels of GOLPH3 protein in PCa cells correlated with its level of transcription.
The localization and levels of expression of GOLPH3 in PCa cells. A. Subcellular localization of GOLPH3 in PCa cells. The upper panels show the location of the nuclei (blue) and GOLPH3 expression (green), and the lower panels show the merged image and the trans-Golgi marker TGN46 (red). B. The levels of expression of GOLPH3 mRNA in various PCa cell lines. C and D. The levels of expression of GOLPH3 protein in various PCa cell lines. The expression of GOLPH3 was normalized to that of GAPDH.
Immunohistochemical expression of GOLPH3 in prostate tissues
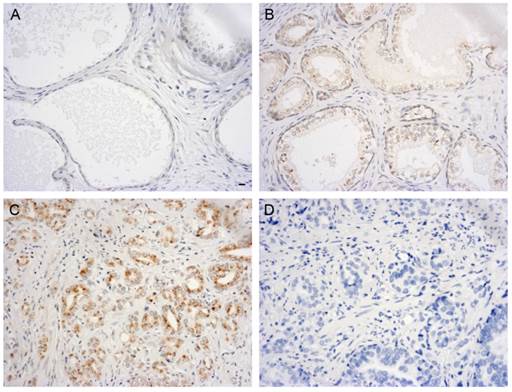
The QPCR and western blot analyses of the PCa cell lines demonstrated that the expression of GOLPH3 was significantly elevated. To analyze the expression of GOLPH3 in normal and diseased prostate tissues in greater detail, we performed immunohistochemistry using TMAs. Figure 2 showed various patterns of GOLPH3 expression in normal prostate, benign prostatic hyperplasia (BPH), and adenocarcinoma tissues. The expression of GOLPH3 protein was typically cytoplasmic. In 2 out of 10 (20%) normal prostate tissues and in 6 out of 20 (30%) BPH tissues, positive GOLPH3 immunostaining could be observed in the cytoplasm. Of the 50 prostate carcinoma samples, 32 (64%) were positive for GOLPH3 expression (P<0.05). The normal prostate and BPH tissues showed low levels of GOLPH3 staining, in contrast to PCa, which exhibited strong GOLPH3 staining. These findings suggest that GOLPH3 protein was significantly expressed in PCa in comparison to the normal prostate gland and BPH.
GOLPH3 expression and pathological parameters of PCa
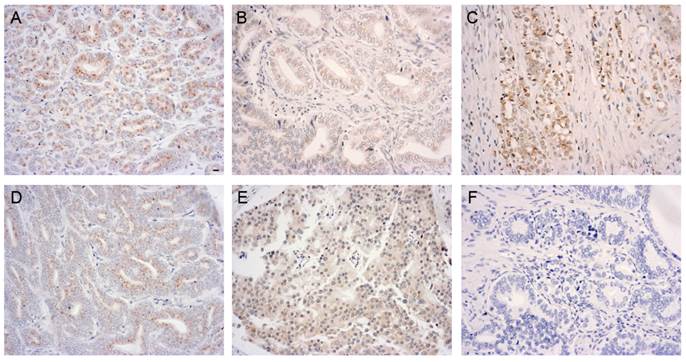
To further determine the association of GOLPH3 with the pathological parameters of PCa, we performed a statistical analysis of the immunohistochemical expression of GOLPH3 protein. The histological grade of the PCa, as defined by the Gleason score, was classified as well-differentiated (Gleason score 2 to 5), moderately differentiated (Gleason score 6 to 7), and poorly differentiated (Gleason score 8 to 10). Gleason scores were obtained from the prostate adenocarcinoma TMA manufacturer. Figure 3 showed various GOLPH3 expression patterns in different histological grades and pathological stages of PCa. Of the 50 PCa specimens (the Gleason scores of two specimens were lacking), the frequency of expression of the GOLPH3 protein in well-differentiated, moderately differentiated, and poorly differentiated groups were 6 out of 10 (60.0%), 10 out of 17 (58.8%) and 14 out of 21 (66.7%), respectively. There was no statistically significant association between GOLPH3 staining and the Gleason score (P>0.05). The expression rates of GOLPH3 in PCa stage T1-2 and T3-4 were 66.7% (18/27) and 60.9% (14/23), respectively. Similarly, statistical analysis showed that the expression of GOLPH3 in PCa was not associated with the pathological stage (P >0.05).
Inhibition of GOLPH3 arrests cell growth in PCa cells
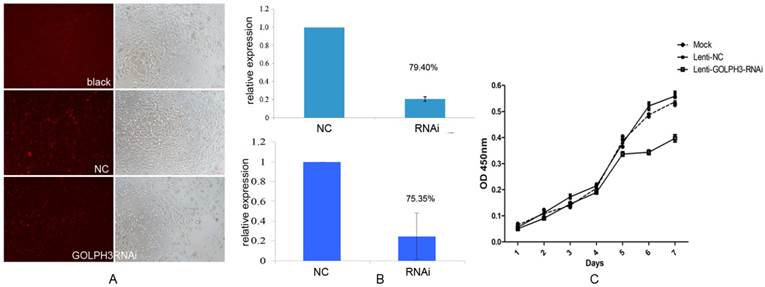
To assess whether the expression of GOLPH3 is important for the proliferation or survival of PCa cells, we first established GOLPH3 deleted stable cell lines by transfection of lentiviruses expressing shRNA targeting the human GOLPH3 gene or control shRNA and examined the effects on the cell proliferation rate. As shown in Figure 4A, as we expected, we obtained a lentiviral GOLPH3 shRNA that effectively silenced human GOLPH3 expression in PCa cells. QPCR and western blot analysis demonstrated that the levels of GOLPH3 mRNA and protein were both reduced by approximately 80% in the stable cell line compared to the scrambled shRNA control (Figure 4B). Furthermore, silencing of GOLPH3 expression resulted in robust inhibition of the proliferation of PC-3 cells. Together with the results shown in Figure 4C, our findings clearly indicate that down regulation of GOLPH3 significantly suppressed the growth in PC-3 cells (P<0.05). This observation indicated that GOLPH3 is a potential gene therapy target for PCa.
Immunohistochemical evaluation of GOLPH3 expression in different human prostate tissues. Immunoreactivity for GOLPH3 was mainly found in the cytoplasm. 200× magnification views of GOLPH3 staining in prostatic tissues. Normal prostate glands did not exhibit any immunostaining for GOLPH3 staining (A). BPH shows very weak staining for GOLPH3 (B), whereas adenocarcinoma tissues show strong staining for GOLPH3 (C). The negative control (D) shows no immunostaining. Scale bars: 10 µm.
Elevated levels of GOLPH3 in various histological grades and pathological stages of PCa tissue. Immunoreactivity for GOLPH3 was mainly found in the cytoplasm. 200× magnification views of GOLPH3 staining in well-differentiated (A, moderate), moderately differentiated (B, weak), poorly differentiated (C, moderate), low-stage (D, weak) and high-stage (E, moderate) adenocarcinoma tissues. The negative control (F) does not show immunostaining. Scale bars: 10 µm.
The effect of silencing of GOLPH3 expression on PC-3 cell proliferation. A. Comparative analysis of the viability of cells in each group seven days after PC-3 cells were seeded. B. QPCR and western blot were performed to validate the efficiency of GOLPH3 interference in PCa cells. C. Cell growth curve of PC-3 cells stably expressing GOLPH3 RNAi. No significant effects of lentiviral infection on the growth of PC-3 cell viability (NC) were detected. Silencing of GOLPH3 expression significantly suppressed PC-3 cell growth (P <0.05), indicating that GOLPH3 as a positive regulatory gene implicated in the growth of PC-3 cells.
Inhibition of GOLPH3 arrests the cell cycle at the G2/M phase
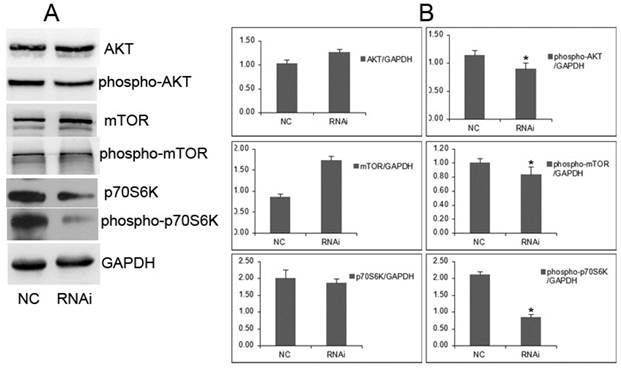
To elucidate the molecular mechanism underlying the effect of inhibition of GOLPH3 on cell growth arrest, we transduced PC-3 cells with the lentivirus expressing GOLPH3 shRNA or a control, and established stable cell lines. We performed a flow-cytometric analysis to determine the impact of silencing of GOLPH3 expression on the distribution of the cell cycle in PC-3 cells. As shown in Figure 5, in agreement with its inhibitory function, knockdown of GOLPH3 increased the percentage of cells in the G2/M phase, suggesting the cells were significantly arrested in the G2/M phase (P < 0.05). These data imply that GOLPH3 may regulate some of the cell cycle proteins in G2/M phase.
GOLPH3 regulates the expression of cyclinB1 and CDK1/2 in PCa cells
To investigate the underlying molecular mechanisms by which GOLPH3 positively regulates cell growth of PCa cell lines, we analyzed several cell cycle regulators by western blots. We found that cyclinB1 and CDK1/2 protein were downregulated in PC-3 cells transfected with GOLPH3 shRNA, compared with cells transfected with control lentiviruses. On contrast, P21 protein was overexpressed (Figure 6). These results demonstrated that GOLPH3 inhibited the G2 to M phase transition concomitantly with upregulation of P21 protein, and downregulation of cyclinB1 and CDK1/2.
GOLPH3 modulates the AKT-mTOR signaling pathways
It will be important to determine whether cellular proliferation is required for GOLPH3-mediated signaling through mTOR. We examined the effect of GOLPH3 on its downstream signaling, such as the AKT-mTOR pathways. In the GOLPH3-knockdown cells, the levels of phosphorylated AKT and mTOR were remarkably reduced, associated with decrease of phosphorylated mTOR and phosphorylated p70S6K (Figure 7). However, the levels of AKT and mTOR were not affected. The proliferation process mediated by GOLPH3 involved the downregulation of phospho-AKT, phospho-mTOR and phospho-p70S6K. These findings suggested that GOLPH3 modulates the phosphorylation state of the AKT-mTOR signaling pathway, which integrates input from multiple signaling pathways to control cell growth, proliferation, differentiation, and survival [16,17].
Cell cycle analysis of PC-3 cells stably expressing GOLPH3RNAi. A. Representative histograms of flow cytometry analysis of cell cycle alterations of PC-3 cells 72 h after lentiviral infection (NC). B. Representative histograms of flow cytometry analysis of cell cycle alterations of PC-3 cells stably expressing GOLPH3RNAi 72 h after treatments. C. Effects of lentiviral infection (NC) and GOLPH3RNAi on cell cycle at 24, 48, and 72 h after treatments. The results are expressed as the mean of three experiments ± SD of the relative cell population (%). *P <0.05 versus control.
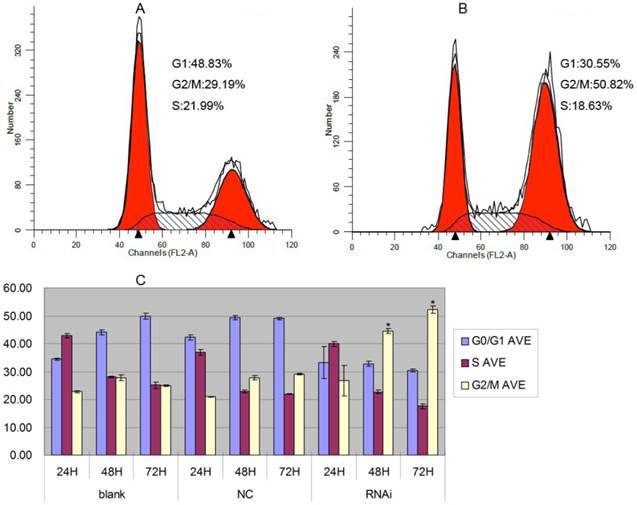
GOLPH3 modulates cell cycle-related proteins. A. Representative photographs from western blot assay. B. The relative levels of expression of P21, cyclinB1 and CDK1/2 proteins in PCa cells were analyzed by western blots. *P <0.05 versus NC control.
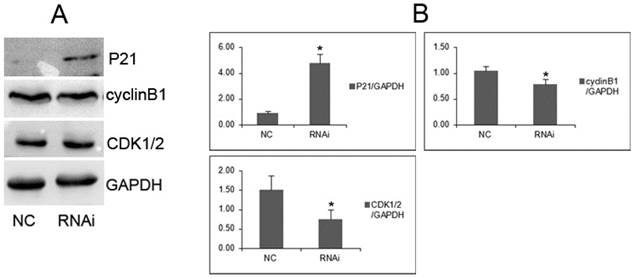
GOLPH3 modulates the phosphorylation status of the AKT-mTOR signaling pathways. A. Representative photographs from western blot assay. B. The relative levels of expression of AKT, mTOR, phospho-AKT, phospho-mTOR, p70S6K, and phospho-p70S6K in PCa cells were analyzed by western blots. *P <0.05. versus NC control.
Discussion
GOLPH3 is a newly identified member of the family of Golgi matrix proteins [1, 4, 18]. To our knowledge, the GOLPH3 protein exerts various functions depending on its intracellular localization. Generally speaking, proteins located in the cell membrane act as receptors, whereas proteins located in the cytoplasm are involved in protein modification and energy metabolism, and proteins located in the cell nucleus are transcription factors. The Golgi plays important roles in the maturation of glycoproteins. Using an immunofluorescence assay, we confirmed that GOLPH3 and TGN46 co-localized in the Golgi complex in PCa cells. As a Golgi-localized protein, GOLPH3 showed a typical Golgi localization and may play an important role. Recent studies in yeast show that Vps74p, a human protein ortholog of GOLPH3, directly interacts with many glycosyltransferases through their N-terminal cytoplasmic domains. It is possible that Vps74p and GOLPH3 play a similar role [19]. In addition, GOLPH3 has a role in the spatial localization 2 N-acetylglucosamine-transferase 1 of α-2,6-sialyltransferase 1, and GOLPH3 depletion changed the subcellular localization of these enzymes [20]. S-nitrosylation of the extracellular matrix metalloproteinase inducer (EMMPRIN) and GOLPH3 has been demonstrated in endothelial cells; this modification regulates extracellular matrix remodeling, signal transduction, protein trafficking, glycosylation and maintenance of the Golgi structure [21]. The finding suggests a novel function for GOLPH3 and highlights an important regulatory role of GOLPH3 in Golgi trafficking. Golgi-targeted GOLPH3 may functionally associate with cancer-related signaling factors. To date, the precise biological role and molecular function of GOLPH3 in the pathogenesis of PCa and other solid tumors is unknown. Other studies have suggested that GOLPH3 may promote migration and invasion of tumor cells [22, 23]. The role of GOLPH3 in the regulation of cancer cell proliferation and growth remains to be determined.
GOLPH3 has been reported to be overexpressed in solid tumors [10], but the present report is the first study of the expression and function of GOLPH3 in PCa. Using QPCR, we confirmed the initial report about the upregulation of GOLPH3 mRNA in PCa cells [24]. In our study, we demonstrated that the expression of GOLPH3 mRNA and protein was elevated in four commonly used PCa cell lines (22Rv1, LNCaP, DU145, and PC-3). Interestingly, among the PCa cells, the expression of GOLPH3 was much higher in androgen-independent cell lines than in androgen-dependent cell lines. Emerging evidence has indicated that GOLPH3 was an androgen-regulated gene, which has been implicated in protein synthesis and trafficking, response to transcription, proliferation, apoptosis, and differentiation [24]. Taken together, these findings suggested that the upregulation of GOLPH3 may be involved in the progression from androgen-dependent to androgen-independent growth.
TMA immunohistochemical studies showed that the expression of GOLPH3 was occasionally observed in benign prostate tissue, whereas GOLPH3 was predominantly found in the cytoplasm of prostate tumor cells. These results indicated that only low levels of GOLPH3 were detected in the normal prostate gland and BPH. In contrast, PCa of each grade revealed a statistically significant overexpression of GOLPH3. The differences in the expression of GOLPH3 in PCa compared with benign prostate tissue may facilitate the clinical diagnoses of PCa. In addition, to investigate whether GOLPH3-positive PCa are associated with higher pathological stage and grade, we performed a statistical analysis of the immunohistochemical data regarding the expression of GOLPH3 protein. The results showed that levels of GOLPH3 expression were not associated with the histological grade or pathological stage of PCa. Upregulated expression of GOLPH3 in PCa was confirmed in different types of PCa cell lines, i.e., 22Rv1, LNCaP, DU145, and PC-3, as previously described. Cumulatively, these observations suggest that GOLPH3 acts as an oncogene and may be a useful target for therapeutic interventions.
Because GOLPH3 was found to be upregulated in PCa cells and tissues, it remains to be determined whether or not GOLPH3 alters the cell cycle and proliferation of PCa cells. In this study, PC-3 cells were transfected with GOLPH3 shRNA or NC. We used seven GOLPH3 specific shRNAs to insure that the knockdown was specific for the GOLPH3 gene and not due to off target effects. By using loss-of-function approaches, we confirmed that the growth of PCa cells was significantly decreased compared with that of their corresponding controls, measured using the CCK-8 assays. The results demonstrated that GOLPH3 is a potent positive regulator of the growth of PCa cells. Furthermore, silencing of GOLPH3 increased the percentage of cells in the G2/M phase. GOLPH3 also regulated cell-cycle progression and survival. GOLPH3 regulated components of the G2/M checkpoint, which are closely associated with tumorigenesis.
To investigate the underlying molecular mechanisms by which GOLPH3 regulates the growth of PCa cells, we determine that cyclinB1 protein, a key regulator of cell cycle (G2/M phase) for both normal and tumor cells, is negatively regulated by downregulation of GOLPH3 in PCa cells. The proliferation of PCa cells, driven by cyclin-dependent kinases (CDKs) and their cyclin partners, is deregulated. As shown previously, knockdown of CDK1 leads to slowing of G2/M, whereas CDK2 depletion causes G1 accumulation; dual CDK1/2 depletion resulted in further G2/M accumulation [25]. Downregulation of cell cycle inhibitor P21 may also inhibit CDK1, which has a critical role at the G2/M transition [26]. These findings suggest that inhibition of cyclinB1 and CDK1/2 meditated by GOLPH3 reduced the proliferation of PCa cells.
GOLPH3 interacts with VPS35, which has been implicated in recycling of receptors [10, 27, 28]; therefore, GOLPH3 possibly promotes downstream signaling of receptor tyrosine kinases (RTK). Indeed, GOLPH3 has been found to enhance mTOR signaling [10]. To investigate the effect of GOLPH3 on RTK and its downstream signaling, we examined the phosphorylation of AKT-mTOR and its downstream signaling. We showed that increased expression of GOLPH3 was associated with PCa proliferation mediated through AKT-mTOR signaling. Amplification of GOLPH3 at chromosomal band 5p13, which correlates with increased phosphorylation of the mTOR substrate p70S6 kinase in lung cancer, served to link the function of GOLPH3 to the activity of mTOR [29]. mTOR signaling is known to be a central regulator of cell growth, proliferation, differentiation, and survival [16, 17]. Other studies have revealed that GOLPH3 is an oncoprotein that modulates mTOR signaling by upregulating the phosphorylation of both mTORC1 and mTORC2 substrates in human cancer cells which influences cell size, enhances cell growth, regulates cell proliferation and survival, and confers increased sensitivity to rapamycin, an inhibitor of the mTOR pathway [10, 29]. In addition, GOLPH3 has been involved in vesicular trafficking, receptor endocytosis and recycling, and protein glycosylation [29]. mTOR mediates the translation of mRNAs related with cell cycle check-points, regulates the expression of survival factors such as c-myc, HIF-1α and VEGF and is involved in the regulation of CDK1/2 [30]. Mechanistically, we showed that the enhanced phosphorylation of AKT-mTOR represents a potential molecular basis for the oncogenic activity of GOLPH3. These findings also raise the possibility that the level of expression of GOLPH3 or its copy number status may serve as a predictive biomarker for PCa.
Taken together, through our systematic study of GOLPH3 expression and function, we found that GOLPH3 was highly expressed in PCa. Silencing of GOLPH3 inhibited the proliferation of PCa cells at the G2/M cell cycle arrest. GOLPH3 can function as an oncogene to promote cell proliferation by enhancing the activity of AKT-mTOR signaling, a serine/threonine protein kinase known to modulate cell growth, proliferation, and survival. The present study supports a novel role for GOLPH3 in PCa cell proliferation, distinct from the previously reported role of GOLPH3 as a promoter for cancer metastasis. Our study also suggests that GOLPH3 may be an important characteristic of PCa and could be used as a disease marker and as a therapeutic target.
Abbreviations
BPH: benign prostatic hyperplasia; CDK: cyclin-dependent kinase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GOLPH3: Golgi phosphoprotein 3; mTOR: mammalian target of rapamycin; NC: negative control; PBS: phosphate-buffered saline; PCa: prostate cancer; PtdIns: phosphatidylinositol; QPCR: quantitative real time-polymerase chain reaction; RNAi: RNA interference; RTK: receptor tyrosine kinases.
Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (81170697).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wu CC, Taylor RS, Lane DR. et al. GMx33: A novel family of trans-Golgi proteins identified by proteomics. Traffic. 2000;1:963-75
2. Bell AW, Ward MA, Blackstock WP. et al. Proteomics characterization of abundant Golgi membrane proteins. J Biol Chem. 2001;276:5152-65
3. Nakashima-Kamimura N, Asoh S, Ishibashi Y. et al. MIDAS/GPP34, a nuclear gene product, regulates total mitochondrial mass in response to mitochondrial dysfunction. J Cell Sci. 2005;118:5357-67
4. Snyder CM, Mardones GA, Ladinsky MS. et al. GMx33 associates with the Trans-Golgi Matrix in a dynamic manner and sorts within tubules exiting the Golgi. Mol Biol Cell. 2006;17:511-24
5. Wood CS, Schmitz KR, Bessman NJ. et al. PtdIns4P recognition by Vps74/GOLPH3 links PtdIns 4-kinase signaling to retrograde Golgi trafficking. J Cell Biol. 2009;187:967-75
6. D'Angelo G, Vicinanza M, Di Campli A. et al. The multiple roles of PtdIns(4)P-not just the precursor of PtdIns (4,5) P2. J Cell Sci. 2008;121:1955-63
7. Dippold HC, Ng MM, Farber-Katz SE. et al. GOLPH3 bridges phosphatidylinositol-4-phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009;139:337-51
8. Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855-67
9. Kang JG, Ko JH, Kim YS. Pros and cons of using aberrant glycosylation as companion biomarkers for therapeutics in cancer. BMB Rep. 2011;44:765-71
10. Scott KL, Kabbarah O, Liang MC. et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459:1085-90
11. Barber JC, Huang S, Bateman MS. et al. Transmitted deletions of medial 5p and learning difficulties; does the cadherin transcluster only become penetrant when flanking genes are deleted? Am J Med Genet A. 2011;155A:2807-15
12. Zeng Z, Lin H, Zhao X. et al. Overexpression of GOLPH3 promotes proliferation and tumorigenicity in breast cancer via suppression of the FOXO1 transcription factor. Clin Cancer Res. 2012;18:4059-69
13. Peng J, Fang Y, Tao Y. et al. Mechanisms of GOLPH3 associated with the progression of gastric cancer: a preliminary study. PLoS One. 2014;9:e107362
14. Ma Y, Wang X, Wu Y. et al. Overexpression of GOLPH3 protein is associated with worse prognosis in patients with epithelial ovarian cancer. Tumour Biol. 2014;35:11845-9
15. Zhang LJ, Wang KB, Liu LS. et al. Overexpression of GOLPH3 is associated with poor prognosis and clinical progression in pancreatic ductal adenocarcinoma. BMC Cancer. 2014;14:571
16. Edlind MP, Hsieh AC. PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl. 2014;16:378-86
17. Wozney JL, Antonarakis ES. Growth factor and signaling pathways and their relevance to prostate cancer therapeutics. Cancer Metastasis Rev. 2014;33:581-94
18. Nagano-Ito M, Yoshikawa S, Tamura M. et al. Identification and characterization of a novel alternative splice variant of mouse GMx33alpha/GPP34. Gene. 2007;400:82-8
19. Graham TR, Burd CG. Coordination of Golgi functions by phosphatidylinositol 4-kinases. Trends Cell Biol. 2011;21:113-21
20. Eckert ES, Reckmann I, Hellwig A. et al. Golgi phosphoprotein 3 triggers signal-mediated incorporation of glycosyltransferases into coatomer-coated (COPI) vesicles. J Biol Chem. 2014;289:31319-29
21. Sangwung P, Greco TM, Wang Y. et al. Proteomic identification of S-nitrosylated Golgi proteins: new insights into endothelial cell regulation by eNOS-derived NO. PLoS One. 2012;7:e31564
22. Dai T, Zhang D, Cai M. et al. Golgi Phosphoprotein 3 (GOLPH3) promotes hepatocellular carcinoma cell aggressiveness by activating NF-κB pathway. J Pathol. 2014;235:490-501
23. Zhang X, Ding Z, Mo J. et al. GOLPH3 promotes glioblastoma cell migration and invasion via the mTOR-YB1 pathway in vitro. Mol Carcinog. 2014 [Epub ahead of print]
24. Lee HS, Park EJ, Oh JH. et al. Bisphenol A exerts estrogenic effects by modulating CDK1/2 and p38 MAP kinase activity. Biosci Biotechnol Biochem. 2014;78:1371-5
25. Satyanarayana A, Hilton MB, Kaldis P. p21 Inhibits Cdk1 in the absence of Cdk2 to maintain the G1/S phase DNA damage checkpoint. Mol Biol Cell. 2008;19:65-77
26. Romanuik TL, Wang G, Holt RA. et al. Identification of novel androgen- responsive genes by sequencing of LongSAGE libraries. BMC Genomics. 2009;10:476
27. Seaman MN. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68-75
28. Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427-436
29. Scott KL, Chin L. Signaling from the Golgi: Mechanisms and models for Golgi Phosphoprotein 3-mediated oncogenesis. Clin Cancer Res. 2010;16:2229-34
30. Jiang BH, Liu LZ. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat. 2008;11:63-76
Author contact
![]() Corresponding authors: Dr. Zhong Wang, Department of Urology, Shanghai Ninth People's Hospital, 639 Zhizaoju Road, Shanghai 200011, China, Tel: +86-21-23271699-5116, Fax: +86-21-63136856, Email: zhongwang2000com or Dr. Xiang Wang, Department of Urology, Shanghai HuaShan Hospital, 12 Middle Wulumuqi Road, Shanghai 200040, China, Tel: +86-21-52888279, Fax: +86-21-62111306, Email:wang.surgerycom
Corresponding authors: Dr. Zhong Wang, Department of Urology, Shanghai Ninth People's Hospital, 639 Zhizaoju Road, Shanghai 200011, China, Tel: +86-21-23271699-5116, Fax: +86-21-63136856, Email: zhongwang2000com or Dr. Xiang Wang, Department of Urology, Shanghai HuaShan Hospital, 12 Middle Wulumuqi Road, Shanghai 200040, China, Tel: +86-21-52888279, Fax: +86-21-62111306, Email:wang.surgerycom

 Global reach, higher impact
Global reach, higher impact