3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2015; 6(9):913-921. doi:10.7150/jca.12162 This issue Cite
Research Paper
Association between Dietary Vitamin C Intake and Risk of Prostate Cancer: A Meta-analysis Involving 103,658 Subjects
1. School of Life Science and Biotechnology, Dalian University of Technology, Dalian, China
2. School of Life Science and Medicine, Dalian University of Technology, Panjin, China
Received 2015-3-18; Accepted 2015-6-11; Published 2015-7-28
Abstract
We attempted to systematically determine the association between dietary intake of vitamin C and risk of prostate cancer. PubMed and Embase were searched to obtain eligible studies published before February 2015. Cohort or case-control studies that reported the relative risk (RR)/odds ratio (OR) estimates with 95% confidence intervals (CIs) for the association between vitamin C intake and prostate cancer risk were included. Eighteen studies regarding dietary vitamin C intake were finally obtained, with a total of 103,658 subjects. The pooled RR of prostate cancer for the highest versus the lowest categories of dietary vitamin C intake was 0.89 (95%CI: 0.83-0.94; p = 0.000) with evidence of a moderate heterogeneity (I2 = 39.4%, p = 0.045). Meta-regression analysis suggested that study design accounted for a major proportion of the heterogeneity. Stratifying the overall study according to study design yielded pooled RRs of 0.92 (95%CI: 0.86-0.99, p = 0.027) among cohort studies and 0.80 (95%CI: 0.71-0.89, p = 0.000) among case-control studies, with no heterogeneity in either subgroup. In the dose-response analysis, an inverse linear relationship between dietary vitamin C intake and prostate cancer risk was established, with a 150 mg/day dietary vitamin C intake conferred RRs of 0.91 (95%CI: 0.84-0.98, p = 0.018) in the overall studies, 0.95 (95%CI: 0.90-0.99, p = 0.039) in cohort studies, and 0.79 (95%CI: 0.69-0.91, p = 0.001) in case-control studies. In conclusion, intake of vitamin C from food was inversely associated with prostate cancer risk in this meta-analysis.
Keywords: vitamin C, dietary intake, prostate cancer, risk, meta-analysis
Introduction
Prostate cancer is the second most common cancer in men all over the world [1]. It has the highest incidence rate and is the second leading cause of cancer death among men in the US, with more than 233,000 new cases diagnosed in 2014 [2]. It is believed that both genetic and the environment may be the contributing factors to prostate carcinogenesis [3-5]. Among those who had migrated to the US, the disease has seen a substantial increase compared to their countrymen back home. This appears to suggest that a change in the environment, noticeably in the form of diet and lifestyle, might have been the contributing factors [6]. Thus, nutritional modification has become the focus in the primary prevention of prostate cancer [7], and this has led to so many studies investigating the association between antioxidants intake and the risk of prostate cancer.
Vitamin C or ascorbic acid is considered to be the most important water-soluble antioxidant that is derived mainly from fruit and vegetable sources [8]. Human cannot synthesize vitamin C and therefore has to depend on the diet as a source of it. Vitamin C has been shown to have cancer prevention effect by reducing oxidative DNA damage, including DNA mutations, and thereby protecting against the harmful effects of carcinogens [9, 10]. Epidemiological studies have yielded inconsistent results regarding the relationship between vitamin C intake and the risk of prostate cancer. Vitamin C intake includes vitamin C from foods and supplements, and dietary vitamin C intake refers to vitamin C from foods only [11]. Two meta-analyses examined the relationship between antioxidants from supplements and risk of prostate cancer. These studies found no association between vitamin C from supplements and prostate cancer risk [12, 13]. However, studies on supplement use might give rise to bias the results, due to the fact that people who use supplements may have more health problems [14, 15] and that the duration of supplements use is relatively short-term [16]. Additionally, the effects of supplementary vitamin C intake might be not the same as that of dietary use because of the different absorption or biological activity [16]. In consideration that most of the relevant studies reported the use of vitamin C from foods source and risk of prostate cancer, and there has been no comprehensive quantitative assessment aiming at this topic, we therefore undertook a meta-analysis to assess the relationship between the dietary vitamin C intake and the occurrence of prostate cancer in men.
Materials and Methods
Search strategy
The PubMed and Embase were searched for relevant studies published before February 2015 using the following terms without restrictions: (“vitamin C” OR “ascorbic acid”) AND (“prostate cancer” OR “prostatic cancer”). Furthermore, the reference lists from the relevant articles or reviews were also searched for additional eligible studies. The latest studies were selected when there were duplicates that report the same data or overlapping data.
Eligibility criteria
Studies were included if they met all the following criteria: 1) cohort, case-control, or nested case-control study; 2) association of dietary vitamin C intake with prostate cancer risk; 3) adjusted relative risk (RR)/odds ratio (OR) with corresponding 95% confidence intervals (CIs) were reported or could be calculated. Two investigators retrieved literatures independently for eligibility.
Quality assessment
The Newcastle-Ottawa scale (NOS) was applied to assess the quality of the eligible studies (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). It consists of three perspectives: selection, comparability, and exposure. The NOS scores represented the quality of the studies. Studies with a score equal to or higher than five points were recognized to be high-quality ones [17], whereas studies with scores less than five points were regarded as low-quality ones which would be further excluded.
Data extraction
Two investigators independently extracted the data. The following information was extracted from each eligible study: first author, year of publication, geographic region, study design, study period, ages of participants, range of vitamin C intake dosage (range of exposure), other variables that might have contributed to the disease that were adjusted for in the original studies, and RR (or OR) estimates with 95%CIs for the highest versus lowest categories of dietary vitamin C intake. Additionally, estimate for each category compared with the lowest category of dietary vitamin C intake was also recorded to assess the dose-response effect. Since most of the included studies did not mention the use of supplement intake, we used estimates of vitamin C intake from food. To avoid the confounding effect of covariates on our analysis, the RRs (or ORs) reflecting the greatest degree of control for potential confounders were extracted in the main analysis.
Statistical analysis
Study-specific RR (OR) estimates with 95%CIs for the highest versus lowest categories of dietary vitamin C intake were pooled using Z-test under fixed-effects model (Mantel-Haenszel method) if no significant heterogeneity existed [18]. Otherwise, the random-effects model (DerSimonian-Laird method) was preferred. Heterogeneity across all the studies was assessed using Q-test and I2 statistics [19]. A p value less than 0.1 and/or I2 > 25% was considered to be significant heterogeneity. In the case of heterogeneity, meta-regression with a single covariate analysis was performed to determine the source of heterogeneity. Subgroup analyses were performed according to study design, geographic region, and range of exposure. Sensitivity analysis was performed by omitting one study per cycle of evaluation aiming at assessing the influence of each individual data set to the pooled RRs.
For the dose-response relationship between vitamin C intake and prostate cancer risk, we used the method proposed by Greenland and Longnecker [20] to compute the study-specific trend and 95%CI from the natural RR and 95%CI across all categories of dietary vitamin C intake. A potential nonlinear dose-response relationship between the intake of vitamin C and risk of prostate cancer was observed using restricted cubic splines with three knots, each set at a different percentage (25%, 50%, and 75% ) of the distribution [21]. Studies that reported the number of total subjects and cases, adjusted RR (OR), and corresponding 95%CI for each intake category (three or more categories) were included in the dose-response meta-analysis. The median level of dietary vitamin C intake in each category was assigned to the corresponding RR with 95%CI for each study. For studies in which the median level for each category was not mentioned, we used the mean value by calculating the average of the lower and upper bound. The lower boundary was set to zero when the lowest category was an open-ended category, and the highest open-ended category was assumed to be the same length as its adjacent one [22].
The Begg's funnel plot and Egger's regression were used to detect publication bias among the involved studies, with p < 0.05 considered as significant publication bias [23, 24]. STATA software, version 12.0 (Stata Corporation, College Station, TX, USA) was used to perform all statistical analyses.
Results
Literature search
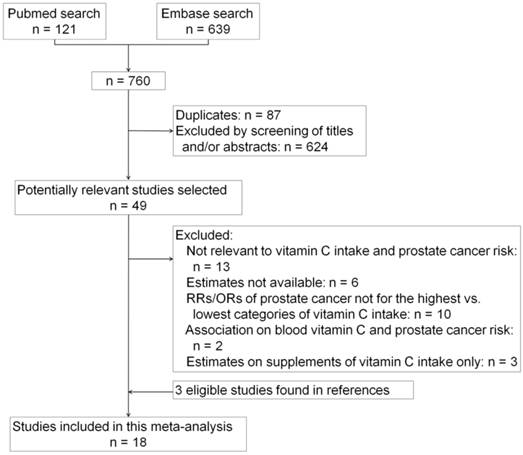
The flow diagram showing the selection of studies obtained from PubMed and Embase searches is presented in Figure 1. A total of 760 studies were initially retrieved from the databases, but after all the duplicated studies were removed, only 673 studies remained. Further elimination of articles that concerned with review, comment, meta-analysis, and meeting abstract, as well as those that were obviously irrelevant after reading the titles and abstracts, only 49 articles remained that potentially investigate the association between vitamin C intake and the risk of prostate cancer. Thirty-four of these articles were excluded because of the following reasons: not relevant to vitamin C intake and the risk of prostate cancer (n = 13); estimates of RR/OR with 95%CI not available (n = 6); RRs/ORs of prostate cancer were not based on the highest versus lowest categories of vitamin C intake (n = 10); association between blood vitamin C levels and the risk of prostate cancer (n = 2); and RRs/ORs on the intake of vitamin C supplements only (n = 3). Three additional eligible articles were obtained from references cited in the relevant articles or reviews. Thus, a total of 18 studies were finally used in this meta-analysis [25-42].
Flow diagram of study selection process.
Study characteristics
The 18 studies aimed at dietary vitamin C intake included 6 cohort studies and 12 case-control studies, and were published between 1992 and 2013, and involved a total of 103,658 subjects (Table 1). Among them, eleven studies were conducted in the United States, six in Europe, and one in South America. All the eligible studies were of high quality owing to the fact that the NOS scores were higher than 5 points among the overall studies. Assessment of the quality of the eligible studies based on the NOS is listed in Table 2.
Characteristics of eligible studies on dietary vitamin C intake and prostate cancer risk.
| Study | Year | Geographic region | Study period | Design | Age, years | Case/ Control | Range of Exposure (mg/d) | Adjustment for Covariates |
|---|---|---|---|---|---|---|---|---|
| Shibaba et al | 1992 | United states | 1981-1989 | Cohort | 68-82 | 208/3,789 | <145(T1); ≥210(T3) | Age, smoking, BMI, and physical activity |
| Daviglus et al | 1996 | United states | 1959-1989 | Cohort | 40-55 | 132/1,767 | ≤74(Q1); >121(Q4) | Age, number of cigarettes smoked per day, dietary cholesterol and saturated fat, ethanol intake, total energy intake, occupation, and education |
| Andersson et al | 1996 | Sweden | 1989-1994 | PCC | cases: 70.7(5.9); control: 70.6(6.2) | 526/536 | 35.7(T1); 86.1(T3) | Age, energy, BMI, physical activity, and nutrient residuals |
| Mayer et al | 1997 | United states | 1990-1993 | HCC | ≥ 45 | 215/593 | Q1-Q4, cut points were not mentioned | Age, education, family history of prostate cancer, BMI, physical activity, and dietary energy |
| Vlajinac et al | 1997 | Yugoslavia | 1990-1994 | HCC | cases: 70.5; control: 71.5 | 101/202 | <84.7(T1); ≥188.7(T3) | Energy, nutrients which were significant between cases and controls, physical activity, specific occupational exposure, nephrolithiasis, other diseases such as chronic bronchitis, chronic rheumatic diseases, hypertension, cardiomyopathia, diabetes mellitus, renal diseases, eye diseases and tuberculosis, greater number of brothers, greater number of sexual partners |
| Key et al | 1997 | UK | 1989-1992 | PCC | mean age of cases and controls were 68.1 | 328/328 | <66.1(T1); ≥104.3(T3) | Energy, social class, height, BMI, age, smoking, family history of prostate cancer, and nutrients intake |
| Demeo-Pellegrini et al | 1999 | Uruguay | 1994-1997 | HCC | 40-89 | 175/233 | ≤85.8(Q1); >161.9(Q4) | Age, residence, urban/rural status, education, family history of prostate cancer, BMI and total energy intake |
| Jain et al | 1999 | United states | 1989-1993 | PCC | cases: 69.8; controls: 69.9 | 617/636 | <121.08(Q1); >243.70(Q4) | Log total energy, vasectomy, age, smoked, marital status, study area, BMI, education, ever-used multivitamin supplements within 1 year, area of study, and log-converted amounts for grains, fruit, vegetables, total plants, total carotenoids, folic acid, dietary fiber, conjugated linoleic acid, vitamin E, retinol, total fat, and linoleic acid |
| Kristal et al | 1999 | United states | 1993-1996 | PCC | 40-64 | 697/666 | Q1-Q4, cut points were not mentioned | Fat, energy, race, age, family history of prostate cancer, BMI, PSA tests in previous 5 years, and education |
| Ramon et al | 2000 | Spain | 1994-1998 | HCC | matched by age (within 5 years) | 217/434 | 104.6(Q1); 165(Q4) | Age, smoking, marital status, number of children, residence, calories, family history, BMI, quartiles of animal fat and α-linolenic acid |
| Cohen et al | 2000 | United states | 1993-1996 | PCC | 40-64 | 628/602 | <70(Q1); ≥150(Q4) | Fat, energy, race, age, family history of prostate cancer, BMI, prostate-specific antigen tests in previous 5 years, education, and intake of fruits and vegetables per week and nutrients per day |
| McCann et al | 2005 | United states | 1986-1991 | PCC | controls were matched to cases on age | 433/538 | ≤139(Q1); >240(Q4) | Age, education, BMI, cigarette smoking status, total energy and vegetable intake |
| Kirsh et al | 2006 | United states | 1993-2001 | Cohort | 55-74 | 1,338/28,023 | 77(Q1); 268(Q5) | Age, total energy, race, study center, family history of prostate cancer, BMI, smoking status, physical activity, total fat intake, red meat intake, history of diabetes, aspirin use, and number of screening examinations during the follow-up period |
| Rohrmann et al | 2007 | United states | 1992-2000 | Cohort | 46-81 | 6,092/18,373 | 79(Q1); 265(Q5) | Age, race or ethnicity, cigarette smoking, BMI, leisure-time physical activity, alcohol consumption, energy intake, intake of protein, and intake of polyunsaturated fatty acids |
| Kristal et al | 2008 | United states | 1994-2003 | Cohort | 54-86 | 876/3,894 | <69.8(Q1); ≥194.0(Q5) | Age, race/ethnicity, waist/hip ratio, smoking, BMI, physical activity, and total energy |
| Bidoli et al | 2009 | Italy | 1991-2002 | HCC | 46-74 | 1,294/1,451 | <95.8(T1); ≥139.9(T3) | Age, study center, period of interview, education, physical activity, BMI, alcohol intake, smoking habits, family history of prostate cancer and total energy intake, according to the residual model |
| Lewis et al | 2009 | United states | 1998-2004 | PCC | cases: 63.3(8.2); controls:62.0(10.7) | 478/382 | ≤90.7(T1); ≥143.3(T3) | Age, education, BMI, smoking history, family history of prostate cancer in first-degree relatives, and total caloric intake |
| Roswall et al | 2013 | Denmark | 1993-2010 | Cohort | 50-64 | 1,571/25,285 | ≤70.6(Q1); >121.5(Q4) | Intake of folate, vitamin E, and beta-carotene for both dietary and supplemental exposure, height, weight, education, intake of red meat, alcohol consumption, selenium intake, smoking, and physical activity |
Abbreviations: HCC, hospital-based case-control study; PCC, population-based case-control study; BMI, body mass index; Q, quartile/quintile; T, tertile.
Range of exposure indicates the cutoff points for the highest and lowest categories of dietary vitamin C intake.
Assessment of the quality of the eligible studies based on NOS1.
| Case-control study | Selection | Comparability6 | Exposure | Total | |||||
| Definition2 | Representativeness3 | Selection4 | Definition5 | Ascertainment7 | Method8 | Rate9 | |||
| Andersson et al(1996) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Mayer et al(1997) | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Vlajinac et al(1997) | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Key et al(1997) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Demeo-Pellegrini et al(1999) | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Jain et al(1999) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Kristal et al(1999) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Ramon et al(2000) | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Cohen et al(2000) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| McCann et al(2005) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Bidoli et al(2009) | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Lewis et al(2009) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Cohort study | Selection | Comparability6 | Outcome | Total | |||||
| Representativeness10 | Selection11 | Ascertainment7 | Demonstration12 | Assessment13 | Duration14 | Adequacy15 | |||
| Shibaba et al(1992) | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 |
| Daviglus et al(1996) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Kirsh et al(2006) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Rohrmann et al(2007) | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Kristal et al(2008) | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Roswall et al(2013) | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
1Assessed with the 9-star Newcastle-Ottawa Scale (NOS); 2Adequate definition of cases (0, 1); 3Consecutive or obviously representative series of cases (0, 1); 4Selection of controls: Community controls (0, 1); 5Definition of controls: No history of disease (0, 1); 6Study controls for the most important factor or any additional factor (0, 1, 2); 7Secure record (0, 1); 8Same method of ascertainment for cases and controls (0, 1); 9Same non-response rate for both groups (0, 1); 10Truly or somewhat representative of the exposed cohort (0, 1); 11Selection of the non exposed cohort (0, 1); 12Demonstration that outcome of interest was not present at start of study (0, 1); 13Assessment of outcome (0, 1); 14Follow-up long enough for outcomes to occur (0, 1); 15Adequacy of follow up of cohorts (0, 1).
High versus low dietary vitamin C intake
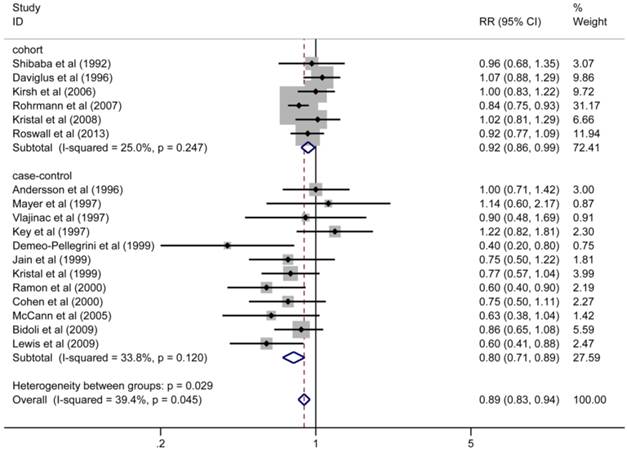
The multivariate-adjusted RRs (ORs) for the highest versus lowest categories of dietary vitamin C intake in each study were pooled using the random-effects model with a moderate heterogeneity (pooled RR = 0.89, 95%CI 0.83-0.94, p = 0.000; I2 = 39.4%, p = 0.045). Meta-regression with a single covariate was performed based on the year of publication, study design, sample size, geographic region, and range of vitamin C intake. We found that the heterogeneity may come from the study design (p < 0.05), which was also confirmed by subgroup analysis (see below).
Subgroup and sensitivity analyses
Subgroup analyses were conducted for those studies that examine the association of dietary vitamin C intake with prostate cancer risk. When stratified by study design, the pooled RRs were statistically significant among cohort studies (RR = 0.92, 95%CI 0.86-0.99, p = 0.027) and case-control studies (RR = 0.80, 95%CI 0.71-0.89, p = 0.000) with no significant heterogeneity in each subgroup (for cohort studies: I2 = 25.0%, p = 0.247; for case-control studies: I2 = 33.8%, p = 0.120) (Figure 2). A relative higher quality for the pooling analysis was achieved because the majority of subjects involving in our analysis were from cohort studies (sample size in cohort studies accounted for 72.4%), which are more powerful for identifying the risk factors and are typically ranked higher in the hierarchy of evidence compared with case-control studies. Stratifying by geographic region, the pooled RRs of prostate cancer for the highest versus lowest categories of dietary vitamin C intake were 0.89 (95%CI: 0.83-0.95) for studies conducted in the United States and 0.90 (95%CI: 0.80-1.02) in Europe. No significant heterogeneity was observed among studies in region-stratified subgroups (p > 0.1). With stratified analysis that was based on the range of exposure, the pooled RRs of prostate cancer were 0.89 (95%CI: 0.81-0.97) in the subgroup with wide exposure range (difference in median vitamin C intake between the highest and lowest categories was equal or more than 150 mg/day) and 0.84 (95%CI: 0.71-1.00) in subgroup with narrow exposure range (difference in median vitamin C intake between the highest and lowest categories was less than 150 mg/day). The main results of subgroup analyses are listed in Table 3. Sensitivity analysis was conducted by omitting one study at a time and recalculating the pooled RR. Overall, the corresponding pooled RRs were not substantially altered, suggesting that the results of this meta-analysis were stable.
Dose-response meta-analysis
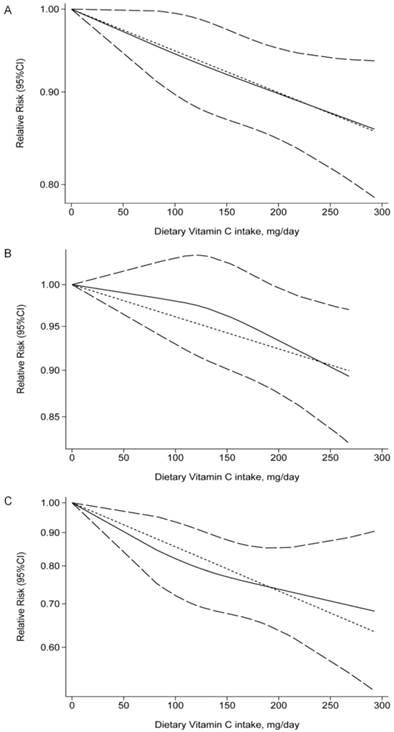
Dose-response relationship between dietary vitamin C intake and the risk of prostate cancer was assessed. Statistical significance (p < 0.05) was determined by nonlinear test for dose-response relationship. A dietary vitamin C intake of 150 mg/day conferred an RR of 0.91 (95%CI: 0.84-0.98, p = 0.018; Figure 3A). A 150 mg/day increment of dietary vitamin C intake reduced prostate cancer risk of 5% (95%CI: 0.90-0.99, p = 0.039) in cohort studies (Figure 3B) and 21% (95%CI: 0.69-0.91, p = 0.001) in case-control studies (Figure 3C), respectively.
Publication bias
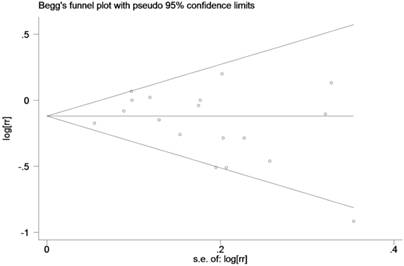
No statistically significance of publication bias was detected in the overall study, as revealed by Begg's funnel plot (p = 0.173; Figure 4) and Egger's regression (p = 0.295).
Association between dietary vitamin C intake and prostate cancer risk stratified by study design, geographic region, and range of exposure for the highest versus lowest categories.
| Subgroups | Number of studies | Test of heterogeneity | Test of association | |||||
|---|---|---|---|---|---|---|---|---|
| Q | p | I2 (%) | RR | 95% CI | Z | p | ||
| Study design | ||||||||
| Cohort | 6 | 6.67 | 0.247 | 25.0 | 0.92 | 0.86-0.99 | 2.21 | 0.027 |
| Case-control | 12 | 16.62 | 0.120 | 33.8 | 0.80 | 0.71-0.89 | 3.92 | 0.000 |
| Geographic region | ||||||||
| United States | 11 | 16.22 | 0.101 | 38.2 | 0.89 | 0.83-0.95 | 3.35 | 0.001 |
| Europe | 6 | 6.64 | 0.249 | 24.7 | 0.90 | 0.80-1.02 | 1.68 | 0.076 |
| Range of exposure | ||||||||
| ≥150 mg/day | 7 | 6.38 | 0.382 | 5.9 | 0.89 | 0.81-0.97 | 2.57 | 0.010 |
| <150 mg/day | 9 | 20.18 | 0.010 | 60.3 | 0.84 | 0.71-1.00 | 2.02 | 0.044 |
Adjusted RRs of prostate cancer for the highest versus lowest categories of dietary vitamin C intake stratified by study design.
Discussion
The current meta-analysis incorporated eighteen studies on dietary intake of vitamin C and the risk of prostate cancer, with a total of 103,658 subjects. The pooled estimates indicated that a higher vitamin C intake from food might provide protection against prostate cancer. Stratification by study design showed that the pooled RRs of both cohort and case-control subgroups for the association between vitamin C intake and the risk of prostate cancer were statistically significant, with no indication of heterogeneity. The dose-response analysis found an inverse linear relation between the dietary intake of vitamin C and the risk of prostate cancer in the overall study, with a 9% reduction in risk for every 150 mg/day increment in vitamin C intake (Figure 3A). When sub-analyzed by study design, the dose-response graphs showed that 5% reduction in prostate cancer risk among the cohort studies (RR = 0.95, 95%CI = 0.90-0.99, p = 0.039) and 21% reduction among the case-control studies (RR = 0.79, 95%CI = 0.69-0.91, p = 0.001) for every 150 mg/day increment in dietary vitamin C intake. Sample size in cohort studies accounted for 72.4%, which is far higher than that observed for the case-control studies, indicating a relative higher quality for the pooled analysis (Figure 2). Subgroup analysis carried out according to geographic region suggested that the protective effect of vitamin C from food against prostate cancer was more conspicuous in the United States (RR = 0.89, 95%CI 0.83-0.95, p = 0.001), where no significant heterogeneity was detected for each region-specific subgroup. Despite the pooled estimate in Europe not being statistically significant, the overall result indicated that higher intake of dietary vitamin C has a trend to prevent the occurrence of prostate cancer in Europe (RR = 0.90, 95%CI 0.80-1.02, p = 0.076).
Dose-response relationship between dietary vitamin C intake and the relative risk of prostate cancer in the overall studies (A), cohort studies (B), and case-control studies (C). Dietary vitamin C intake were modeled with a linear trend in a random-effects meta-regression model. The solid line represents association between dietary vitamin C intake and prostate cancer risk. Long dashed lines indicate 95% confidence intervals.
Begg's funnel plot to explore the publication bias in the overall studies (z = 1.36, p = 0.173).
Vitamin C is considered to have a potential role in the chemoprevention of cancer, due to its function as a scavenger of free radicals, as well as the role it plays in the recycling of vitamin E and in reducing oxidative DNA damage [9, 10, 43]. In vitro studies have shown that vitamin C could inhibit the growth and viability of prostate cancer cells [44]. As human cannot synthesize vitamin C, but depends on a dietary or supplementary source, such vitamin C intake is recognized as essential for primary cancer prevention [45]. Numerous epidemiological studies have explored the use of vitamin C in preventing the initiation of different cancers. Data from the previous meta-analyses have suggested that vitamin C intake is associated with reduced morbidity from breast cancer [46], and reduced risks of colorectal adenoma [47], lung cancer [48], and endometrial cancer [49]. Many studies examining the association of vitamin C intake with prostate cancer risk have yielded inconsistent results. A previous study in which meta-analysis have been performed, reported that vitamin C supplement did not reduce the incidence and mortality of prostate cancer with 2 trials involved [12]. Another meta-analysis which included 3 trials to evaluate the effect of the use of supplement vitamin C on the occurrence of prostate cancer, also failed to detect any association [13]. However, the number of subjects included in the previous analyses for the association of supplement vitamin C with prostate cancer was too small to do summarized analyses. In addition, results on supplement use of vitamin C and prostate cancer risk may not be consistent with results on intake from food due to the different intake duration, absorptive pattern, and biological activity in vivo [16]. Additionally, diet has been reported to potentially play a role in a man's risk for prostate cancer [6, 50]. The latest review with regard to the dietary factors and prostate cancer incidence indicated that vitamin C might function both as pro-oxidant and antioxidant for the initiation and development of prostate cancer, and the relationship between dietary vitamin C intake and prostate cancer risk needed clarification [51]. To our knowledge, there has been no meta-analysis examining the effect of vitamin C intake on the risk of prostate cancer in a dietary manner which refers to vitamin C from foods only. Owing to the mentioned above, we have systemically performed a meta-analysis to evaluate the association between dietary vitamin C intake and the risk of prostate cancer based on the RRs/ORs for the highest versus lowest categories and the dose-response analyses.
We observed a moderate heterogeneity between studies in the overall analysis. The heterogeneity between studies disappeared in each subgroup when stratification was used, suggesting that the source of the heterogeneity might have come from study design. Subsequent meta-regression also confirmed this result. Sensitivity analysis revealed the pooled RRs were not altered by omitting a single study each time, indicating that the results were stable. To assess the publication bias, the Begg's and Egger's tests were performed. The results of these tests suggested that no publication bias existed.
There were some limitations in the current meta-analysis. First, the inherent confounding factors in the included studies could not be solved by meta-analysis. Although the estimates from all the eligible studies were adjusted for other possible risk factors for prostate cancer, there might be unknown confounders in the controls of either the case-control or cohort studies that could not be excluded, which might have given rise to bias in the results. For instance, it is suggested that the effect of dietary vitamin C might be attenuated after adjusting for total vegetable intake [52]. In the 18 eligible studies, only three studies adjusted for total vegetable intake [32, 35, 36], while the other study did not make such adjustment. Second, the width of the cutoff points for the highest versus lowest categories of dietary vitamin C intake was different among studies, and this might also have influenced the pooled analysis. Therefore, we undertook the dose-response analysis, which can avoid the influence of different cut-off points, to show the RR per unit increase (150 mg/day) in the dose-response graph. Third, it is known that cohort studies are more powerful for identifying the risk factors and are typically ranked higher in the hierarchy of evidence, compared with the case-control studies. The case-control studies were included in our analysis despite the fact that only a relatively small proportion of these studies in sample size were included (27.6% among the overall population; Figure 2). Considering that the case-control studies are susceptible to recall bias and selection bias, we performed the sub-analyses by study design in both pooled and dose-response statistics. Finally, all the studies used for this analysis were concerned with the intake of dietary vitamin C only, since the number of subjects concerned in the use of vitamin C supplements and risk of prostate cancer were too small to be summarized. Thus, the supplements should be assessed when there are enough data.
In conclusion, findings from the present meta-analysis showed that intake of vitamin C from food was inversely associated with prostate cancer risk. The dose-response analysis found an inverse linear relationship between dietary vitamin C intake and the risk of prostate cancer, with a 9% reduction in risk for each 150 mg/day increment. It has been suggested that dietary intake of vitamin C-riched fruit and vegetables might prevent the onset of prostate cancer. However, the evaluation of the role of vitamin C intake in prostate cancer carcinogenesis should be confirmed in-depth, and large randomized clinical trials for vitamin C intake are highly preferred to get a more precise estimate for the exposure factor associated with prostate cancer.
Acknowledgements
We thank Dr. Alan K. Chang for the contribution to the manuscript preparation. This research was supported by grants (31171353, 31271500 to H.W and 81301504 to M.W) from National Natural Science Foundation of China and grants (973 Program 2011CB504201 to H.W) from the Ministry of Science and Technology of China.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mandair D, Rossi RE, Pericleous M, Whyand T, Caplin ME. Prostate cancer and the influence of dietary factors and supplements: a systematic review. Nutr Metab (Lond). 2014;11:30
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29
3. Haj-Ahmad TA, Abdalla MA, Haj-Ahmad Y. Potential Urinary miRNA Biomarker Candidates for the Accurate Detection of Prostate Cancer among Benign Prostatic Hyperplasia Patients. J Cancer. 2014;5:182-91
4. Ao X, Liu Y, Bai XY, Qu X, Xu Z, Hu G. et al. Association between EHBP1 rs721048(A>G) polymorphism and prostate cancer susceptibility: a meta-analysis of 17 studies involving 150,678 subjects. Onco Targets Ther. 2015;8:1671-80
5. Pan H, Niu W, He L, Wang B, Cao J, Zhao F. et al. Contributory role of five common polymorphisms of RAGE and APE1 genes in lung cancer among Han Chinese. PLoS One. 2013;8:e69018
6. Sonn GA, Aronson W, Litwin MS. Impact of diet on prostate cancer: a review. Prostate Cancer Prostatic Dis. 2005;8:304-10
7. Blumenfeld AJ, Fleshner N, Casselman B, Trachtenberg J. Nutritional aspects of prostate cancer: a review. Can J Urol. 2000;7:927-35 discussion 36
8. Putchala MC, Ramani P, Sherlin HJ, Premkumar P, Natesan A. Ascorbic acid and its pro-oxidant activity as a therapy for tumours of oral cavity -- a systematic review. Arch Oral Biol. 2013;58:563-74
9. Lee KW, Lee HJ, Surh YJ, Lee CY. Vitamin C and cancer chemoprevention: reappraisal. Am J Clin Nutr. 2003;78:1074-8
10. Han X, Li J, Brasky TM, Xun P, Stevens J, White E. et al. Antioxidant intake and pancreatic cancer risk: the Vitamins and Lifestyle (VITAL) Study. Cancer. 2013;119:1314-20
11. Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29:3775-82
12. Jiang L, Yang KH, Tian JH, Guan QL, Yao N, Cao N. et al. Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta-analysis of randomized controlled trials. Nutr Cancer. 2010;62:719-27
13. Stratton J, Godwin M. The effect of supplemental vitamins and minerals on the development of prostate cancer: a systematic review and meta-analysis. Fam Pract. 2011;28:243-52
14. Bender MM, Levy AS, Schucker RE, Yetley EA. Trends in prevalence and magnitude of vitamin and mineral supplement usage and correlation with health status. J Am Diet Assoc. 1992;92:1096-101
15. Ferrucci LM, McCorkle R, Smith T, Stein KD, Cartmel B. Factors related to the use of dietary supplements by cancer survivors. J Altern Complement Med. 2009;15:673-80
16. Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC. et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223-9
17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-5
18. Bai XY, Li S, Wang M, Qu X, Hu G, Xu Z. et al. Association of monocyte chemoattractant protein-1 (MCP-1)-2518A>G polymorphism with susceptibility to coronary artery disease: a meta-analysis. Ann Hum Genet. 2015;79:173-87
19. Pietrzak A, Mosiewicz J, Chodorowska G, Brzozowska A, Michalak-Stoma A, Szumilo J. et al. Eruption of palmoplantar pustular psoriasis in patient treated with anti-androgen therapy for prostate cancer and aggravation of lesions after statin treatment. Central European Journal of Medicine. 2014;9:657-62
20. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301-9
21. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-83
22. Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E. et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617
23. Bannerman B, Xu L, Jones M, Tsu C, Yu J, Hales P. et al. Preclinical evaluation of the antitumor activity of bortezomib in combination with vitamin C or with epigallocatechin gallate, a component of green tea. Cancer Chemotherapy and Pharmacology. 2011;68:1145-54
24. Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO. et al. Photodynamic therapy of cancer: An update. CA Cancer Journal for Clinicians. 2011;61:250-81
25. Shibata A, Paganini-Hill A, Ross RK, Henderson BE. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br J Cancer. 1992;66:673-9
26. Daviglus ML, Dyer AR, Persky V, Chavez N, Drum M, Goldberg J. et al. Dietary beta-carotene, vitamin C, and risk of prostate cancer: Results from the Western Electric Study. Epidemiology. 1996;7:472-7
27. Andersson SO, Wolk A, Bergstrom R, Giovannucci E, Lindgren C, Baron J. et al. Energy, nutrient intake and prostate cancer risk: a population-based case-control study in Sweden. Int J Cancer. 1996;68:716-22
28. Meyer F, Bairati I, Fradet Y, Moore L. Dietary energy and nutrients in relation to preclinical prostate cancer. Nutr Cancer. 1997;29:120-6
29. Vlajinac HD, Marinkovic JM, Ilic MD, Kocev NI. Diet and prostate cancer: a case-control study. Eur J Cancer. 1997;33:101-7
30. Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT. A case-control study of diet and prostate cancer. Br J Cancer. 1997;76:678-87
31. Deneo-Pellegrini H, De Stefani E, Ronco A, Mendilaharsu M. Foods, nutrients and prostate cancer: a case-control study in Uruguay. Br J Cancer. 1999;80:591-7
32. Jain MG, Hislop GT, Howe GR, Ghadirian P. Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer. 1999;34:173-84
33. Kristal AR, Stanford JL, Cohen JH, Wicklund K, Patterson RE. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:887-92
34. Ramon JM, Bou R, Romea S, Alkiza ME, Jacas M, Ribes J. et al. Dietary fat intake and prostate cancer risk: a case-control study in Spain. Cancer Causes Control. 2000;11:679-85
35. Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61-8
36. McCann SE, Ambrosone CB, Moysich KB, Brasure J, Marshall JR, Freudenheim JL. et al. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York. Nutr Cancer. 2005;53:33-41
37. Kirsh VA, Hayes RB, Mayne ST, Chatterjee N, Subar AF, Dixon LB. et al. Supplemental and dietary vitamin E, beta-carotene, and vitamin C intakes and prostate cancer risk. J Natl Cancer Inst. 2006;98:245-54
38. Rohrmann S, Giovannucci E, Willett WC, Platz EA. Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am J Clin Nutr. 2007;85:523-9
39. Kristal AR, Arnold KB, Schenk JM, Neuhouser ML, Goodman P, Penson DF. et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2008;167:925-34
40. Bidoli E, Talamini R, Zucchetto A, Bosetti C, Negri E, Lenardon O. et al. Dietary vitamins E and C and prostate cancer risk. Acta Oncol. 2009;48:890-4
41. Lewis JE, Soler-Vila H, Clark PE, Kresty LA, Allen GO, Hu JJ. Intake of plant foods and associated nutrients in prostate cancer risk. Nutr Cancer. 2009;61:216-24
42. Roswall N, Larsen SB, Friis S, Outzen M, Olsen A, Christensen J. et al. Micronutrient intake and risk of prostate cancer in a cohort of middle-aged, Danish men. Cancer Causes Control. 2013;24:1129-35
43. Zhao F, Wang M, Li S, Bai X, Bi H, Liu Y. et al. DACH1 inhibits SNAI1-mediated epithelial-mesenchymal transition and represses breast carcinoma metastasis. Oncogenesis. 2015;4:e143
44. Willis MS, Wians FH. The role of nutrition in preventing prostate cancer: a review of the proposed mechanism of action of various dietary substances. Clin Chim Acta. 2003;330:57-83
45. Wisard M, Pescia G. Urology. Revue Medicale Suisse. 2010;6:145-9
46. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-31
47. Xu X, Yu E, Liu L, Zhang W, Wei X, Gao X. et al. Dietary intake of vitamins A, C, and E and the risk of colorectal adenoma: a meta-analysis of observational studies. Eur J Cancer Prev. 2013;22:529-39
48. Luo J, Shen L, Zheng D. Association between vitamin C intake and lung cancer: a dose-response meta-analysis. Sci Rep. 2014;4:6161
49. Bandera EV, Gifkins DM, Moore DF, McCullough ML, Kushi LH. Antioxidant vitamins and the risk of endometrial cancer: a dose-response meta-analysis. Cancer Causes Control. 2009;20:699-711
50. Dunn JE. Cancer epidemiology in populations of the United States--with emphasis on Hawaii and California--and Japan. Cancer Res. 1975;35:3240-5
51. Lin PH, Aronson W, Freedland SJ. Nutrition, dietary interventions and prostate cancer: the latest evidence. BMC Med. 2015;13:3
52. Vance TM, Su J, Fontham ET, Koo SI, Chun OK. Dietary antioxidants and prostate cancer: a review. Nutr Cancer. 2013;65:793-801
Author contact
![]() Corresponding authors: Huijian Wu, PhD, School of Life Science and Biotechnology, Dalian University of Technology, No. 2, Ling Gong Road, Dalian, 116024, China. Tel: 86-411-84706105; Fax: 86-411-84706105; E-mail: wuhjedu.cn or Miao Wang, PhD, School of Life Science and Biotechnology, Dalian University of Technology, No. 2, Ling Gong Road, Dalian, 116024, China. Tel: 86-411-84706105; Fax: 86-411-84706105; E-mail: wangmedu.cn
Corresponding authors: Huijian Wu, PhD, School of Life Science and Biotechnology, Dalian University of Technology, No. 2, Ling Gong Road, Dalian, 116024, China. Tel: 86-411-84706105; Fax: 86-411-84706105; E-mail: wuhjedu.cn or Miao Wang, PhD, School of Life Science and Biotechnology, Dalian University of Technology, No. 2, Ling Gong Road, Dalian, 116024, China. Tel: 86-411-84706105; Fax: 86-411-84706105; E-mail: wangmedu.cn

 Global reach, higher impact
Global reach, higher impact