3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2015; 6(10):970-975. doi:10.7150/jca.12471 This issue Cite
Research Paper
Prognostic Impact of ABO Blood Group on the Survival in Patients with Ovarian Cancer
1. Xiamen Cancer Center, Department of Obstetrics and Gynecology, the First Affiliated Hospital of Xiamen University, Xiamen, People's Republic of China
2. Department of Basic Medical Science, Medical College, Xiamen University, Xiamen People's Republic of China
3. Sun Yat-sen University Cancer Center, Department of Radiation Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, People's Republic of China
4. Xiamen Cancer Center, Department of Radiation Oncology, the First Affiliated Hospital of Xiamen University, Xiamen, People's Republic of China
* Juan Zhou, Li-Chao Yang and Zhen-Yu He contributed equally to this work.
Received 2015-4-22; Accepted 2015-6-12; Published 2015-8-8
Abstract
Purpose: The impact of ABO blood group on the survival of patients with ovarian cancer remains uncertain. The aim of this study was to evaluate the prognostic value of the ABO blood group in ovarian cancer patients.
Methods: 256 ovarian cancer patients who received a cytoreductive surgery were retrospectively reviewed. The prognostic impact of the ABO blood group with respect to overall survival (OS) was analyzed.
Results: The median follow-up time was 57 months and the 5-year OS was 70.1%. The 5-year OS were 55.0%, 83.3%, 82.5%, and 70.0% in patients with A, B, AB, and O blood type, respectively (p = 0.003). Patients with blood type A had a poorer 5-year OS than patients with blood type non-A (55.0% vs. 75.0%, p = 0.001), especially in patients with age > 50 years (40.0% vs. 62.5%, p = 0.004). Univariate Cox analyses showed that blood type A was significantly associated with OS than those with non-A types (hazard ratio (HR) 2.210, 95% confidence interval (CI) 1.373-3.557, p = 0.001). Blood type A remained an independent prognostic factor for OS than those with non-A blood types in multivariate analyses (HR 2.235, 95% CI 1.360-3.674, p = 0.002).
Conclusion: ABO blood group is associated with survival in patients with ovarian cancer, patients with blood type A had a significantly worse OS than patients with non-A blood types, especially in patients with age > 50 years.
Keywords: Ovarian cancer, ABO blood group, prognosis, cytoreductive surgery, overall survival
Introduction
Ovarian cancer is a highly fatal gynecologic cancer and the fifth leading cause of cancer mortality in women, with 14,030 deaths occurring in the United States per year (1). In China, ovarian cancer is the tenth cancer incidence rate of 7.95 per 100,000 women during 2009 (2). Most cases of ovarian cancer are diagnosed at an advanced stage and quickly progress, resulting in a poor outcome.
At present, prognostic factors for ovarian cancer reflect the intrinsic biology of a tumor (e.g. histological subtype, grade of differentiation), cancer stage, residual disease and the performance status (3-5). Although prognostic factors for ovarian cancer have conducted intensive studies, including in the field of molecular biology, but for the prognosis of ovarian cancer can be different despite similar stages and grades. A better understanding of an ideal biomarker with readily available, inexpensive and reproducible of ovarian cancer could improve the prognosis of patients and provide therapies that are more appropriate.
Recently, the associations between ABO blood group and survival have been evaluated in several malignancy, including pancreatic cancer (6), esophageal squamous cell carcinoma (7), colon cancer (8), nasopharyngeal carcinoma (9), and breast cancer (10). Previous studies suggested that blood type A was associated with an increased risk of ovarian cancer (11,12), while another study presence of the B antigen was positively associated with ovarian cancer incidence (13). However, to date, studies of the impact of ABO blood group on the survival of patients with ovarian cancer remained uncertain. Therefore, the aim of the retrospective analysis was to analyze the relationship between ABO blood type and the survival of patients with ovarian cancer underwent cytoreductive surgery as their primary treatment in Chinese population.
Materials and methods
Patients
Two hundred fifty-six patients with primary ovarian cancer who underwent cytoreductive surgery at the Sun Yat-Sen University Cancer Center between December 2004 and March 2012 were retrospectively analyzed. Cytoreductive surgery was considered to have achieved optimal debulking when the residual disease was < 1 cm. Patients with synchronous or metachronous tumors, borderline tumors and advanced stage (stage IV) ovarian cancer were excluded. Other patients with missing data were also excluded. Patients who with indications for chemotherapy did not complete at least four cycles of chemotherapy were excluded. Patients were considered eligible only when the following data were available. The study was approved by the ethics committee of Sun Yat-Sen University Cancer Center. All patients provided written consent for storage of their information in the hospital database, and for the research use of the information.
Clinicopathological factors
Clinicopathological factors were used to assess the risk of death. Factors examined included age, histological type, histological type grade, lymph node status, FIGO stage, CA125 at diagnosis, and ABO blood group.
Follow-up and survival endpoints
Follow up was performed every 3-6 months. The follow-up visits consisted of a physical examination and laboratory studies at least every 6 months or when clinically indicated. The endpoint of the study was overall survival (OS). OS was calculated as the period from the date of diagnosis to the date of death from any cause or the date of last follow-up.
Statistical analysis
The χ2 and Fisher's exact tests were used to analyze the differences between qualitative data. Calculation of survival rates were plotted by the Kaplan-Meier method, and compared using the log-rank test. Univariate and multivariate Cox regression analyses were performed. Multivariable analyses were performed for factors which were significantly associated with OS in univariate analyses. All data were analyzed the SPSS statistical software package, version 16.0 (IBM Corporation, Armonk, NY, USA). A value of p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 256 eligible patients with ovarian cancer were identified at our institution during the study period from December 2004 to March 2012. The median age of the patients was 50 years (range, 19-76 years). The distribution of the ABO blood type was 60 blood type A patients (23.4%), 52 blood type B patients (20.3%), 120 blood type O patients (46.9%), and 24 blood type AB patients (9.4%). Table 1 summarizes the characteristics of the study population. χ2 test showed that ABO blood type positively correlated with age (p < 0.001) and CA-125 at diagnosis (p = 0.046).
Effect of ABO blood group on survival
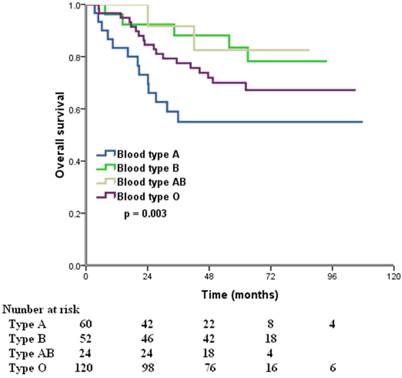
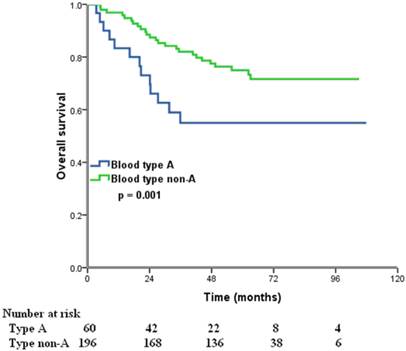
The median follow-up time was 57 months (range, 8.2-107.9 months) at the last clinical follow-up. A total of 76 patients died, 73 of whom died of ovarian cancer-related diseases, 3 died of other diseases. The 5-year OS for all patients was 70.1%. In the Kaplan-Meier analysis, ABO blood type was associated with OS, the 5-year OS were 55.0%, 83.3%, 82.5%, and 70.0% in patients with A, B, AB, and O blood type, respectively (p = 0.003) (Figure 1). Furthermore, we compared the prognostic impact of the ABO groups with type A and non-A types, patients with non-A types had a better 5-year OS than patients with blood type A (75.0% vs. 55.0%, p = 0.001) (Figure 2).
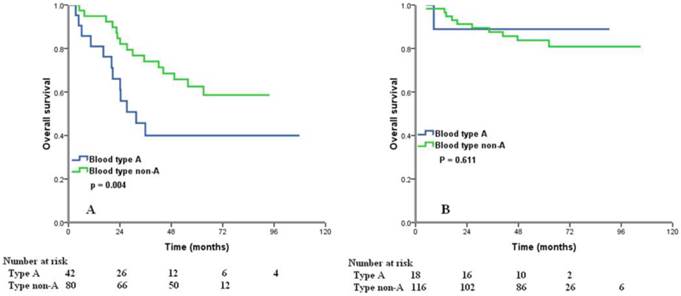
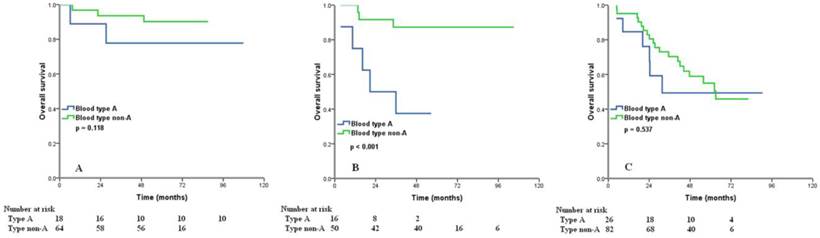
We examined the prognostic effect of the ABO blood type according to different age group and different FIGO stage. Significant differences in 5-year OS between the type A and non-A types ovarian cancers were observed in patients with age > 50 years (40.0% vs. 62.5%, p = 0.004) (Figure 3A). However, for age ≤50 years patients, ABO groups showed no association with OS (p = 0.611) (Figure 3B). In patients with FIGO stage II, type A tumors had worse OS than those with non-A types (5-year OS 37.5% vs. 87.3%, p < 0.001) (Figure 4B), but there was no significant difference from the OS of FIGO stage I (p = 0.118) (Figure 4A) and III (p = 0.537) (Figure 4C) according to different blood group.
To determine whether ABO blood type could serve as an independent prognostic factor, we examined OS using the Cox proportional hazards model. Compared with patients with blood type A, the hazard ratios (HR) for OS in univariate analysis among patients with blood type B, AB, and O were 0.318 (95% confidence interval [CI] 1.153-0.662, p = 0.002), 0.280 (95% CI 0.098-0.802, p = 0.018), and 0.555 (95% CI 0.335-0.920, p = 0.220), respectively. However, no significant differences in OS were observed for patients with blood type O or AB in univariate analysis when compared with patients with blood type B. We next examined the effect of ABO blood group in terms of type A and non-A types. Blood type A was significantly associated with OS than those with non-A types (HR 2.210, 95%CI 1.373-3.557, p = 0.001) in univariate analysis. In addition, the higher risks of death for patients with type A vs. non-A types remained significant in multivariate analyses (HR 2.235, 95% CI 1.360-3.674, p = 0.002). Other significant prognostic factors included age, histological grade, and FIGO stage.
Overall survival for patients with ovarian cancer according to different ABO blood types.
Overall survival for patients with ovarian cancer with blood type A and non-A blood types.
Correlation between ABO blood group and clinicopathological characteristics
| Characteristic | A (n = 60) | B (n = 52) | O (n = 120) | AB (n = 24) | p-value |
|---|---|---|---|---|---|
| Age (y) | |||||
| ≤ 50 | 18 | 30 | 68 | 18 | < 0.001 |
| > 50 | 42 | 22 | 52 | 6 | |
| Histology | |||||
| Serous | 52 | 42 | 98 | 16 | 0.210 |
| Non-serous | 8 | 10 | 22 | 8 | |
| Grading | |||||
| Well | 8 | 10 | 8 | 2 | 0.160 |
| Moderate | 34 | 22 | 58 | 12 | |
| High | 18 | 20 | 54 | 10 | |
| Lymph node stats | |||||
| Negative | 44 | 30 | 78 | 20 | 0.101 |
| Positive | 16 | 22 | 42 | 4 | |
| FIGO stage | |||||
| I | 18 | 18 | 36 | 10 | 0.385 |
| II | 16 | 16 | 26 | 8 | |
| III | 26 | 18 | 58 | 6 | |
| CA-125 at diagnosis (U/ml) | |||||
| ≤100 | 24 | 16 | 34 | 4 | 0.046 |
| >100-300 | 12 | 6 | 20 | 4 | |
| >300-600 | 2 | 8 | 16 | 8 | |
| > 600 | 22 | 22 | 50 | 8 |
Univariate and multivariate Cox regression analyses in patients with ovarian cancer
| Characteristic | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p -value | HR | 95% CI | p -value | |
| Age (y) | ||||||
| ≤50 | 1 | 1 | ||||
| > 50 | 3.228 | 1.964-5.303 | < 0.001 | 3.047 | 1.831-5.069 | < 0.001 |
| Histology | ||||||
| Serous | 1 | 1 | ||||
| Non-serous | 0.201 | 0.073-0.550 | 0.002 | 0.470 | 0.155-1.425 | 0.182 |
| Grading | ||||||
| Well | 1 | 1 | ||||
| Moderate | 3.667 | 0.873-15.395 | 0.076 | 3.199 | 0.754-13.578 | 0.115 |
| High | 7.637 | 1.854-31.468 | 0.005 | 6.971 | 1.670-29.108 | 0.008 |
| Lymph node stats | ||||||
| Negative | 1 | 1 | ||||
| Positive | 2.758 | 1.755-4.333 | < 0.001 | 1.504 | 0.853-2.651 | 0.158 |
| FIGO stage | ||||||
| I | 1 | 1 | ||||
| II | 2.210 | 1.003-4.871 | 0.049 | 3.625 | 1.625-8.087 | 0.002 |
| III | 4.627 | 2.343-9.138 | < 0.001 | 4.255 | 2.145-8.440 | < 0.001 |
| CA-125 at diagnosis (U/ml) | ||||||
| ≤100 | 1 | 1 | ||||
| >100-300 | 2.097 | 1.068-4.116 | 0.031 | 2.020 | 0.554-2.073 | 0.064 |
| >300-600 | 0.492 | 0.166-1.455 | 0.200 | 0.567 | 1.244-3.785 | 0.342 |
| > 600 | 1.666 | 0.950-2.920 | 0.075 | 1.072 | 1.831-5.068 | 0.836 |
| Blood type | ||||||
| Non-A | 1 | 1 | ||||
| A | 2.210 | 1.373-3.557 | 0.001 | 2.235 | 1.360-3.674 | 0.002 |
Overall survival for patients with ovarian cancer with blood type A and non-A blood types in patients with age > 50 years (A) and age ≤ 50 years (B).
Overall survival for patients with ovarian cancer with blood type A and non-A blood types in patients with FIGO stage I (A), II (B), and III (C).
Discussion
The present study assessed the cytoreductive surgery results from Chinese patients with ovarian cancer to determine the prognostic value of different ABO blood group. Our results showed that patients with A blood group predicted a poorer OS than that patients with non-A blood group.
The role of inherited blood group antigens in cancer risk and progression has been intensely studied across many different types of solid organ and hematologic malignancies (13-16). At present, the impacts of ABO blood group on the survival of patients with ovarian cancer are limited. To the best of our knowledge, only one investigation is available that has reviewed the relationship between the ABO blood group and prognosis in ovarian cancer patients. In 92 patients with ovarian cancer, blood type A was found to be occur more frequently than in those with the other blood types and patients with blood group A was associated with a poor prognosis (17). 256 patients enrolled in the present study, and the proportions of blood types A, B, O, and AB were approximately similar to those of the cases in the Nurses' Health Study with the proportions were 31.2%, 17.5%, 41.0%, and 10.3%, respectively (13). And our study further clarified the prognostic value of ABO blood groups in ovarian cancer with patient survival in Chinese population.
Recently studies have demonstrated that the risk for ovarian cancer of being significantly higher in patients with blood group A (11,12). Owing to these data, we hypothesized that the predisposing environmental and genetic background associated with blood group A might develop ovarian cancer to a poorer prognosis. Our findings are quite similar to previous studies in nasopharyngeal carcinoma (9) and breast cancer (18), while the other studies showed that non-AB blood types in colon cancer (8), O blood type in bladder cancer (19) and esophageal squamous cell carcinoma (20) were poor prognostic factors. Conversely, studies indicated that ABO blood group was not associated with survival of triple negative breast cancer (21), gastric adenocarcinoma (22), and renal cell carcinoma (23). Proposed reasons for these conflicting results include heterogeneity of study populations, differences in ABO distribution or other demographics, and limited study sizes.
It is remained uncertain why ABO blood groups affect the survival of patients with ovarian cancer. However, growing studies discussed the possible mechanisms of the ABO blood groups affect the survival. The associations between the ABO blood group and tumor prognosis including inflammation, immunosurveillance for cancer cells, intracellular adhesion, and membrane signaling have been proposed (24). It was also found that ABO blood group antigen expression on cancer cells was modified by hypermethylation of ABO gene promoter (25), which might be related with the tumor invasion and metastasis. Previous studies showed that the number of methylated gene promoters increased with age (26,27), which may partly explain the reason for patients with age > 50 years had worse OS in this study.
The ABO gene is located on chromosome 9q34 encodes glycosyltransferases, which is frequently lost in ovarian cancer (28). The gene encodes for glycosyltransferases, which catalyze the step-by-step transfer of nucleotide donor sugars to the H antigen to form the A and B antigen (29). Aberrant glycosylation represents a hall mark of cancer development and progression (30). Thus, we hypothesize that patients with blood type A have impacted tumor cell invasion and immune responses of ovarian cancer cells and more susceptible to disease failure. Further studies of the impact of blood type on tumor behavior or response to therapy would be important to elucidate the potential impact of blood type on tumor biology and might help guide individualized therapy for ovarian cancer.
There are some limitations of the current study. This study was a retrospective study performed in a single center with a relatively small number of patients enrolled, thus the findings may not be applicable to the general population, and we could not have a definite conclusion about the ABO blood group and survival of different FIGO stage ovarian cancer. Further studies are required to evaluate the correlation between ABO blood group and survival of different FIGO stage ovarian cancer. In addition, the patients enrolled in the present study was underwent cytoreductive surgery which achieved optimal debulking when the residual disease was < 1 cm. Therefore, the metastatic cases with FIGO stage IV were excluded and not discussed in this study.
Conclusion
In conclusion, the results of the present study show that ABO blood group is a prognostic factor for survival in ovarian cancer patients after cytoreductive surgery. Patients with blood type A had a significantly worse OS than those with non-A types, especially in patients with age > 50 years. However, our result should be verified by further studies, and the related mechanism should be studied to elucidate the association between the ABO blood group and tumor genetic or biological differences.
Abbreviations
FIGO, The International Federation of Gynecology and Obstetrics; OS, overall survival; HR, hazard ratios; CI, confidence interval.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81402527), the Sci-Tech Office of Guangdong Province (No. 2013B021800157), the Education Scientific Research Project of Young Teachers in Fujian Province (No. JB13131), and Natural Science Foundation of Fujian Province (No. 2015J01550).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30
2. Chen W, Zheng R, Zhang S. et al. The incidences and mortalities of major cancers in China, 2009. Chin J Cancer. 2013;32(3):106-12
3. Agarwal R, Kaye SB. Prognostic factors in ovarian cancer: how close are we to a complete picture? Ann Oncol. 2005;16(1):4-6
4. Clark TG, Stewart ME, Altman DG. et al. A prognostic model for ovarian cancer. Br J Cancer. 2001;85(7):944-52
5. Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19(1):3-10
6. Rahbari NN, Bork U, Hinz U. et al. AB0 blood group and prognosis in patients with pancreatic cancer. BMC Cancer. 2012;12:319
7. Sun P, Chen C, Zhang F. et al. The ABO blood group predicts survival in esophageal squamous cell carcinoma in patients who ever smoked: a retrospective study from China. Tumour Biol. 2014;35(7):7201-8
8. Cao X, Wen ZS, Sun YJ. et al. Prognostic value of ABO blood group in patients with surgically resected colon cancer. Br J Cancer. 2014;111(1):174-80
9. Ouyang PY, Su Z, Mao YP. et al. Prognostic value of ABO blood group in southern Chinese patients with established nasopharyngeal carcinoma. Br J Cancer. 2013;109(9):2462-6
10. Cihan YB. Significance of ABO-Rh blood groups in response and prognosis in breast cancer patients treated with radiotherapy and chemotherapy. Asian Pac J Cancer Prev. 2014;15(9):4055-60
11. Poole EM, Gates MA, High BA. et al. ABO blood group and risk of epithelial ovarian cancer within the Ovarian Cancer Association Consortium. Cancer Causes Control. 2012;23(11):1805-10
12. Yuzhalin AE, Kutikhin AG. ABO and Rh blood groups in relation to ovarian, endometrial and cervical cancer risk among the population of South-East Siberia. Asian Pac J Cancer Prev. 2012;13(10):5091-6
13. Gates MA, Wolpin BM, Cramer DW. et al. ABO blood group and incidence of epithelial ovarian cancer. Int J Cancer. 2011;128(2):482-6
14. Wolpin BM, Chan AT, Hartge P. et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101(6):424-31
15. Ben Q, Wang K, Yuan Y. et al. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: a case-control study. Int J Cancer. 2011;128(5):1179-86
16. Pandey M, Gautam A, Shukla VK. ABO and Rh blood groups in patients with cholelithiasis and carcinoma of the gall bladder. BMJ. 1995;310(6995):1639
17. Marinaccio M, Traversa A, Carioggia E. et al. Blood groups of the ABO system and survival rate in gynecologic tumors. Minerva Ginecol. 1995;47(3):69-76 [Article in Italian]
18. Stamatakos M, Kontzoglou K, Safioleas P. et al. Breast cancer incidence in Greek women in relation to ABO blood groups and Rh factor. Int Semin Surg Oncol. 2009;6:14
19. Klatte T, Xylinas E, Rieken M. et al. Impact of ABO blood type on outcomes in patients with primary nonmuscle invasive bladder cancer. J Urol. 2014;191(5):1238-43
20. Yang X, Huang Y, Feng JF. Is there an association between ABO blood group and overall survival in patients with esophageal squamous cell carcinoma? Int J Clin Exp Med. 2014;7(8):2214-8
21. Yu J, Gao F, Klimberg VS, Margenthaler JA. ABO blood type/Rh factor and the incidence and outcomes for patients with triple-negative breast cancer. Ann Surg Oncol. 2012;19(10):3159-64
22. Qiu MZ, Zhang DS, Ruan DY. et al. A relationship between ABO blood groups and clinicopathologic characteristics of patients with gastric adenocarcinoma in China. Med Oncol. 2011;28(Suppl 1):S268-73
23. de Martino M, Waldert M, Haitel A. et al. Evaluation of ABO blood group as a prognostic marker in renal cell carcinoma (RCC). BJU Int. 2014;113(5b):E62-6
24. Nozoe T, Ezaki T, Baba H. et al. Correlation of ABO blood group with clinicopathologic characteristics of patients with esophageal squamous cell carcinoma. Dis Esophagus. 2004;17(2):146-9
25. Gao S, Worm J, Guldberg P. et al. Genetic and epigenetic alterations of the blood group ABO gene in oral squamous cell carcinoma. Br J Cancer. 1996;73(4):420-3
26. Kwabi-Addo B, Chung W, Shen L. et al. Age-related DNA methylation changes in normal human prostate tissues. Clin Cancer Res. 2007;13(13):3796-802
27. Marsit CJ, Houseman EA, Schned AR. et al. Promoter hypermethylation is associated with current smoking, age, gender and survival in bladder cancer. Carcinogenesis. 2007;28(8):1745-51
28. Devlin J, Elder PA, Gabra H. et al. High frequency of chromosome 9 deletion in ovarian cancer: evidence for three tumour-suppressor loci. Int J Cancer. 2004;109(2):230-7
29. Yazer MH. What a difference 2 nucleotides make: a short review of ABO genetics. Transfus Med Rev. 2005;19(3):200-9
30. Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci U S A. 2002;99(16):10231-3
Author contact
![]() Corresponding author: San-Gang Wu, Xiamen Cancer Center, Department of Radiation Oncology, the First Affiliated Hospital of Xiamen University, Xiamen, People's Republic of China. Tel. +86 592 2139531; Fax. +86 592 2317301; E-mail. unowu12345com and Jia-Yuan Sun, Sun Yat-sen University Cancer Center, Department of Radiation Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, People's Republic of China. Tel. +86 20 87343543; Fax. +86 20 87343392; E-mail. sunjyorg.cn
Corresponding author: San-Gang Wu, Xiamen Cancer Center, Department of Radiation Oncology, the First Affiliated Hospital of Xiamen University, Xiamen, People's Republic of China. Tel. +86 592 2139531; Fax. +86 592 2317301; E-mail. unowu12345com and Jia-Yuan Sun, Sun Yat-sen University Cancer Center, Department of Radiation Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, People's Republic of China. Tel. +86 20 87343543; Fax. +86 20 87343392; E-mail. sunjyorg.cn

 Global reach, higher impact
Global reach, higher impact