3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2015; 6(10):1041-1048. doi:10.7150/jca.12819 This issue Cite
Research Paper
A Muti-center, Randomized Phase II Study of Oxaliplatin and S-1 versus Capecitabine and Oxaliplatin in Patients with Metastatic Colorectal Cancer
1. Department of Internal Medicine, Hallym University Medical Center, Hallym University College of Medicine, Anyang, South Korea
2. Department of Internal Medicine, Chonnam National University College of Medicine, Gwangju, South Korea
3. Department of Internal Medicine, Keimyung University College of Medicine, Daegu, South Korea
4. Department of Internal Medicine, Gangneung Asan Hospital, Gangneung, South Korea
5. Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, South Korea
6. Department of Surgery, Hallym University Medical Center, Hallym University College of Medicine, Anyang, South Korea
7. Department of Radiology, Hallym University Medical Center, Hallym University College of Medicine, Anyang, South Korea
Received 2015-5-29; Accepted 2015-7-27; Published 2015-8-29
Abstract
Background: Capecitabine plus oxaliplatin (XELOX) is considered one of the primary chemotherapy regimens for patients with metastatic colorectal cancer (CRC). Oxaliplatin plus S-1 (OS) has also demonstrated significant efficacy in CRC. We performed this randomized phase II study to evaluate the efficacy and toxicity of XELOX versus OS as first-line chemotherapy in patients with metastatic CRC.
Methods: Patients were assigned randomly to receive either OS or XELOX chemotherapy. Oxaliplatin was administered intravenously to all patients at a dose of 130 mg/m2 on day 1. Patients received either S-1 (40 mg/m2) or capecitabine (1,000 mg/m2), twice a day for 2 weeks, followed by a 1-week rest.
Results: Forty-two patients were assigned to the OS arm and 44 to the XELOX arm. The overall response rate was 33.3% (95% CI, 18.8-47.2) in the OS arm and 40.9% (95% CI, 25.5-54.4) in the XELOX arm (P = 0.230). The disease control rate was significantly higher in the OS arm than the XELOX arm [92.9% (95% CI, 83.7-100) versus 77.3% (95% CI, 64.5-89.4), P = 0.044]. With a median follow up of 17.9 months, the median progression-free survival was 6.1 months in the OS arm and 7.4 months in the XELOX arm, respectively (P = 0. 599). The median survival time was 18.7 months in the OS arm and 20.1 months in the XELOX arm (P = 0.340). The most common grade 3/4 hematologic toxicity was thrombocytopenia in both arms (19.0% for OS and 28.6% for XELOX). Grade 3/4 neutropenia was observed more frequently in the XELOX arm than the OS arm (16.7% vs. 2.4%, P = 0.026).
Conclusion: Both OS and XELOX were effective and well tolerated in patients with metastatic CRC. Our results indicate that the combination of oxaliplatin and S-1 is a possible additional therapeutic strategy for such patients.
Keywords: Colorectal neoplasm, Oxaliplatin, S-1, Capecitabine, Randomized controlled trial
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer-related death in the world [1]. For more than four decades, 5-fluorouracil (5-FU) combined with leucovorin (LV) has been the mainstay of palliative chemotherapy in patients with metastatic CRC [2]. Since the 1990s, the introduction of irinotecan or oxaliplatin has extended the spectrum of therapeutic options. The combination of oxaliplatin or irinotecan with 5-FU plus LV has been considered the standard regimen for first-line treatment of metastatic CRC [3-6]. However, this is an inconvenient therapeutic option due to the requirement for continuous vascular infusion of 5-FU.
Capecitabine is an orally administered fluoropyrimidine that was rationally designed to generate 5-FU preferentially at the tumor site. Capecitabine demonstrated a safety profile superior to that of 5-FU/LV, with a significantly lower incidence of diarrhea, stomatitis, nausea, alopecia, and grade 3/4 neutropenia [7]. Capecitabine monotherapy as first-line chemotherapy for patients with metastatic CRC has shown an overall response rate (ORR) of approximately 20% [8]. Since capecitabine has been adopted as a substitute for infused 5-FU/LV to overcome the inconvenience of 5-FU, subsequent data have found capecitabine plus oxaliplatin (known as XELOX or CAPOX) to be a comparable therapeutic regimen to infused 5-FU/LV plus oxaliplatin (known as FOLFOX-4 or FUOX) [9-11].
S-1 is another oral fluoropyrimidine that combines tegafur with gimeracil and oteracil. It has also been evaluated in patients with CRC. In phase II studies, S-1 monotherapy as first-line treatment of metastatic CRC resulted in ORRs of 19-40% with tolerable toxicities [12-14]. Subsequent phase I/II and phase II studies demonstrated that the combination of oxaliplatin with S-1 (known as OS or SOX), instead of 5-FU/LV, was also effective and well tolerated in patients with metastatic CRC [15-16].
We performed a randomized phase II trial to evaluate the efficacy and toxicity of the XELOX versus OS regimen as first-line chemotherapy in patients with metastatic CRC.
Patients and methods
Study design
This was a multi-center, open-label, randomized phase II trial of OS versus XELOX combination chemotherapies in previously untreated patients with recurrent or metastatic CRC. Randomization was stratified by institution, Eastern Cooperative Oncology Group (ECOG) performance status score (PS) (0 or 1 versus 2) and recurrent disease versus metastatic disease. Patients were assigned randomly (1:1 ratio with block size 4) using a table of random digits to receive either the OS or XELOX regimen. Five institutions in South Korea participated in this study. The study protocol was approved by the Institutional Review Board of each participating institution. Written informed consent was obtained from all patients before enrollment, and the study was conducted according to the Good Clinical Practice guidelines. This study was registered with ClinicalTrials.gov as number NCT00677144.
Eligibility
For enrollment in this study, patients were required to meet the following inclusion criteria: histologically confirmed CRC; presence of metastatic disease; age of 19 years or older; ECOG PS of 0-2; estimated life expectancy of more than 3 months; and adequate hematological (white blood cell count ≥ 4,000/µL or absolute neutrophil count ≥ 1,500/µL, platelets ≥ 100,000/µL, and hemoglobin level ≥ 9.0 g/dl), renal (serum creatinine < 1.5 mg/dL), and hepatic functions (serum bilirubin and serum transaminase levels < 2.5-fold the upper normal limit).
The presence of at least one unidimensionally measurable lesion (≥ 10 mm) according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.017 was also required for enrollment in this study. Patients who had completed adjuvant or neo-adjuvant chemotherapy at least 6 months prior to recruitment were eligible. However, patients with recurrent disease following adjuvant S-1 or capecitabine-based chemotherapy were not eligible for this study regardless of the time to recurrence. Patients with central nervous system metastasis, obvious bowel obstruction, overt gastrointestinal bleeding, active infection, or serious co-morbidities were excluded from this study.
Pretreatment evaluation
Baseline evaluations included a medical history, a physical examination, ECOG PS, a complete blood count with differential count, serum chemistry and electrolytes, urine analysis, and three-dimensional computed tomography with the administration of an intravenous contrast medium.
Treatment scheme
Oxaliplatin was administered intravenously at a dose of 130 mg/m2 over 2 h on day 1 for patients in both arms. Depending on the arm to which they were assigned, patients were given 40 mg/m2 S-1 [body surface area (BSA) < 1.25 m2, 40 mg; 1.25 ≤ BSA < 1.5, 50 mg; BSA ≥ 1.5, 60 mg] or 1,000 mg/m2 capecitabine twice a day for 2 weeks followed by a 1-week rest. This schedule was repeated every 3 weeks until the occurrence of disease progression, the development of unacceptable toxicity, or patient refusal.
Dose modification
The dose of a specific agent was adjusted when the cause of toxicity could be distinguished. When both agents were believed to have caused toxicity, the doses of both agents were reduced. Treatment was interrupted in patients who experienced grade 2 or higher toxicity and was not resumed until the toxicity resolved or had improved to grade 1. The dose of oxaliplatin was reduced by 25% of the initial dose in patients who experienced any related grade 3 toxicity or a second occurrence of same grade 2 toxicity. If peripheral neuropathy persisted between courses, the next treatment cycle was started at 75% of the previous dose of oxaliplatin. Because of two dose strengths supplied, the dose of S‑1 or capecitabine was reduced by approximately 20% in patients who experienced any grade 3 toxicity or a second occurrence of same grade 2 toxicity.
In patients who experienced grade 4 toxicity or a second occurrence of same grade 3 toxicity, the dose of oxaliplatin was reduced by 50% of the initial dose. The dose of S‑1 or capecitabine was reduced by approximately 40% in patients who experienced any related grade 4 toxicities or a second occurrence of same grade 3 toxicity. After dose reduction of a specific drug, no increase in dosage of that drug was allowed. Treatment was discontinued if, despite the dose reduction, same toxicity occurred for a fourth time at grade 2 toxicity, a third time at grade 3, or a second time at grade 4. In addition, if the toxicity had not improved to grade 0 or 1 after 3 weeks of continued treatment, the patient was removed from the study.
Response and toxicity evaluation
Tumor response assessments were performed after every two cycles of treatment. Tumor responses were assessed according to the RECIST guidelines version 1.0 [17]. A complete response (CR) was defined as the complete disappearance of all target and nontarget lesions. A partial response (PR) was defined as a decrease of at least 30% in the sum of the longest diameters of the target lesions, referenced against the baseline sum of the longest diameters of the target lesions, together with stabilization or a decrease in size of the nontarget lesions. Progressive disease (PD) required at least a 20% increase in the tumor measurement sum, a substantial increase in the nontarget lesions, or development of any new lesions. Stable disease (SD) was defined as insufficient shrinkage to qualify for a PR and as insufficient increase to qualify for PD. Tumor responses were determined by an independent response review committee. All PR and CR were confirmed no less than 4 weeks after the criteria for a response were first met. The National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTC AE) version 3.0 were used to assess toxicity. After completion of the protocol, patients were followed up every 3 months until the occurrence of disease progression or death.
Statistical analyses
To evaluate the efficacy of OS and XELOX, the primary endpoint was the overall response rate (ORR) in patients with recurrent or metastatic CRC. The secondary endpoints were the assessments of safety profile, duration of response (DoR), progression-free survival (PFS), and survival time (ST) for each treatment regimen. The accrual number was calculated using Simon's optimal (MiniMax) two-stage design [18]. Assuming P0 = 0.3 and P1 = 0.5 with an α error = 0.05 and β error = 0.20, the initial requirement was the accrual of 19 patients in each arm. The study was continued if at least seven tumor responses were observed during the first stage. The second stage required an additional 20 patients to be enrolled. Assuming a 10% drop-out rate, 44 enrolled patients were required in each arm, thus a total of 88 across both arms. The DoR (from the first assessment of CR or PR to the documentation of disease progression), PFS (time from enrollment to the documentation of disease progression or death from any cause), and ST (time from enrollment to death from any cause), along with the 95% confidence interval (CI) for the median time to event, were estimated using the Kaplan-Meier method and compared by log-rank test.
Results
Patient characteristics
Between April 2008 and August 2011, 88 patients were screened for the study. However, two patients were confirmed as being ineligible for the study, because they had no measurable lesions. Thus, 86 patients were assigned randomly to either the OS arm (n = 42) or the XELOX arm (n = 44) (Fig. 1). Patients were well balanced between the two arms in terms of baseline characteristics (Table 1).
Treatment
The details of the treatment administration are summarized in Table 2. There were no statistical differences in the number of treatments administered or relative dose intensity of the drugs. In the OS arm, 42 patients received a total of 280 cycles, with a median number of six cycles (range, 1-39 cycles) per patient. The most common cause for discontinuation of treatment in this arm was disease progression (24 patients). Treatment delay was necessary in 20 patients (47.6%). For oxaliplatin, doses were modified in 25 patients (59.5%), and doses of S-1 were reduced in 23 patients (54.8%). The mean relative dose intensities ± standard deviation of oxaliplatin and S-1 were 0.84 ± 0.13 and 0.83 ± 0.15, respectively.
Basic characteristics of patients.
| Characteristics | OS (n=42) | XELOX (n=44) | P-value | |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Median age, years (range) | 67 (46-83) | 66 (29-76) | 0.637 | |
| Gender | 0.609 | |||
| Male | 28 (66.7) | 27 (61.4) | ||
| Female | 14 (33.3) | 17 (38.6) | ||
| ECOG performance status | 0.816 | |||
| 0 | 20 (47.6) | 22 (50.0) | ||
| 1 | 20 (47.6) | 21 (47.7) | ||
| 2 | 2 (4.8) | 1 (2.3) | ||
| Disease state | 0.566 | |||
| Recurrent | 11 (26.2) | 14 (31.8) | ||
| Metastatic | 31 (73.8) | 30 (68.2) | ||
| Primary site | 0.134 | |||
| Colon | 18 (42.8) | 25 (56.8) | ||
| Recto-sigmoid | 7 (16.7) | 10 (22.7) | ||
| Rectum | 17 (40.5) | 9 (20.5) | ||
| No. of metastatic organs | 0.202 | |||
| 1 | 22 (52.4) | 29 (65.9) | ||
| ≥ 2 | 20 (47.6) | 15 (34.1) |
In the XELOX arm, 44 patients received a total of 294 cycles of treatment, with a median number of five cycles (range, 1-19 cycles) per patient. The most common cause for discontinuation of treatment in this arm was disease progression (22 patients). Treatment delay was necessary in 25 patients (56.8%). Reduced doses of oxaliplatin were necessary in 28 patients (63.6%), and the dose of capecitabine was reduced in 27 patients (61.4%). The mean relative dose intensities ± standard deviation of oxaliplatin and capecitabine were 0.82 ± 0.14 and 0.81 ± 0.16, respectively.
Summary of overall treatment.
| OS (n=42) | XELOX (n=44) | |
|---|---|---|
| No. of treatment cycles | ||
| Total | 280 | 294 |
| Median | 6 | 5 |
| Range | 1 - 39 | 1 - 19 |
| Relative dose intensity | ||
| (mean ± standard deviation) | ||
| Oxaliplatin | 0.84 ± 0.13 | 0.82 ± 0.14 |
| S-1 or Capecitabine | 0.83 ± 0.15 | 0.81 ± 0.16 |
CONSORT diagram
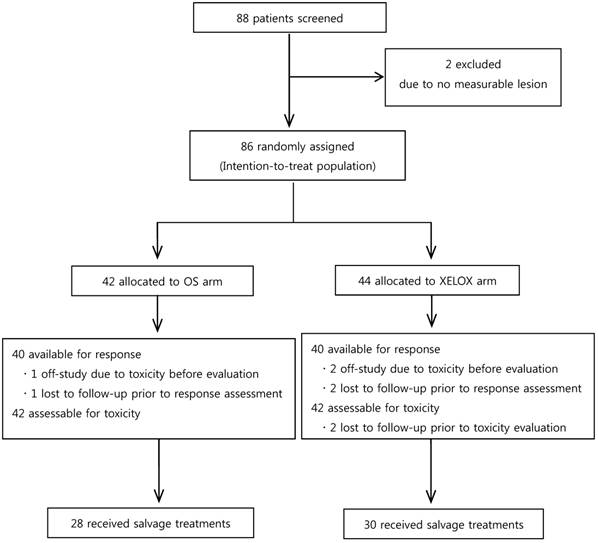
Efficacy
Three patients (one in the OS arm and two in the XELOX arm) refused further treatment, and another three (one in the OS arm and two in the XELOX arm) discontinued treatment due to toxicity prior to assessment of the tumor response (Figure 1). As a result, 40 patients in each arm were included in the tumor response assessment. The best tumor responses based on the intention-to-treat (ITT) population are described according to the treatment arms in Table 3. The ORR was 33.3 % (95% CI, 18.8-47.2) in the OS arm and 40.9% (95% CI, 25.5-54.4) in the XELOX arm (P = 0.230). The disease control rate (DCR) was significantly higher in the OS arm [92.8% (95% CI, 83.7-100) vs. 77.3% (95% CI, 64.5-89.4) in the XELOX arm (P = 0.044)]. The median DoR was 8.2 months (95% CI, 4.6-15.7) in the OS arm and 6.8 months (95% CI, 5.4-8.2) in the XELOX arm (P = 0.33).
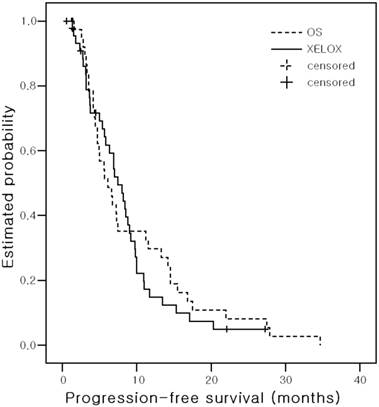
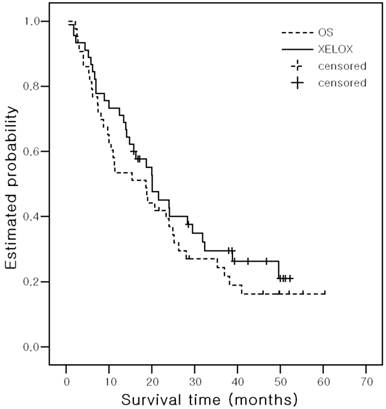
With a median follow up of 17.9 months (range, 0.56 - 60.4 months), the median PFS was 6.1 months (95% CI, 4.0-8.2) in the OS arm and 7.4 months (95% CI, 5.8 - 9.1) in the XELOX arm (P = 0. 599) (Figure 2). The median ST was 18.7 months (95% CI, 8.7-28.6) in the OS arm and 20.1 months (95% CI, 13.8-26.4) in the XELOX arm (P = 0.340) (Figure 3). The estimated 2-year survival rate was 36.9% in the OS arm and 40.1% in the XELOX arm.
Safety
Safety was assessed in 84 patients (42 per arm) who were treated with their allocated study regimen for at least one cycle. The toxicity profiles are listed in Table 4. The most common grade 3/4 hematologic toxicity was thrombocytopenia in both arms (19.0% in the OS arm and 28.6% in the XELOX arm, P = 0.306). Grade 3/4 neutropenia was observed more frequently in the XELOX arm than in the OS arm (2.4% in the OS arm vs. 16.7% in the XELOX arm, P = 0.026). Non-hematologic toxicities were usually mild (mostly grade 1/2), showing no significant differences between the two arms. As anticipated, hand foot syndrome (HFS) of any grade was observed frequently in the XELOX arm (4.8% in the OS arm vs. 23.8% in the XELOX arm, P = 0.013). Grade 3/4 HFS and peripheral neuropathy were observed only in the XELOX arm (4.8% and 7.1%, respectively). There were no treatment-related deaths in either arm.
Salvage treatment
Among 86 patients, 58 (67.4%) received further treatment after OS or XELOX (Figure 1). Twenty-eight patients (66.7%) in the OS arm and 30 (68.2%) in the XELOX arm received second-line chemotherapy. The most common regimen administered in both arms was infused 5-FU/LV plus irinotecan (known as FOLFIRI); 24 patients in the OS arm and 25 in the XELOX arm received the FOLFIRI regimen.
Kaplan-Meier estimates of progression-free Survival
Kaplan-Meier estimates of survival Time
Efficacy based on the intention-to-treat population.
| Best tumor response | OS (n=42) | XELOX (n=44) | P-values | |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Complete response | 3 (7.1) | 5 (11.4) | ||
| Partial response | 11 (26.2) | 13 (29.5) | ||
| Stable disease | 25 (59.5) | 16 (36.4) | ||
| Progressive disease | 1 (2.4) | 6 (13.6) | ||
| Non-evaluable | 2 (4.8) | 4 (9.0) | ||
| Summary | ||||
| Overall response rate (%) | 33.3 (95% CI, 18.8 - 47.2) | 40.9 (95% CI, 25.5 - 54.4) | 0.230 | |
| Disease control rate (%) | 92.9 (95% CI, 83.7 - 100) | 77.3 (95% CI, 64.5 - 89.4) | 0.044 | |
| Median duration of response (months) | 8.2 (95% CI, 4.6 - 17.7) | 6.8 (95% CI, 5.4 - 8.2) | 0.330 | |
| Median progression-free survival (months) | 6.1 (95% CI, 4.0-8.2) | 7.4 (95% CI, 5.8-9.1) | 0.599 | |
| Median survival time (months) | 18.7 (95% CI, 8.7-28.6) | 20.1 (95% CI, 13.8-26.4) | 0.340 |
Adverse events.
| Toxicities | OS (n = 42) | XELOX (n = 42) | P-values | |||||
|---|---|---|---|---|---|---|---|---|
| All grades No. (%) | Grade 3/4 No. (%) | All grades No.(%) | Grade 3/4 No. (%) | All grades | Grade 3/4 | |||
| Hematologic | ||||||||
| Leukopenia | 18 (42.9) | 2 (4.8) | 21 (50.0) | 1 (2.4) | 0.512 | 0.557 | ||
| Neutropenia | 18 (42.9) | 1 (2.4) | 27 (64.3) | 7 (16.7) | 0.049 | 0.026 | ||
| Anemia | 39 (92.9) | 4 (9.5) | 36 (85.7) | 5 (11.9) | 0.290 | 0.724 | ||
| Thrombocytopenia | 26 (54.2) | 8 (19.0) | 32 (76.2) | 12 (28.6) | 0.157 | 0.306 | ||
| Non-hematologic | ||||||||
| Asthenia | 17 (40.5) | 3 (7.1) | 12(28.6) | 2 (4.8) | 0.251 | 0.645 | ||
| Anorexia | 25 (59.5) | 2 (4.8) | 24 (57.1) | 1 (2.4) | 0.825 | 0.557 | ||
| Nausea | 18 (42.9) | 3 (7.1) | 19 (45.2) | 4 (9.5)) | 0.826 | 0.457 | ||
| Vomiting | 16 (38.1) | 2 (4.8) | 17 (40.5) | 4 (9.5) | 0.823 | 0.397 | ||
| Diarrhea | 11 (26.2) | 3 (7.1) | 11 (26.2) | 2 (4.8) | 1.0 | 0.645 | ||
| Constipation | 8 (19.0) | 0 | 4 (9.5) | 0 | 0.212 | 1.0 | ||
| Stomatitis | 4 (9.5) | 1 (2.4) | 2 (4.8) | 0 | 0.397 | 0.314 | ||
| Hand-foot syndrome | 2 (4.8) | 0 | 10 (23.8) | 2 (4.8) | 0.013 | 0.152 | ||
| Peripheral neuropathy | 21 (50.0) | 0 | 22 (52.4) | 3 (7.1) | 0.827 | 0.078 | ||
| Hyperbilirubinemia | 16 (38.1) | 1 (2.4) | 15 (35.7) | 2 (4.8) | 0.821 | 0.557 | ||
| Elevated AST/ALT | 17 (40.5) | 0 | 24 (57.1) | 1 (2.4) | 0.127 | 0.314 | ||
Abbreviations: AST, aspartic acid transaminase; ALT, alanine transaminase
Discussion
This study was the first randomized trial comparing S-1 or capecitabine in combination with oxaliplatin in patients with metastatic CRC. However, it should be noted that a multi-center, randomized phase III study with the same regimens was initiated shortly after patient enrollment started in our trial [19]. Results from our study found the OS regimen to be comparable to the XELOX regimen as a first-line palliative chemotherapy for patients with metastatic CRC.
Since capecitabine in combination with oxaliplatin (known as XELOX, CAPOX, or CapeOX) was found to be comparable to infused 5-FU/LV plus oxaliplatin (usually known as FOLFOX or FUOX), the XELOX regimen has been used as a more convenient first-line chemotherapy in patients with metastatic CRC. Oxaliplatin is typically administered intravenously at a dose of 130 mg/m2 (day 1) or 70 mg/m2 (days 1, 8) every 3 weeks, and 1,000 mg/m2 capecitabine is administered orally, twice daily on days 1-14, with a 1-week interval. The reported ORR, median PFS or time to progression (TTP), and median ST in phase II or III trials of XELOX were 37-55%, 6.0-8.9 months, and 16.8-19.8 months, respectively [9-11].
Before conducting this randomized phase II trial, we had performed a phase II study evaluating OS in patients with metastatic CRC [16]. The observed ORR was 54%, and the median TTP and median ST were 8.5 months and 27.2 months, respectively. The OS regimen was well tolerated in patients. These results were favorable comparable with those obtained using FOLFOX or XELOX regimens in other phase II or III studies.[3,4,9-11] Therefore, we decided to conduct this randomized phase II study using the OS versus the XELOX regimen.
In this study evaluating patients with recurrent or metastatic CRC, no significant difference was observed in the efficacy between the OS and XELOX arms. Based on the ITT population, patients in the OS arm showed an ORR of 33.3% and a median PFS of 6.1 months. These results are comparable to those reported in a phase I/II evaluating the OS regimen in patients with metastatic CRC [15]. However, our results in the OS arm were somewhat inferior to those observed in our previous phase II trial [16]. A couple of different patient characteristics may explain these results. In the previous phase II trial, patients were relatively younger than those in the OS arm of the current study (median age 56 vs. 67 years). In addition, most patients (81.2%) of the phase II study had a very good PS (ECOG 0), compared with 47.6% in the current study. In the XELOX arm of the current study, the ORR was 40.9%, and the median PFS was 7.4 months, similar to the results reported in other phase II or III trials incorporating capecitabine plus oxaliplatin [9-11,19-24]. Those studies reported an ORR of 37-55%, with a median PFS or TTP of 6.0-8.9 months, in patients with metastatic CRC.
As mentioned previously, the results of a randomized phase III trial of OS (referred to as SOX in the report) versus XELOX (named as CapeOX in the paper) had been published [19]. In that trial, 168 patients were randomly assigned to receive OS and 172 to receive XELOX to evaluate the efficacy (determined as PFS) of OS vs. XELOX. Patients received the same dose of each drug as in our study. The median PFS, the primary endpoint, was 8.5 months in the OS group and 6.7 months in the XELOX group [hazard ratio (HR): 0.79 (95% CI, 0.60 - 1.04), P non-inferiority < .0001, P log-rank = 0.09]. In terms of secondary endpoints, ORR and time to treatment failure (TTF), the OS regimen resulted in superior outcomes over the XELOX regimen. The reported ORR was 47% in the OS arm and 36% in the XELOX arm (P = 0.029), and the median TTF was 6.9 months in the OS arm and 5.6 months in the XELOX arm (P = 0.036). The authors of this study recently reported the updated results [20]. With a median follow-up of 17.9 months, the updated median PFS was 7.1 months (95% CI, 6.4-8.0) in the OS group and 6.3 months (95% CI, 4.9-6.7) in the XELOX group [HR: 0.83 (95% CI, 0.66-1.04, P = 0.10]. The median ST was 19.0 months (95% CI, 15.3-23.0) in the OS group and 18.4 months (95% CI, 14.1-20.7) in the XELOX group [HR: 0.86 (95% CI, 0.68-1.08), P = 0.19]. Subgroup analyses according to principal demographic factors such as sex, age, ECOG PS, primary tumor location, measurability, previous adjuvant therapy, number of metastatic organs, and liver metastases showed no interaction between any of these characteristics and the treatment.
In the current study, although there were no significant statistical differences, the ORR (33.3% vs. 40.9%) and median PFS (6.1 vs. 7.4 months) were slightly inferior in the OS arm than the XELOX arm. These results might be attributed to the difference in the extent of metastatic disease between the two arms at the time of enrollment: 47.6% of patients in the OS arm had metastatic disease in two or more organs, compared with 34.1% in the XELOX arm. In terms of DCR, however, the OS arm was slightly superior to the XELOX arm (92.9 vs. 77.2%, P = 0.044). The median ST was 18.7 months in the OS arm and 20.1 months in the XELOX arm, and the estimated 2-year survival rate was 36.9% in the OS arm and 40.1% in the XELOX arm. In concordance with the phase III study [19,20], our results indicate that the OS regimen is comparable to the XELOX regimen as first-line chemotherapy in patients with metastatic CRC.
The treatments were generally well tolerated by most patients, which is consistent with recent trials evaluating OS or XELOX in patients with metastatic CRC [9-11,15,16,19, 21-24]. Except for neutropenia, there was no significant difference in the overall incidence of adverse hematologic effects between the two arms. Grade 3/4 neutropenia was observed more frequently in the XELOX arm (16.7% vs. 2.4%, P = 0.026). The most common grade 3/4 hematologic toxicity was thrombocytopenia in both arms (19.0% in OS and 28.6% in XELOX, P = 0.306). Thrombocytopenia increased in a cumulative manner, which was one of the major reasons for treatment plan alterations in both arms. The higher incidences of neutropenia and thrombocytopenia in the XELOX arm were associated with the more frequent treatment delay or dose reduction. The incidence of grade 3/4 thrombocytopenia in the OS group was similar to that (13-28%) reported in other studies [15,16,19]. However, for reasons unknown, its incidence (28.6%) in the XELOX arm appears to be higher than that observed in other studies (4-14%) with the same dose and schedule of XELOX [9-11,19]. Contrary to our findings, the incidences of grade 3/4 neutropenia and thrombocytopenia in the phase III study by Hong et al [19]. were significantly higher in the OS arm than the XELOX arm (29% vs. 15% for neutropenia and 22% vs. 7% for thrombocytopenia, respectively). These findings might be explained in part by the difference in the number of treatment cycles administered between the two arms: patients in the OS arm received more cycles of chemotherapy than those in the XELOX arm [1194 (median, 8) vs. 1084 (median, 6) cycles for oxaliplatin and 1520 (median, 9) vs. 1206 (median, 6) for S-1 or capecitabine, respectively].
In terms of non-hematologic toxicities, anorexia, nausea, elevation of liver enzymes, and peripheral neuropathy were common adverse effects in both arms. Peripheral neuropathy occurred in approximately 50% of patients in both arms. Its incidence was lower than those (~80%) reported in the phase III trial of OS and XELOX [19], which might be attributed to the difference in the number of treatments administered between the two studies. Grade 3 peripheral neuropathy tended to be associated more with XELOX than OS (7.1% vs. 0%, P = 0.078). As expected, hand-foot syndrome of any grade was reported more commonly in the XELOX group (4.8% vs. 23.8%, P = 0.013).
In conclusion, both OS and XELOX were effective and well tolerated palliative treatment regimens in patients with recurrent or metastatic CRC. Our results indicate that the combination of oxaliplatin and S-1 represents an additional possible convenient therapeutic option for such patients. Future clinical trials for combining this regimen with target agents are warranted.
Acknowledgements
Oxaliplatin and S-1 were provided by sanofi Korea (Seoul, South Korea) and Jeil Pharmaceutical (Seoul, South Korea), respectively.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90
2. Thrion P, Michiels S, Pignon JP, Buyse M, Braud AC, Carlson RW. et al. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: An updated meta-analysis. J Clin Oncol. 2004;2:3766-75
3. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J. et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-47
4. Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C. et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136-47
5. Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P. et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-47
6. Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476-87
7. Cassidy J, Twelves C, Van cutsem E, Hoff P, Bajetta E, Boyer M. et al. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol. 2002;13:566-75
8. Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M. et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;9:4097-106
9. Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T. et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22:2084-91
10. Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R. et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006-12
11. Díaz-Rubio E, Tabernero J, Gómez-España A, Massutí B, Sastre J, Chaves M. et al. Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol. 2007;25:4224-30
12. Ohtsu A, Baba H, Sakata Y, Mitachi Y, Horikoshi N, Sugimachi K, Taguchi T. Phase II study of S-1, a novel oral fluoropyrimidine derivative, in patients with metastatic colorectal carcinoma. S-1 Cooperative Colorectal Carcinoma Study Group. Br J Cancer. 2000;83:141-5
13. Van den Brande J, Schöffski P, Schellens JH, Roth AD, Duffaud F, Weigang-Köhler K. et al. EORTC Early Clinical Studies Group early phase II trial of S-1 in patients with advanced or metastatic colorectal cancer. Br J Cancer. 2003;88:648-53
14. Shirao K, Ohtsu A, Takada H, Mitachi Y, Hirakawa K, Horikoshi N. et al. Phase II study of oral S-1 for treatment of metastatic colorectal carcinoma. Cancer. 2004;100:2355-61
15. Yamada Y, Tahara M, Miya T, Satoh T, Shirao K, Shimada Y. et al. Phase I/II study of oxaliplatin with oral S-1 as first-line therapy for patients with metastatic colorectal cancer. Br J Cancer. 2008;98:1034-8
16. Zang DY, Lee BH, Park H, Song HH, Kim HJ, Jung JY. et al. Phase II study with oxaliplatin and S-1 for patients with metastatic colorectal cancer. Ann Oncol. 2009;20:892-6
17. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-16
18. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1-10
19. Hong YS, Park YS, Lim HY, Lee J, Kim TW, Kim KP. et al. S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for first-line treatment of patients with metastatic colorectal cancer: a randomiased, non-inferiority phase 3 trial. Lancet Oncol. 2012;13:1125-32
20. Kim ST, Hong YS, Lim HY, Lee J, Kim TW, Kim KP. et al. S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for the first-line treatment of patients with metastatic colorectal cancer: updated results from a phase 3 trial. BMC Cancer. 2014;14:883-9
21. Porschen R, Arkenau HT, Kubicka S, Greil R, Seufferlein T, Freier W. et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol. 2007;25:4217-23
22. Makatsoris T, Kalofonos HP, Aravantinos G, Papadimitriou C, Kastritis E, Rigatos SK. et al. A phase II study of capecitabine plus oxaliplatin (XELOX): a new first-line option in metastatic colorectal cancer. Int J Gastrointest Cancer. 2005;35:103-9
23. Scheithauer W, Kornek GV, Raderer M, Schüll B, Schmid K, Kovats E. et al. Randomized multicenter phase II trial of two different schedules of capecitabine plus oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2003;21:1307-12
24. Shields AF, Zalupski MM, Marshall JL, Meropol NJ. Treatment of advanced colorectal carcinoma with oxaliplatin and capecitabine: a phase II trial. Cancer. 2004;100:531-7
Author contact
![]() Corresponding author: Dae Young Zang, M.D., Ph.D., Division of Hematology-Oncology, Department of Internal Medicine, Hallym University Sacred Heart Hospital, 896 Pyeongchon-dong, Dongan-gu, Anyang 431-070, South Korea; Tel: +82-31-380-3871; Fax: +82-31-386-2269; E-mail: fhdzangac.kr
Corresponding author: Dae Young Zang, M.D., Ph.D., Division of Hematology-Oncology, Department of Internal Medicine, Hallym University Sacred Heart Hospital, 896 Pyeongchon-dong, Dongan-gu, Anyang 431-070, South Korea; Tel: +82-31-380-3871; Fax: +82-31-386-2269; E-mail: fhdzangac.kr

 Global reach, higher impact
Global reach, higher impact