3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2015; 6(11):1155-1159. doi:10.7150/jca.13107 This issue Cite
Research Paper
CD20 Over-Expression in Hodgkin-Reed-Sternberg Cells of Classical Hodgkin Lymphoma: the Neglected Quest
1. Department of Pathology, Soroka University Medical Center, Beer-Sheva and Faculty of Health Sciences, Ben Gurion University of the Negev, Beer-Sheva, Israel
2. Department of Oncology, Soroka University Medical Center, Beer-Sheva and Faculty of Health Sciences, Ben Gurion University of the Negev, Beer-Sheva, Israel
Received 2015-7-1; Accepted 2015-8-8; Published 2015-9-15
Abstract
We have scrutinized a previously analyzed cohort of classical Hodgkin lymphoma patients for evidence of a CD20 over-expression. This was pursued in order to determine whether all the 24 (12.6%) CD20+++ patients had clinical and/or biological profiles which would warrant a separate consideration and treatment or would carry a different outcome from our 166 CD20 (-) classical Hodgkin lymphoma patients. Except for an older age and a significantly lower expression of non-sialyl-CD15 antigen, both previously described in classical Hodgkin lymphoma, no justification to exclude these CD20+++ patients from the cohort at large is apparent. We suggest that the generally accepted view to the contrary be revised. In addition, we propose alternative interpretations for the low expression of CD20 found in a majority of Hodgkin-Reed-Sternberg cells in classical Hodgkin lymphoma.
Keywords: CD20, classical Hodgkin lymphoma, CD15, older patients, fixation effect
Introduction
Classical Hodgkin lymphoma (cHL) originates from mature B cells, as its tumor cells demonstrate clonal immunoglobulin heavy and light chain gene rearrangements. However, it seems that the Hodgkin-Reed-Sternberg (H-RS) tumor cells of cHL have lost their capacity to express B cell markers on the cell membrane [1]. CD20 is encoded by the MS4A1 (membrane-spanning 4 domains, subfamily A, member 1) gene. This gene encodes a B lymphocyte surface molecule which plays a role in the development and differentiation of B cells into plasma cells. One notable consequence of this loss is that the CD20 expression on the tumor cells is predominantly negative or, at best, faintly and/or heterogeneously positive in less than 20% of the H-RS cells [2].
Although the above mentioned immunophenotype is in the consensus, several authors have accepted higher rates of the CD20 expression as consistent with the diagnosis of cHL [3-10].
Most authorities, however, would now exclude the diagnosis of cHL in the presence of more than 20%, CD20 homogeneous or strongly positive, and will favor in these instances diagnoses of lymphocyte predominant HL, T-cell/histiocyte-rich large B-cell lymphoma, diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma or the gray zone lymphomas [11-15].
We have, together with others, seen such cases which have been otherwise typical of cHL and have shown an excellent response to cHL therapy. We therefore set out to review our cHL cases showing more than 20%, CD20 strong/homogeneous phenotype and to compare them with the rest of our cohort. Our population of cHL patients has been the subject of a previous study [16].
Materials and methods
Within a random cohort of cHL, previously published [Institutional Ethics Committee approval: SOR-0276-11], and including 190 patients, we found 24 (12.6%) who were distinct for a marked and/or homogeneous expression of CD20, encompassing between 20 and 70% of the H-RS cells (CD20+++). In the remaining 166 (87.4%), the CD20 was negative or weak (<20%) in the tumor cells [CD20 (-)].
Our adult patients had been primarily treated with MOPP (Mechlorethamine, Vincristine, Procarbazine and Prednisone) or ABVD (Doxorubicine, Bleomycine, Vinblastine and Dacarbazine) in 88% of cases. Children were treated with COPP/ABVD.
The CD20+++ group was compared with the rest of the population for the clinical and for biological traits of their H-RS cells in order to determine possible distinctive qualities of this subset of cHL. The various characteristics were compared, using contingency tables analysis and the Chi-square test. The Kaplan-Meier overall and relapse-free survival was employed to explore the effect of CD20 expression and the log Rank test was used to define statistically significant differences. The Cox proportional hazard analysis was used to test the effect of age, stage, systemic symptoms, LeuM1 and CD20 expression on the risk of dying from cHL. All statistical analyses were performed, using the SPSS, 21 Version for Windows and p values of .05 or less were considered statistically significant.
Eleven of the patients were lost to follow-up and thus data were missing, including those of three patients for whom no age was available.
Results
Of the 190 patients, 112 (59.9%) were male and 75 were female; 77 (52.4%) presented with B symptoms; 34 (24.6%) had bulky disease, while 104 did not. The histologic type was: nodular sclerosis - 102 (61.8%); mixed cellularity - 59 (35.8%); lymphocyte depleted - 4 (2.4%). Lymphocyte-rich HL and indeterminate cHL cases were poorly characterized and were excluded.
No difference in the primary chemotherapy was found between the two groups. Radiotherapy was distributed widely, and was not analyzed.
The clinical outcome revealed no evidence of tumor at the last visit in 110 (55.6%) patients; and alive with disease or dead of disease in 70 (35.3%). Relapse was evident in 42 patients (34.4%); no evidence of relapse was found in 80 patients (65.6%).
The CD20+++ phenotype was found in 24 patients (12.6%). CD20 was negative or faint in <20% in 166 (87.4%) [CD20 (-)].
Table 1 shows a comparison of CD20+++ with the rest of the cohort, with emphasis on clinical features. More patients younger than 45 belonged to the CD20 (-) group to a significant degree (p=.016). All the other clinical characteristics observed, including gender, stage, bulky disease, systemic symptoms, the histological types and outcome showed no statistically significant differences between the two categories.
Classical Hodgkin patients, CD20 expression, clinical features
| CD20+++ | CD20 (-) | p value | ||
|---|---|---|---|---|
| Age | <45 | 9 (8) | 104 (92) | |
| >=45 | 11 (21.2) | 41 (78.8) | .016 | |
| Gender | M | 12 (11.7) | 91 (88.3) | |
| F | 8 (11.8) | 60 (88.2) | .98 | |
| B sympt | Yes | 11 (14.3) | 66 (85.7) | |
| No | 7 (10.8) | 58 (89.2) | .53 | |
| Bulky | Yes | 6 (18.2) | 27 (81.8) | |
| No | 10 (10) | 90 (90) | .21 | |
| Type | Nod Sc | 13 (13.3) | 85 (86.7) | |
| MC-LD | 6 (10.3) | 52 (89.7) | .59 | |
| Stage B | I-IIA | 4 (17.4) | 19 (82.6) | |
| IIB-IVB | 9 (11.4) | 70 (88.6) | .45 | |
| Outcome | NED | 12 (11.5) | 92 (88.5) | |
| AWD-DOD | 9 (13.8) | 56 (86.2) | .75 |
B sympt - systemic symptoms
Nod Sc - nodular sclerosis type
MC-LD - mixed cellularity- lymphocyte depleted types
NED - no evidence of tumor
AWD-DOD - alive with disease or dead of tumor
In Table 2, we present a comparison between the study group and the remainder, regarding the standard immune markers of cHL, as well as those related with apoptosis. No significant difference was evident in the expression of CD30, FH6, Cas3, Cas8, Bcl-2, Bax and p53. In contrast, several CD15 antigens demonstrated a marked difference: LeuM1 and 80H5 were positive for non-sialyl-CD15 antigens in significantly more patients with negative/weak CD20 expression. On the other hand, Lex1, reacting with the sialyl-CD15 antigen was negative in more patients with the CD20 (-) expression.
In Table 3, we describe the expression of EBV/LMP1 and of measles virus antigens as they compare between the study group and the CD20 (-) group: no significant difference was found.
Table 4. The mean age was significantly lower in 145 patients with CD20 (-) when compared with the study group. In contrast, no significant difference in the mean follow-up periods was evident between the two groups.
CD20 expression in classical Hodgkin lymphoma - markers
| CD20+++ | CD20 (-) | p value | ||
|---|---|---|---|---|
| CD30 | Pos | 20 (12.7) | 137 (87.3) | |
| Neg | 4 (13.8) | 25 (86.2) | .88 | |
| LeuM1 | Pos | 15 (9.1) | 149 (90.9) | |
| Neg | 7 (33.3) | 14 (66.7) | .001 | |
| 80H5 | Pos | 16 (10) | 144 (90) | |
| Neg | 7 (30.4) | 16. (69.6) | .006 | |
| Lex1 | Pos | 5 (33.3) | 10 (66.7) | |
| Neg | 15 (10.3) | 131 (89.7) | .03 | |
| FH6 | Pos | 2 (28.6) | 5 (71.4) | |
| Neg | 15 (10.8) | 126 (89.4) | .25 | |
| Cas3 | Pos | 11 (11.1) | 88 (88.9) | |
| Neg | 7 (13.2) | 46 (86.8) | .70 | |
| Cas8 | Pos | 9 (18.4) | 40 (81.6) | |
| Neg | 11 (10.4) | 95 (89) | .168 | |
| Bcl-2 | Pos | 11 (13.3) | 72 (86.7) | |
| Neg | 11 (11.3) | 86 (88.7) | .70 | |
| p53 | Pos | 19 (12.8) | 129 (87.2) | |
| Neg | 5 (12.8) | 34 (87.2) | .99 |
80H5 - antibody to non-sialyl-CD15
Lex1 - antibody to sialyl-CD15
FH6 - antibody to sialyl-CD15
CD20 expression in classical Hodgkin lymphoma - viral antigens
| CD20+++ | CD20 (-) | P value | ||
|---|---|---|---|---|
| LMP1 | Pos | 4 (7.1) | 52 (92.9) | |
| Neg | 19 (14.4) | 113 (85.6) | .16 | |
| MV | Pos | 12 (12.1) | 87 (87.9) | |
| Neg | 6 (15.8) | 32 (84.2) | .57 | |
| H14 NP | Pos | 8 (9.9) | 73 (90.1) | |
| Neg | 16 (15.8) | 85 (84.2) | .24 | |
| AL 3922 | Pos | 9 (9.7) | 84 (90.3) | |
| Neg | 14 (18.2) | 63 (81.8) | .107 | |
| AL88H | Pos | 2 (5.1) | 37 (94.9) | |
| Neg | 13 (12.7) | 89 (87.3) | .19 | |
| AL 3961 | Pos | 6 (11.5) | 46 (88.5) | |
| Neg | 18 (14.2) | 109 (85.8) | .64 |
MV - expression of 3-5 measles virus antigens
H14NP and AL 3922 - antibodies for NP measles antigens
AL 88H and AL3961 - antibodies for hemagglutinin antigens
CD20 expression in classical Hodgkin lymphoma - the effect of age and follow-up period
| CD20 | n | Mean | St deviation | St Error mean | P value | |
|---|---|---|---|---|---|---|
| Age | +++ | 20 | 47.25 | 21.86 | 4.88 | |
| (-) | 145 | 35.8 | 16.54 | 1.37 | .034 | |
| FU | +++ | 19 | 65.00 | 62.89 | 14.43 | |
| (-) | 135 | 79.45 | 69.58 | 5.98 | .39 |
Table 5 shows the distribution of three of the main immune markers in our study. By far the most frequent combination is CD30+; CD15+; CD20 (-), in 126 cases (65.6%). CD20+++ was found in 15 cases: CD30+; CD15+++; CD20+ in 14 cases (7.3%) and CD30 (-); CD15+++; CD20+ in one case.
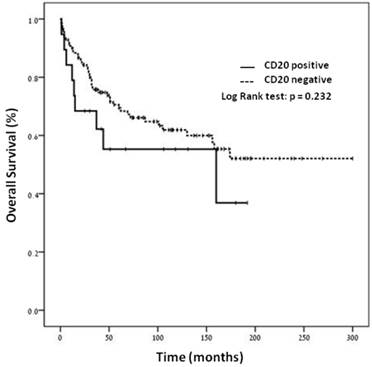
Figure 1. No statistically significant difference was found in overall survival between the two subsets [CD20+++ versus CD20(-)] of patients, using the Kaplan-Meyer analysis.
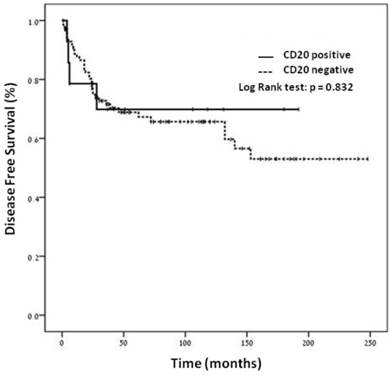
Figure 2. No statistically significant difference was shown in the disease-free survival by the Kaplan-Meier analysis between the two categories of patients.
Table 6. The Cox proportional hazard analysis model was carried out on the association of prognostic factors with dying of cHL. The group of patients studied was representative of cHL (advanced stages and age older than 45 carried a significantly higher risk of dying of the disease). The Cox analysis did not establish CD20 expression as an independent prognostic factor.
Distribution of three major immune markers in classical Hodgkin lymphoma
| CD30 | CD15 | CD20 | N = 192 |
|---|---|---|---|
| + | + | - | 126 (65.6%) |
| + | - | - | 9 (4.7%) |
| + | + | + | 14 (7.3) |
| - | + | + | 1 (0.5) |
| Others | 42 (21.8%) | ||
Cox proportional hazard analysis model on the association of prognostic factors with dying from classical Hodgkin lymphoma in 69 patients
| Factor | Regression Coefficient | 95% Confidence Intervals | p-value |
|---|---|---|---|
| Age1 | 3.218 | 1.575 - 6.574 | 0.001 |
| Stage2 | 1.598 | 0.981 - 2.602 | 0.060 |
| B symptoms3 | 0.337 | 0.123 - 0.923 | 0.034 |
| Leu M14 | 2.116 | 0.721 - 6.207 | 0.172 |
| CD205 | 1.361 | 0.523 - 3.540 | 0.527 |
Age 45 or more versus age less than 451
Stages IIB-IVB versus stages IA-IIA2
Systemic B symptoms: presence as compared with absence3
Leu M1 expression: negative versus positive4
CD20 positive as compared with CD20 negative5
The overall survival curves for the 152 patients stratified according to the CD20 status. By Kaplan-Meier analysis.
The disease free survival curves for the 116 patients stratified according to the CD20 status. By Kaplan-Meier analysis
Discussion
The purpose of this study was to determine the features of our classical Hodgkin lymphoma (cHL) patients with more than 20% strong and/or homogeneous CD20 expression (CD20+++).
The CD20+++ study group showed similar clinical and immunophenotypic findings regarding most features under investigation, when compared with the control group.
The clinical characteristics which were not statistically significant, when comparing between the two groups included gender, bulky disease, B symptoms and stage, all of which have prognostic significance. They included in addition the follow-up period and the outcome.
With regard to immunophenotyping, CD30 showed no difference between the study group and the remainder, nor did apoptosis-related or viral antigens, including EBV/LMP1.
Isolated features were found to differ between the two groups. Clinically, the only divergence was in the age group: most patients (92%) younger than 45 belonged to the CD20-negative or weak expression group to a significant degree (p=.016). Similarly, the mean age of 145 patients from the control group was significantly lower (35.80+-16.54 - p=.034). Comparable results have been obtained by other authors, but with an emphasis on an association between older age and CD20+++ expression [5].
CD20 functionally couples with the B cell antigen receptor (BCR) on the surface of activated B lymphocytes. They dissociate just before internalization of the BCR. Some have suggested that following this event, CD20 disappears. A problematic distribution of CD20 in fixed cells, due to extracellular brittle CD20 epitopes in the presence of fixation may be at the origin of this uncertain piece of information. As a consequence, staining is very weak. Nevertheless, it is clear that CD20, unlike the BCR, remains on the cell surface [17].
As anatomo-pathologists, we certainly can concur with the concept that in a small proportion of cases, varying from one laboratory to the other, lymph nodes fixation may have inconstant results.
Deans et al [18] further showed that the BCR associates with CD20 in non-stimulated cells. Lack of CD20 detection in the early stages may be due to the size of the protein and to the few epitopes empty for cell surface marking. The authors suggest that the success of the biotin attachment to surface CD20 varies and the staining is often poor [18].
Thus, although as a rule, CD20 stains very strongly B lymphocytes in benign and malignant lymphoid tissues, it is not completely excluded that the weak to absent expression of CD20 in the HRS cells of cHL may be a variety of fixation distortion. It is not excluded that a similar artifact may also well be related with the CD20 faint expression in the small B lymphocytes of chronic lymphatic leukemia/small lymphocytic lymphoma. It is of note that CD20 expression is not the only B-cell marker to have been described as faint or negative in cHL. This decreased expression has been related to a crippled IgH gene rearrangement. A loss of B-lineage-specific gene expression program has also been hypothesized [19]. However, a role for epigenetic silencing of B cell-specific genes has been recently demonstrated [20]. As for the OCT.2 and BOB.1 B-cell transcription factors they show an often weaker expression in cHL, which is however consistent with their presence [21].
The other attribute which discriminates the study from the control groups belongs to the differential expression of the CD15 antigens. Thus, the positive staining with the LeuM1 and 80H5 antibodies against the non-sialyl CD15 antigen, showed a significant preferential expression for CD20 that is weak or negative (p=.001 and p=.006, respectively). While negative staining with Lex1 antibody against the sialyl-CD15 antigen showed a preferential relation with a weak or negative CD20 expression. This finding has been described previously, though not in so many details [22] and it has been related by these authors with a differential composition of the lymph node population in cHL. The authors have related the predominance of CD15 antigen (supposedly non-sialyl-CD15 antigens) to a lymph node population rich in granulocytes. On the other hand, a strongly expressed CD20 was associated with a paucity in the reactive inflammatory infiltrate [16, 22]
We describe several combinations of the three markers: CD30; CD15 and CD20. They differ from those described by von Wasielewski et al [10], who found less than 5% of H-RS cell with strong CD20 positivity, while our study group included 24 such cases (12.6%).
In our research, the expression of CD20 is not an independent prognostic factor for dying of cHL. Other authors have associated CD20 expression to a worse prognosis [23]. Still others could not find an association with the outcome, as in our study [3, 9]. In only one instance did CD20 expression carry a good prognosis [4].
We conclude that, in the present investigation, the only attributes which distinguish our study group (CD20+++) from the control category are an older age and the absence of expression of the non-sialyl-CD15 antigen. In the previous papers, these features did not induce the authors into rejecting the diagnosis of cHL. The two groups are otherwise similar.
Our data sustain, therefore, that our study group is an integral part of cHL, though slightly distinct from the cohort at large, perhaps due to a variable fixation effect. This suggests that a cHL case with CD20+++ expression, should, for practical purposes be diagnosed as cHL, though with due caution.
Acknowledgements
We thank Prof. S. Ariad for helpful suggestions.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kuppers R, Engert A, Hansmann ML. Hodgkin lymphoma. J Clin Invest. 2012;122:3439-3447
2. Tzankov A, Zimpfer A, Pehs A-C. et al. Expression of B-cell markers in classical Hodgkin lymphoma: a tissue microarray analysis of 330 cases. Mod Pathol. 2003;16:1141-1147
3. Rassidakis GZ, Medeiros LJ, Viviani S. et al. CD20 expression in Hodgkin and Reed-Sternberg cells of classical Hodgkin's disease: association with presenting features and clinical outcome. J Clin Oncol. 2002;20:1278-1287
4. Tzankov A, Krugmann J, Fend F. et al. Prognostic significance of CD20 expression in classical Hodgkin lymphoma: a clinicopathological study of 119 cases. Clinical Cancer Res. 2003;9:1381-1387
5. Aldred V, Vassallo J, Campos AHJFM. et al. Letter to the editor: CD20 expression by Hodgkin-Reed-Sternberg cells in classical Hodgkin lymphoma is related to reduced overall survival in young adult patients. Leuk Lymphoma. 2008;49:2198-2202
6. Elsayed AA, Asano N, Ohshima K. et al. Prognostic significance of CD20 expression and Epstein-Barr virus (EBV) association in classical Hodgkin lymphoma in Japan: a clinicopathological study. Pathol Int. 2014;64:336-345
7. Canioni D, Deau-Fischer B, Taupin P. et al. Prognostic significance of new immunohistochemical markers in refractory classical Hodgkin lymphomas: a study of 59 cases. PLoS One. 2009;4:e6341 1-7
8. Isaacson PG, Ashton-Key M. Phenotype of Hodgkin's and Reed-Sternberg cells. Lancet. 1996;347(8999):481
9. Vassallo J, Metze K, Traina F. et al. Further remarks on the expression of CD20 in classical Hodgkin lymphoma. Haematologica. 2002;87:ELT17
10. von Wasielewski R, Mengel M, Fischer R. et al. Classical Hodgkin disease. Clinical impact of the immunophenotype. Am J Pathol. 1997;151:1123-1130
11. Rudiger T, Ott G, Ott MM. et al. Differential diagnosis between classic Hodgkin's lymphoma, T-cell-rich B-cell lymphoma and paragranuloma by paraffin immunohistochemistry. Am J Surg Pathol. 1998:1184-1191
12. Boudova L, Torlakovic E, Delabie J. et al. Nodular lymphocyte predominant Hodgkin lymphoma with nodules resembling T-cell/histiocyte-rich B-cell lymphoma: differential diagnosis between nodular lymphocyte-predominant Hodgkin lymphoma and T-cell/histiocyte-rich B-cell lymphoma. Blood. 2003;102:3753-3758
13. Cazals-Hatem D, Andre M, Mounier N. et al. Pathologic and clinical features of 77 Hodgkin's lymphoma patients treated in a lymphoma protocol (LNH87). A GELA study. Am J Surg Pathol. 2001;25:297-306
14. Harris NL. Shades of gray between large B-cell lymphomas and Hodgkin lymphomas: differential diagnosis and biological implications. Mod Pathol. 2013;26(Suppl 1):s57-70
15. Yamamoto W, Nakamura N, Tomita N. et al. Clinicopathological analysis of mediastinal large B-cell lymphoma and classical Hodgkin lymphoma of the mediastinum. Leuk Lymphoma. 2013;54:967-972
16. Benharroch D, Pilosof S, Gopas J. et al. Primary refractory and relapsed classical Hodgkin lymphoma - significance of differential CD15 expression in Hodgkin-Reed-Sternberg cells. J Cancer. 2012;3:322-327
17. Petrie RJ, Deans JP. Colocalization of the B cell receptor and CD20 followed by activation-dependent dissociation in distinct lipid rafts. J Immunol. 2002;169:2886-2891
18. Polyak MJ, Li H, Shariat N. et al. CD20 homo-oligomers physically associates with the B cell antigen receptor. Dissociation upon receptor engagement and recruitment of phosphoproteins and calmodulin-binding proteins. J Biol Chem. 2008;283:18545-18552
19. Schwering I, Brauninger A, Klein U. et al. Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2003;101:1505-1512
20. Ushmorov A, Leithauser F, Sakk O. et al. Epigenetic processes play a major role in B-cell specific gene silencing in classical Hodgkin lymphoma. Blood. 2006;107:2493-2500
21. Garcia-Cosio M, Santon A, Martin P. et al. Analysis of transcription factor OCT.1, OCT.2 and BOB.1 expression using tissue arrays in classical Hodgkin lymphoma. Mod Pathol. 2004;17:1531-1538
22. Zukerberg LR, Collins AB, Ferry JA, Harris NL. Coexpression of CD15 and CD20 by Reed-Sternberg cells in Hodgkin disease. Am J Pathol. 1991;139:475-483
23. Portlock CS, Donnelly GB, Qin J. et al. Adverse prognostic significance of CD20 positive Reed-Sternberg cells in classical Hodgkin disease. Brit J Haematol. 2004;125:701-708
Author contact
![]() Corresponding author: Prof. Daniel Benharroch, Dept. of Pathology, Soroka University Medical Center, P.O.Box 151, Beer-Sheva, 84101, Israel. Tel: 97286400920. Fax:86232770. e-mail: benarochac.il
Corresponding author: Prof. Daniel Benharroch, Dept. of Pathology, Soroka University Medical Center, P.O.Box 151, Beer-Sheva, 84101, Israel. Tel: 97286400920. Fax:86232770. e-mail: benarochac.il

 Global reach, higher impact
Global reach, higher impact