3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2016; 7(1):14-21. doi:10.7150/jca.13126 This issue Cite
Research Paper
To Operate or Not: Prediction of 3-Month Postoperative Mortality in Geriatric Cancer Patients
1. Department of Medical Oncology, Chang Gung Memorial Hospital, LinKou
2. Department of Surgery, Chang Gung Memorial Hospital, LinKou
3. Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University
4. Department of Medical Oncology, Chang Gung Memorial Hospital, Chiayi
5. Department of Radiation Oncology, Chang Gung Memorial Hospital, Chiayi
6. Department of Radiology, Chang Gung Memorial Hospital, Kaoshiung
7. Department of Medical Oncology, Chang Gung Memorial Hospital at Keelung, Chang Gung University College of Medicine, Taiwan
Received 2015-7-3; Accepted 2015-9-8; Published 2016-1-1
Abstract
Context: Appropriate selection of aging patient who fit for cancer surgery is an art-of-state.
Objectives: This study aimed to identify predictive factors pertinent to 3-month postoperative mortality in geriatric cancer patients.
Methods: A total of 8,425 patients over 70 years old with solid cancer received radical surgery between 2007 and 2012 at four affiliated hospitals of the Chang Gung Memorial Hospital were included. The clinical variables of patients who died within 3 months post-surgery were analyzed retrospectively. Recursive partitioning analysis (RPA) was performed by randomly selecting 50% of the patients (testing set) to identify specific groups of patients with the lowest and highest probability of 3-month postoperative mortality. The remaining 50% were used as validation set of the model.
Results: Patients' gender, Eastern Cooperative Oncology Group performance (ECOG scale), Charlson comorbidity index (CCI), American Society of Anesthesiologist physical status, age, tumor staging, and mode of admission were independent variables that predicted 3-month postoperative mortality. The RPA model identified patients with an ECOG scale of 0-2, localized tumor stage, and a CCI of 0-2 as having the lowest probability of 3-month postoperative mortality (1.1% and 1.3% in the testing set and validation set, respectively). Conversely, an ECOG scale of 3-4 and a CCI >2 were associated with the highest probability of 3-month postoperative mortality (55.2% and 47.8% in the testing set and validation set, respectively).
Conclusion: We identified ECOG scale and CCI score were the two most influencing factors that determined 3-month postoperative mortality in geriatric cancer patients.
Keywords: postoperative mortality, solid cancer, geriatric patients, predictive factors
Introduction
Radical surgical resection is the most common, and sometimes the only, curative modality for solid cancer patients. However, surgery may compromise outcome and even lead to death in medically unfit or frail patients (1-6). Old age is a reliable negative predictor of outcome after cancer surgery (7). In a nationwide study in the United States evaluating the impact of age on postoperative outcome for colorectal cancer patients over a ten years period, elder patients were associated with a higher hospital cost, increased length of stay, and higher in-hospital mortality and morbidity compared to younger patients (8). The difference in postoperative mortality rates between older and younger patients became wider as age increases. In a European based study, the postoperative mortality for patients aged 85-89 years and those 90 years and older after cancer surgery was 2-fold and 3-fold higher than patients aged 80-84 years, respectively (9). In contrast, many studies suggested that, with careful preoperative selection, surgical treatment can be performed at acceptable risk and with good outcomes in elderly patients of various types of cancer (10-19).These disparities suggest that age is not the sole predictor of postoperative mortality, which can also be affected by various other factors such as comorbidity and functional status (20-22).
As populations are aging and cancer incidences increase worldwide, there is an urgent clinical need to address the merits and demerits of surgical treatment in elderly cancer patients. Overtreatment may result in high postoperative mortality due to disregarding aging patients' frailty. On the other hand, elderly patients are less likely to be offered standard cancer treatment because of the unfamiliarity of medical personnel with caring for elderly patients (23), or concern about their ability to tolerate treatment (24); thus, the outcome is suboptimal owing to undertreatment (25). Since the high risk of postoperative mortality for aging patients should be weighed against the potential benefit, proper selection of aging patients who qualify for surgery, and identifying vulnerable subjects who do not qualify, may assist in the decision-making process as well as the designing of treatment alternative (7). To address this, we analyzed preoperative clinical variables of patients 70 years and older to identify factors that are correlated with mortality within 3-months of cancer surgery.
Materials and methods
Patient selection
A total of 37,288 patients who underwent operations for solid cancers between January 2007 and December 2012 at four hospitals affiliated with the Chang Gung Memorial Hospital system (CGMH) (The Linkou, Keelung, Chiayi, and Kaohsiung branches of the CGMH) were included in this study. All patients with either pathologically- or radiographically suspicious malignancies underwent radical resection of their primary cancers with curative intent. Patients who received palliative resection or bypass surgery were excluded, as were patients with skin cancers and superficial urinary bladder cancers. Overall, 890 of 37,288 patients (2.4%) died within 3 months post-surgery. The total numbers of patients <70 and ≥70 years of age were 28,836 and 8,452, respectively, and the numbers of those patients who died within 3-month postoperative months were 447 (1.6%) and 443 (5.2%), respectively (p<0.001). The characteristics of patients 70 years and older were analyzed retrospectively to identify preoperative variables pertinent to 3-month postoperative mortality. The study design is presented in Figure 1. The study was approved by the Institutional Review Boards at all CGMH branches, in compliance with the Helsinki Declaration (1996).
Study flow chart
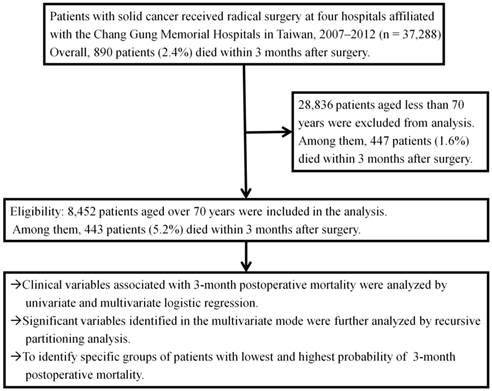
Data collection
The administrative and clinical data collected before surgery included patients' demographics, American Society of Anesthesiologist physical status (ASA score), and the Charlson comorbidity index (CCI). Demographic of every patient including age, gender, Eastern Cooperative Oncology Group performance status (ECOG scale), admission mode (elective or emergency), existence of past cancer history, preexisting comorbidities, cancer site by anatomic location, histological grade of differentiation, and clinical tumor stage were recorded by primary care clinicians preoperatively using a prospectively-formulated electronic record form. This form has been provided by the institutional cancer center with the intent to improve quality of care for cancer patients since 2006 after implementation of the Cancer Prevention and Treatment Act in Taiwan. Data quality is maintained for completeness and accuracy by individual multidisciplinary teams and well-trained cancer center personnel. Tumor stage was recorded as localized, regional, advanced, and unclassified using the Surveillance, epidemiology, and end results program (SEER) summary stage classification (26). ASA scores were provided by anesthesiologists at preanesthetic evaluation, whereas CCIs were calculated from tabulated electronic record forms using The International Classification of Diseases (Ninth Revision) coding. A modified CCI that excluding the scores for patient age and type of cancer was used in this study. Patients with a diagnosis of more than one tumor or those receiving multiple surgeries for their primary tumors within the study period were analyzed from the date of surgery for the first tumor or the first surgery. All included patients were followed until death or June 30, 2014. Survival time was determined from the time of surgery to death or the date last known to be alive. All dates of death were obtained from the National Registry of Death database in Taiwan.
Statistical analysis
Basic demographic data were summarized as n (%) for categorical variables and medians with interquartile ranges (25-75%) for continuous variables. Distribution of clinical variables was tabulated as n (%) by 3-month postoperative mortality status and compared using the chi-square test. Differences between continuous variables were analyzed using t-tests for normally distributed data and Wilcoxon rank sum tests for non-normally distributed data. Possible clinical variables for 3-month postoperative mortality after cancer surgery were examined by univariate and multivariate logistic regressions. Significant variables identified in the multivariate mode were further analyzed by recursive partitioning analysis (RPA) (27), which is a decision tree method for identifying specific groups of patients with a greater probability of a specific outcome. The first node of RPA included all patients; the node was split if the Chi-square statistical test was significant for any variable beyond the 0.05 probability level. Each splitting resulted in two homogeneous subgroups with respect to the 3-month postoperative mortality status. Terminal nodes were defined as those with fewer than 50 patients or when no possible partition exceeded the adjusted minimum significant value. RPA was performed by randomly selecting 50% of the patients, defined as the testing set, to identify optimal patient classifications, whereupon the remaining 50% of the patients, defined as the validation set, were used to estimate the hazard ratio (HR) of 3-month postoperative mortality among these classifications by univariate logistic regression. This was done with the intent of generating preliminary data toward a model that would predict those patients likely to experience 3-month postoperative mortality after cancer surgery. The SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. All statistical assessments were two-sided. A p value smaller than 0.05 was considered significant.
Results
In total, 443 patients (5.2%) died within 3 months after cancer surgery. All the clinical variables are summarized in Table 1. The 3-month postoperative mortality was significantly higher in patients who were male, older than 80, those with a primary tumor site from visceral organs (thorax, central nerve system, stomach, hepatobiliary and pancreas), poor ECOG scale, poorly differentiated grade of tumor, non-elective admission type, higher ASA score, and higher number of comorbidities.
No difference in terms of previous cancer history was observed between the two patient groups.
Univariate and multivariate analyses of clinical variables to predict 3-month postoperative mortality are presented in Table 2. The 3-month postoperative mortality was significantly different with respect to gender (5.9% in male vs. 4.4 % in female), primary tumor location (2.0% in patients with breast or thyroid cancer; 4.8% in patients with colorectal, gynecologic, or urologic cancer; 5.0% in head, neck, and thorax cancer; 7.5% in gastric and hepato-biliary-pancreatic cancer, and 9.4% in patients with tumors of the central nervous system), tumor stage (2.7%, 5.5%, 13.9%, and 10.2% in localized, regional, advanced, and unclassified stages, respectively), histological grade of differentiation (3.6%, 4.4%, 6.8%, and 7.4% in well-, moderately-, poorly-, and unclassified differentiation, respectively), ECOG scale (2.6%, 3.7%, 29.6%, and 57.6% in ECOG scale 0-1, 2, 3, and 4, respectively), admission type (4.9% in elective admission vs. 15.5% in non-elective admission), CCI (3.4%, 4.7%, 7.2%, 18.5%, and 33.8% in CCI 0, 1, 2, 3-4 and 5-8, respectively), ASA scores (2.6%, 6.2%, and 43.2% with ASA scores 1-2, 3, and 4-5, respectively) and age (4.0%, 8.7%, and 11.1% in ages 70-79, 80-89 and over 90, respectively).
Only gender, tumor stage, ECOG scale, admission type, CCI, ASA score, and age were independent variables that predicted 3-month postoperative mortality after cancer surgery as determined by the multivariate analysis.
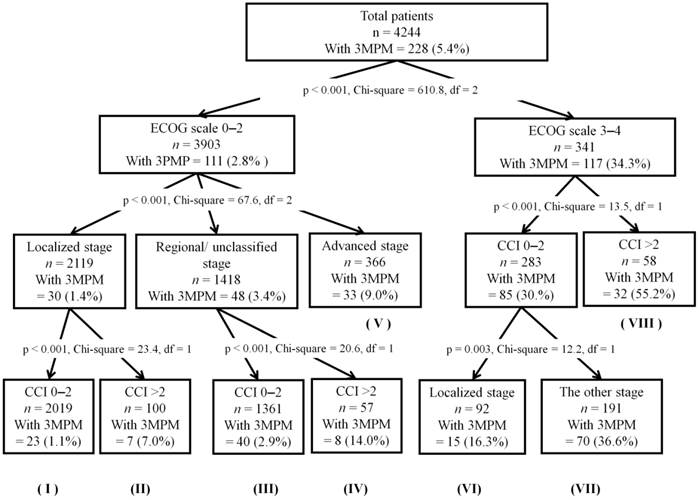
The result of the RPA model is illustrated in Figure 2. In the testing set, patients were divided into eight classifications ranging from the lowest (1.1%) to the highest (55.2%) probability of 3-month postoperative mortality after cancer surgery, based on the decision tree method. Patients with a good ECOG scale (0-1) and fewer comorbidities (CCI 0-2), defined as the reference group, had the lowest probability of 3-month postoperative mortality after cancer surgery. The highest probability of 3-month postoperative mortality (55.2% and 47.8% in the training set and validation set, respectively) after cancer surgery was observed among patients with a poor ECOG scale (3-4) and multiple comorbidities (CCI >2). In the validation set, significant differences were observed between the reference group and other groups according to RPA, with HRs ranging from 1.85 to 67.3 (Table 3).
Basic patient demographic data
| Variable | Categories | Overall, n (%) | Alive at 3 months after surgery, n (%) | Dead within 3 months after surgery, n (%) | P value |
|---|---|---|---|---|---|
| Total | 8,452 (100) | 8,009 (100) | 443 (100) | ||
| Gender | Male | 4,753 (56.2) | 4,471 (55.8) | 282 (63.7) | <0.001a |
| Female | 3,699 (43.8) | 3,538 (44.2) | 161 (36.3) | ||
| Age | Mean (IQR) | 75 (72-80) | 76.3 (71-81) | 78.4 (72-84) | <0.001b |
| 70-79 | 6320 (74.8) | 6,066 (75.7) | 254 (57.3) | <0.001a | |
| 80-89 | 2006 (23.7) | 1,831 (22.9) | 175 (39.5) | ||
| 90-99 | 126 (1.5) | 112 (1.4) | 14 (3.2) | ||
| ECOG scale | 0-1 | 5,473 (64.8) | 5,331 (66.6) | 142 (32.1) | <0.001a |
| 2 | 2,315 (27.4) | 2,229 (27.8) | 86 (19.4) | ||
| 3 | 598 (7.1) | 421 (5.3) | 177 (40.0) | ||
| 4 | 66 (0.8) | 28 (0.3) | 38 (8.6) | ||
| CCI | 0 | 4,548 (53.8) | 4,392 (54.8) | 156 (35.2) | <0.001 a |
| 1 | 2,420 (28.6) | 2,306 (28.8) | 114 (25.7) | ||
| 2 | 988 (11.7) | 917 (11.4) | 71 (16.0) | ||
| 3 | 310 (3.7) | 253 (3.2) | 57 (12.9) | ||
| 4 | 118 (1.4) | 96 (1.2) | 22 (5.0) | ||
| 5 | 73 (0.5) | 30 (0.4) | 15 (3.4) | ||
| 6 | 16 (0.2) | 10 (0.1) | 6 (1.4) | ||
| 7 | 4 (0.04) | 2 (0.02) | 2 (0.5) | ||
| 8 | 3 (0.03) | 3 (0.03) | 0 (0) | ||
| ASA score | 1 | 131 (1.5) | 125 (1.6) | 6 (1.4) | <0.001a |
| 2 | 3,159 (37.4) | 3,079 (38.4) | 80 (18.1) | ||
| 3 | 5,067 (60.0) | 4,751 (59.3) | 316 (71.3) | ||
| 4 | 93 (1.1) | 52 (0.6) | 41 (9.3) | ||
| 5 | 2 (0.02) | 2 (0.02) | 0 (0) | ||
| Primary cancer site | Thorax | 1,117 (13.0) | 1,064 (13.3) | 53 (12.0) | <0.001a |
| CNS | 96 (1.1) | 87 (1.1) | 9 (2.0) | ||
| Esophagus | 39 (0.5) | 34 (0.4) | 5 (1.1) | ||
| Stomach and small bowel | 891 (10.5) | 823 (10.4) | 68 (15.3) | ||
| Colorectum | 3,378 (40.0) | 3,213 (40.1) | 165 (37.2) | ||
| Liver-pancreas-biliary | 853 (10.1) | 791 (9.9) | 62 (14.0) | ||
| Gynecological | 271 (3.2) | 260 (3.2) | 11 (2.5) | ||
| Genitourol. | 1,259 (14.9) | 1,200 (15.0) | 59 (13.3) | ||
| Breast | 436 (5.2) | 432 (5.4) | 4 (0.9) | ||
| Thyroid | 112 (1.3) | 105 (1.3) | 7 (1.6) | ||
| Previous cancer history | Yes | 1,170 (13.8) | 1,107 (13.8) | 63 (14.2) | 0.43a |
| No | 7,282 (86.2) | 6,902 (86.2) | 380 (85.8) | ||
| Tumor grade | Well | 845 (10.0) | 815 (10.2) | 30 (6.8) | <0.001a |
| Moderately | 4,429 (52.4) | 4,236 (52.9) | 193 (43.6) | ||
| Poorly | 1,538 (18.2) | 2,164 (27.0) | 157 (35.4) | ||
| Unclassified | 857 (10.1) | 794 (9.9) | 63 (14.2) | ||
| Tumor stage | Localized | 4,457 (52.7) | 4,335 (54.1) | 122 (27.5) | <0.001a |
| Regional | 2,539 (30.0) | 2,399 (30.0) | 140 (31.6) | ||
| Advanced | 877 (10.4) | 755 (9.4) | 122 (27.5) | ||
| Unclassified | 579 (6.9) | 520 (6.5) | 59 (13.3) | ||
| Admission type | Elective | 8,174 (96.7) | 7,774 (97.1) | 400 (90.3) | <0.001a |
| Emergency | 278 (3.3) | 235 (2.9) | 43 (9.7) |
IQR, interquartile range; CNS, central nerve system; ECOG scale, Eastern Cooperative Oncology Group performance status; CCI, Charlson comorbidity index; ASA score, American Society of Anesthesiologist physical status; Genitourol., genitourological.
a Chi-square test, bUnpaired two-sided t-test
Recursive partitioning analysis of the testing set (n = 4,244). The classification mode is represented by a roman numeral below each node of the decision-tree, and was used for univariate logistic regression analysis in the validation set (see Table 3). 3MPM, 3-month postoperative mortality; df, degrees of freedom; ECOG scale, Eastern Cooperative Oncology Group performance status; CCI, Charlson comorbidity index.
Univariate and multivariate analysis of 3-month postoperative mortality
| Variable | Categories | No of patients dead within 3 months of surgery/total patients, (%) | Univariate, HR (95% CI) | P value | Multivariate, HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Gender | Male | 282/4753 (5.9) | 1 | 1 | ||
| Female | 161/3699 (4.4) | 0.72 (0.59-0.88) | 0.001 | 0.72 (0.57-0.90) | 0.004 | |
| Age | 70-79 | 254/6320 (4.0) | 1 | 1 | ||
| 80-89 | 175/2006 (8.7) | 2.28 (1.87-2.79) | <0.001 | 1.84 (1.46-2.31) | <0.001 | |
| 90 | 14/126 (11.1) | 2.99 (1.69-5.28) | <0.001 | 1.59 (0.82-3.07) | 0.17 | |
| ECOG scale | 0-1 | 142/5473(2.6) | 1 | 1 | ||
| 2 | 86/2315 (3.7) | 1.45 (1.10-1.90) | 0.008 | 1.09 (0.81-1.47) | 0.56 | |
| 3 | 177/598 (29.6) | 15.8 (12.4-20.1) | <0.001 | 9.11 (6.88-12.1) | <0.001 | |
| 4 | 38/66 (57.6) | 50.9 (30.4-85.3) | <0.001 | 16.3 (8.27-32.1) | <0.001 | |
| CCI | 0 | 156/4548 (3.4) | 1 | 1 | ||
| 1 | 114/2420 (4.7) | 1.39 (1.09-1.78) | 0.009 | 1.26 (0.96-1.65) | 0.094 | |
| 2 | 71/988 (7.2) | 2.18 (1.63-2.91) | <0.001 | 1.5 (1.08-2.08) | 0.015 | |
| 3-4 | 79/428 (18.5) | 6.73 (4.76-8.53) | <0.001 | 4.47 (3.17-6.32) | <0.001 | |
| 5-8 | 23/68 (33.8) | 14.4 (8.49-24.4) | <0.001 | 8.09 (4.22-15.5) | <0.001 | |
| ASA score | 1-2 | 86/3290 (2.6) | 1 | 1 | ||
| 3 | 316/5067 (6.2) | 2.48 (1.94-3.16) | <0.001 | 1.05 (0.78-1.39) | 0.76 | |
| 4-5 | 41/95 (43.2) | 28.3 (17.9-44.8) | <0.001 | 2.3 (1.21-4.39) | 0.012 | |
| Primary tumor site | Breast, Thyroid | 11/548 (2.0) | 1 | 1 | ||
| CRC, GYN, GU | 235/4908 (4.8) | 2.46 (1.33-4.52) | 0.004 | 1.32 (0.68-2.56) | 0.41 | |
| HN, Esophagus, Lung, others | 58/1156 (5.0) | 2.58 (1.34-4.95) | 0.004 | 1.64 (0.81-3.32) | 0.17 | |
| HPB, Stomach, Small bowel | 130/1744 (7.5) | 3.93(2.11-7.33) | <0.001 | 1.93 (0.98-3.77) | 0.056 | |
| CNS | 9/96 (9.4) | 5.05 (2.03-12.5) | <0.001 | 0.56 (0.19-1.59) | 0.27 | |
| Previous cancer history | No | 380/7282 (5.2) | 1 | 1 | ||
| Yes | 63/1170 (5.4) | 1.03 (0.79-1.36) | 0.81 | 1.10(0.81-1.50) | 0.54 | |
| Tumor grade | Well | 30/845 (3.6) | 1 | 1 | ||
| Moderately | 193/4429 (4.4) | 1.24 (0.84-1.83) | 0.29 | 0.91 (0.58-1.41) | 0.66 | |
| Poorly | 157/2321 (6.8) | 1.97(1.32-2.94) | 0.001 | 1.38 (0.87-2.20) | 0.17 | |
| Unclassified | 63/857 (7.4) | 2.16 (1.38-3.37) | 0.001 | 1.47 (0.88-2.48) | 0.15 | |
| Tumor stage | Localized | 122/4457 (2.7) | 1 | 1 | ||
| Regional | 140/2539 (5.5) | 2.07 (1.62-2.66) | <0.001 | 1.66 (1.26-2.20) | <0.001 | |
| Advanced | 122/877 (13.9) | 5.74 (4.42-7.47) | <0.001 | 4.60 (3.41-6.20) | <0.001 | |
| Unclassified | 59/579 (10.2) | 4.03 (2.92-5.57) | <0.001 | 2.63 (1.76-3.94) | <0.001 | |
| Admission type | Elective | 400/8174 (4.9) | 1 | 1 | ||
| Emergency | 43/278 (15.5) | 3.56 (2.53-5.00) | <0.001 | 1.86 (1.20-2.88) | 0.005 |
ECOG scale, Eastern Cooperative Oncology Group performance status; CCI, Charlson comorbidity index; ASA score, American Society of Anesthesiologist physical status; CRC, colorectum; GYN, gynecological; GU, genitourological; HN, head and neck; HPB, hepato-pancreatico-biliary; CNS, central nerve system.
Univariate logistic regression analysis for patients with 3-month postoperative mortality as determined by the classification model (Figure 2) based on recursive partitioning analysis: the validation set (n = 4,208)
| Classification | No. of patients who died within 3 months of surgery/total patients, n (%) | Hazard ratio (95% CI) of patients who died within 3 months of surgery | P value |
|---|---|---|---|
| I | 27/2009 (1.3) | 1 (reference) | |
| II | 11/125 (8.8) | 7.08 (3.43-14.6) | <0.001 |
| III | 32/1303 (2.5) | 1.85 (1.10-3.10) | 0.02 |
| IV | 6/69 (8.7) | 6.99 (2.79-17.5) | <0.001 |
| V | 41/379 (10.8) | 8.90 (5.41-14.7) | <0.001 |
| VI | 22/79 (27.8) | 28.3 (15.2-52.7) | <0.001 |
| VII | 54/198 (27.3) | 27.5 (16.8-45.0) | <0.001 |
| VIII | 22/46 (47.8) | 67.3 (33.7-134.4) | <0.001 |
CI: confidence interval
Discussion
We analyzed prospectively collected preoperative clinical data of 8,452 cancer patients 70 years or older underwent surgery. We found the overall 3-month postoperative mortality after cancer surgery was 5.2% in patients 70 years and older, which was 3.3-fold higher than in patients under 70 years old (p<0.001). In addition to age, gender, tumor stage, ECOG scale, ASA score, admission type, and comorbidities were independent factors predicting 3-month postoperative mortality by multivariate analysis. The combination of the above factors can provide better risk stratification of 3-month postoperative mortality in elder patients slated to undergo cancer surgery.
Consistent with previous studies (1-6), univariate analysis in our study population showed that age is an independent factor associated with patients' postoperative mortality. However, our multivariate model revealed that the difference in 3-month postoperative mortality between patients aged 70-79 years and those 90 years and over was insignificant, although the lower number of patients in the 90 years and older group may partially limit the statistical power. More importantly, the impact of age on postoperative mortality was diminished after adjusting for other covariates such as comorbidities, performance status, and emergency or elective admission; these factors significantly influenced the outcome of postoperative mortality. Our study showed that age alone is not a sufficient predictor of 3-month postoperative mortality after cancer surgery.
Oncogeriatric patients are often associated with an increasing prevalence of frailty, multiple comorbidities, and decline of functional reserve. Appropriate comprehensive preoperative evaluation of functional status, nutritional status, cognitive abilities, and associated comorbidities can assist in the identification of patients at risk of postoperative mortality (28-31). However, some of the geriatric assessments and frailty scoring used by well-trained geriatric physicians were cumbersome (31); therefore, they were not widely applied in routine clinical practice. The ECOG scale and ASA score are commonly used to measure the functional status in oncologic practice (32-34). In a recent study comparing the ECOG scale and ASA score as a measure of functional status for predicting the length of hospitalization after colon cancer surgery (35), both scores similarly predicted postoperative length of stay. Furthermore, using both scores simultaneously better predicted postoperative length of stay than a single score (35).
The CCI is one of the most commonly used comorbidity indexes (36, 37), and it was initially used to assess the role of comorbidity on mortality risks in longitudinal studies (36). Higher CCI has since been shown to be associated with a poor outcome in cancer patients undergoing surgery (38). Mayr and colleagues (39) compared ASA score, ECOG scale, and CCI for risk adjustment of postoperative 90-day mortality for patients with bladder cancer; each of the three scores significantly increased the predictive accuracy of postoperative mortality. In line with previous reports, our study showed that the ECOG scale, CCI, and ASA scores were independent prognostic factors for predicting post-operative mortality in a variety of cancer types. Considering their accuracy and the ease of their acquisition, we believe the ECOG scale, CCI, and ASA scores should be widely used as predictors of postoperative mortality for elderly patients in routine clinical practice.
In our study, primary tumor localization, tumor stage, and histological differentiation grade were all significant predictors of postoperative mortality after cancer surgery on univariate analysis. Tumor-related variables may influence a patient's surgical outcome in two ways: First, the severity of surgical procedures can lead to the destruction of vital organs and impairment of functional abilities such as cognition, digestion, and respiration. Second, the type of cancer in each individual determines life expectancy. Accordingly, we found that the probabilities of 3-month postoperative mortality in colorectal, esophageal, hepato-biliary-pancreatic, and central nervous system cancer patients are 2.5-, 2.6-, 3.9-, and 5.1-fold greater, respectively, than that of breast and thyroid cancers on univariate analysis. In line with previous reports (40, 41), our study showed that emergency hospital admission carried a 2.5-fold postoperative risk of death over elective admission in all age groups, and the risk was especially higher in elderly patients (3.3-fold mortality rate compared to elective surgery in patients over 75 years old). Our analysis revealed that, in addition to patients' characteristics, tumor-related variables also influenced postoperative mortality in solid cancer patients undergoing cancer surgery.
This study showed that postoperative mortality in oncogeriatric patients was influenced by multiple factors related to patient and tumor characteristics, not solely age. The RPA classification model was developed by combining patient and tumor factors pertinent to postoperative mortality risk in this study, and the model was validated as accurate in terms of risk stratification for postoperative mortality in oncogeriatric patients. Using the RPA model, we determined that the majority of patients over 70 years old (47.6% and 47.7% of all patients in the testing and validation sets, respectively) had the lowest probability of postoperative mortality (1.1% and 1.3% in the testing and validation sets, respectively). This was consistent with the postoperative mortality rate (1.6%) in patients aged under 70 years old.
Furthermore, the RPA model identified a small subset of elderly patients (1.4% and 1.1% of all patients in the testing and validation sets, respectively) with a significantly higher probability of postoperative mortality (55.2% and 47.8% in the training set and validation set, respectively) with a ECOG scale 3-4 and CCI score >2. Our model provides a clear estimation of 3-month postoperative mortality risk. We believe that the risk model will assist patients and clinicians with making treatment decisions and providing appropriate postoperative care.
The strengths of our study included large patient numbers from multiple institutes across Taiwan over a 6-year duration, and all clinical variables were collected from a prospectively- formulated electronic record form. Additionally, all independent predictive factors were easy to access and available before or soon after the time of surgery; therefore, this risk stratification model can be used in routine clinical practice to predict 3-month postoperative mortality in geriatric patients with solid cancer.
There are some limitations in this study. First, the number of events was small and the occurrence rate was low; hence, this risk stratification model may not represent a patient's true mortality risk. Second, we used the universal factors of all patients to construct the risk model, and it was not possible to stratify postoperative risk among patients according to specific cancers that were treated by different surgical methods. In addition, our study model was built on Taiwanese patient population. The clinical practice and healthcare system in Taiwan may differ from other countries. Therefore, this model may not be applied to all cancer patients worldwide. Last and most importantly, our analysis only included patients who underwent surgery; as such, there was a selection bias towards elderly patients who were offered and received surgical treatment.
Conclusions
The 3-month postoperative mortality in elderly cancer patients was affected by multiple factors. We identified ECOG scale and CCI score, rather than age per se, were the two most influencing factors that determined 3-month postoperative mortality in geriatric cancer patients. Age should not be the sole factor for selecting elderly patients for cancer surgery.
Acknowledgements
The authors thank all the members of the Cancer Center, Chang Gung Memorial Hospital, for assisting with data collection and Prof. Alex Chang, from Johns Hopkins Singapore, for invaluable advice.
Competing Interests
This research was not funded by any public, commercial, or nonprofit agency. No competing financial interests exist.
References
1. Al-Refaie WB, Parsons HM, Habermann EB. et al. Operative outcomes beyond 30-day mortality: Colorectal cancer surgery in oldest old. Ann Surg. 2011;253:947-952
2. Abbass MA, Slezak JM, DiFronzo LA. Predictors of early postoperative outcomes in 375 consecutive hepatectomies: a single-institution experience. Am Surg. 2013;79(10):961-967
3. Lagier A, Mimouni-Benabu O, Baumstarck K. et al. The influence of age on postoperative complications after total laryngectomy or pharyngolaryngectomy. Eur J Surg Oncol. 2014;40(2):202-207
4. Tzeng CW, Cooper AB, Vauthey JN, Curley SA, Aloia TA. Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB (Oxford). 2014;16(5):459-468
5. Powell HA, Tata LJ, Baldwin DR. et al. Early mortality after surgical resection for lung cancer: an analysis of the English National Lung cancer audit. Thorax. 2013;68(9):826-834
6. Iversen LH, Ingeholm P, Gögenur I, Laurberg S. Major reduction in 30-day mortality after elective colorectal cancer surgery: a nationwide population-based study in Denmark 2001-2011. Ann Surg Oncol. 2014;21(7):2267-2273
7. Korc-Grodzicki B, Downey RJ, Shahrokni A. et al. Surgical considerations in older adults with cancer. J Clin Oncol. 2014;32(24):2647-2653
8. Jafari MD, Jafari F, Halabi WJ. et al. Colorectal Cancer Resections in the Aging US Population: A trend toward decreasing rates and improved outcomes. JAMA Surg. 2014 doi: 10.1001/jamasurg.2013.4930
9. Damhuis RA, Meurs CJ, Meijer WS. Postoperative mortality after cancer surgery in octogenarians and nonagenarians: results from a series of 5,390 patients. World J Surg Oncol. 2005;3:71
10. Hodul P, Tansey J, Golts E. et al. Age is not a contraindication to pancreaticoduodenectomy. Am Surg. 2001;67(3):270-275
11. Mahdi H, Lockhart D, Maurer KA. Impact of age on 30-day mortality and morbidity in patients undergoing surgery for endometrial cancer. Gynecol Oncol. 2015 doi: 10.1016/j.ygyno.2015.01.543
12. Okami J, Higashiyama M, Asamura H. et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol. 2009;4(10):1247-1253
13. Lahat G, Dhuka AR, Lahat S. et al. Complete soft tissue sarcoma resection is a viable treatment option for select elderly patients. Ann Surg Oncol. 2009;16(9):2579-2586
14. Peters TT, van Dijk BA, Roodenburg JL, van der Laan BF, Halmos GB. Relation between age, comorbidity, and complications in patients undergoing major surgery for head and neck cancer. Ann Surg Oncol. 2014;21(3):963-970
15. Mita K, Ito H, Hashimoto M. et al. Postoperative complications and survival after gastric cancer surgery in patients older than 80 years of age. J Gastrointest Surg. 2013;17(12):2067-2073
16. Gerstenhaber F, Grossman J, Lubezky N. et al. Pancreaticoduodenectomy in elderly adults: is it justified in terms of mortality, long-term morbidity, and quality of life? J Am Geriatr Soc. 2013;61(8):1351-1357
17. Peters TT, van Dijk BA, Roodenburg JL. et al. Predictors of postoperative complications and survival in patients with major salivary glands malignancies: A study highlighting the influence of age. Head Neck. 2014;36(3):369-374
18. Kow AW, Sadayan NA, Ernest A. et al. Is pancreaticoduodenectomy justified in elderly patients? Surgeon. 2012;10(3):128-136
19. Markar SR, Low DE. Physiology, not chronology, dictates outcomes after esophagectomy for esophageal cancer: outcomes in patients 80 years and older. Ann Surg Oncol. 2013;20(3):1020-1026
20. Ra J, Paulson EC, Kucharczuk J. et al. Postoperative mortality after esophagectomy for cancer: development of a preoperative risk prediction model. Ann Surg Oncol. 2008;15(6):1577-1584
21. Koppert LB, Lemmens VE, Coebergh JW. et al. Impact of age and co-morbidity on surgical resection rate and survival in patients with oesophageal and gastric cancer. Br J Surg. 2012;99(12):1693-1700
22. van Gestel YR, Lemmens VE, de Hingh IH. et al. Influence of comorbidity and age on 1-, 2-, and 3-month postoperative mortality rates in gastrointestinal cancer patients. Ann Surg Oncol. 2013;20(2):371-380
23. Audisio RA, Bozzetti F, Gennari R. et al. The surgical management of elderly cancer patients: Recommendations of the SIOG surgical task force. Eur J Cancer. 2004;40:926-938
24. Zbar AP, Gravitz A, Audisio RA. Principles of surgical oncology in the elderly. Clin Geriatr Med. 2012;28:51-71
25. Bouchardy C, Rapiti E, Fioretta G. et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol. 2003;21:3580-3587
26. Young JL Jr, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA (eds). SEER Summary Staging Manual - 2000: Codes and Coding Instructions, National Cancer Institute, NIH Pub. No. 01-4969, Bethesda, MD. 2001
27. Zhang H, Singer B. Recursive partitioning in the health sciences. Analysis of censored data: survival trees. New York, Springer, pp 93-104 1999
28. Ellis G, Whitehead MA, O'Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011(7):CD006211
29. Kristjansson SR, Nesbakken A, Jordhøy MS. et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76(3):208-217
30. Hamaker ME, Vos AG, Smorenburg CH, de Rooij SE, van Munster BC. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist. 2012;17(11):1439-1449
31. Ommundsen N, Wyller TB, Nesbakken A. et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. 2014;19(12):1268-1275
32. Oken MM, Creech RH, Tormey DC. et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655
33. Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 2002(32A):1135-1148
34. Owens WD, Felts JA, Spitznagel EL Jr. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239-243
35. Young J, Badgery-Parker T, Dobbins T. et al. Comparison of ECOG/WHO Performance Status and ASA Score as a Measure of Functional Status. J Pain Symptom Manage. 2015;49(2):258-264
36. Newschaffer CJ, Bush TL, Penberthy LE. et al. Does comorbid disease interact with cancer? An epidemiologic analysis of mortality in a cohort of elderly breast cancer patients. J Gerontol A Biol Sci Med Sci. 1998;53:372-378
37. Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582-1587
38. Dias-Santos D, Ferrone CR, Zheng H, Lillemoe KD, Castillo CF. The Charlson age comorbidity index predicts early mortality after surgery for pancreatic cancer. Surgery. 2015 S0039-6060(14)00793-4
39. Mayr R, May M, Martini T. et al. Predictive capacity of four comorbidity indices estimating perioperative mortality after radical cystectomy for urothelial carcinoma of the bladder. BJU Int. 2012;110(6 Pt B):E222-227
40. Gooiker GA, Dekker JW, Bastiaannet E. et al. Risk factors for excess mortality in the first year after curative surgery for colorectal cancer. Ann Surg Oncol. 2012;19(8):2428-2434
41. Dekker JW, Gooiker GA, Bastiaannet E. et al. Cause of death the first year after curative colorectal cancer surgery; a prolonged impact of the surgery in elderl colorectal cancer patients. Eur J Surg Oncol. 2014;40(11):1481-1487
Author contact
![]() Corresponding authors: Yung-Chang Lin, MD and Ta-Sen Yeh, MD, PhD, Department of Medical Oncology, Chang Gung Memorial Hospital, 5 Fu-Hsing Street, Kwei-Shan Shiang, Taoyuan, Taiwan. Tel: 886-3281200 Ext 2517 Fax: 886-3-3285818; E-mail: yclinoforg.tw or tsy471027org.tw
Corresponding authors: Yung-Chang Lin, MD and Ta-Sen Yeh, MD, PhD, Department of Medical Oncology, Chang Gung Memorial Hospital, 5 Fu-Hsing Street, Kwei-Shan Shiang, Taoyuan, Taiwan. Tel: 886-3281200 Ext 2517 Fax: 886-3-3285818; E-mail: yclinoforg.tw or tsy471027org.tw

 Global reach, higher impact
Global reach, higher impact