Impact Factor
ISSN: 1837-9664
J Cancer 2016; 7(6):694-701. doi:10.7150/jca.14185 This issue Cite
Research Paper
Pathologic Diagnosis of Pancreatic Adenocarcinoma in the United States: Its Status and Prognostic Value
1. Department of Medical Oncology, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, 651 Dongfeng Road East, Guangzhou 510060, China.
2. Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD 21231, USA.
3. Department of VIP, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, 651 Dongfeng Road East, Guangzhou 510060, China.
4. Department of Experimental Research, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, 651 Dongfeng Road East, Guangzhou, China.
*Miaozhen Qiu and Huijuan Qiu contributed equally to this paper.
Received 2015-10-20; Accepted 2016-1-22; Published 2016-3-26
Abstract
Purpose: Even with the development of new biopsy methods, diagnosis of pancreatic cancer is sometimes without histological evidence. The aim of our study is to find out the status of pancreatic cancer patients who are diagnosed without pathologic confirm and the prognostic value of pathologic diagnosis.
Methods: We identified 52,759 pancreatic adenocarcinoma patients from the Surveillance, Epidemiology, and End Results (SEER) database. Logistic regression model was used to identify factors relating to no pathologic diagnosis. Multivariable Cox regression model identified potential prognostic factors. All statistical tests were two-sided.
Results: There were 6206 (11.76%) patients without pathologic diagnosis. Older age, reported from nursing/convalescent home/hospice or physician's office/private medical practitioner, early year of diagnosis, larger tumor size, pancreatic head cancer, unmarried patients, uninsured and stage I disease all contributed to no pathologic diagnosis. Median cause specific-survival for patients with and without pathologic diagnosis were 7.72 and 3.52 months, respectively. The HR for pathologic diagnosis was 0.92 (95% CI: 0.89-0.95), P<0.001.
Conclusions: Pathologic diagnosis was an independent prognostic factor for pancreatic adenocarcinoma patients. New diagnostic methods are needed to get the pathologic diagnosis.
Keywords: Pancreatic adenocarcinoma, Pathologic diagnosis, Prognosis, SEER.
Introduction
Pancreatic cancer is the fourth most common cause of cancer related death in the United States (1). Despite progresses in the treatment have been made (2-4), the prognosis for pancreatic cancer patients is still dismal (5-7), possibly because of the increasing incidence rate, prevalence of obesity as well as aging population (8-11). The American Cancer Society estimates that in 2015, there will be 48,960 new cases of pancreatic cancer and 40,560 deaths in the United States (1).
Pathologic diagnosis is the golden standard for cancer diagnosis. The majority of patients with pancreatic cancers have metastatic or locally advanced disease at the time of diagnosis, therefore these patients are no longer candidates for surgical resection (12). Pancreatic biopsy is a common procedure to obtain pathology diagnosis for pancreatic cancer patients. The biopsy can be made using fine needle aspiration (FNA) with either endoscopic ultrasound (EUS) or computed tomographic (CT) guidance (13-15). Potential risks of biopsy include bleeding and infection (12, 14, 16). In some cases when the FNA biopsy cannot be obtained, other acceptable methods of biopsy exist, such as intra-ductal biopsy via endoscopic cholangioscopy, percutaneous approach or laparoscopic biopsy (17, 18). However, since pancreas is a retroperitoneal organ, not all the patients can undertake the biopsy. Even for those who receive the biopsy, there is risk of false negative (12, 14, 15, 17, 19-21). For all kinds of reasons, there are some patients without pathologic diagnosis but the radiography examination or tumor markers such as CA 19-9 indicate the presence of pancreatic cancer (22).
How many pancreatic adenocarcinoma patients are diagnosed without microscopically confirm? What factors are related to no pathologic diagnosis? Does it affect the prognosis of pancreatic cancer patients? Till now there is no report on it.
Materials and Methods
Database
The Surveillance, Epidemiology, and End Results (SEER) database is a population-based cancer registry across several disparate geographic regions. The exact dataset we used for analysis was “Incidence-SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2014 Sub (1973-2012 varying)”.
Outcome variables
Variable definitions information on age at diagnosis, sex, year of diagnosis, race/ethnicity, marital status, primary site, tumor grade and differentiation, American Joint Committee on Cancer (AJCC) 6th Tumor-Node-Metastasis (TNM) stage, methods of diagnosis confirmation, insurance status and overall survival were coded and available in SEER database.
Under the item of “Diagnostic confirmation”, there were three subgroups: microscopically confirmed (including code 1: positive histology, code 2: positive cytology and code 4: positive microscopic confirmation, method not specified), not microscopically confirmed (including code 5: positive laboratory test/marker study, code 6: direct visualization without microscopic confirmation, code 7: radiology and other imaging techniques without microscopic confirmation and code 8: clinical diagnosis only other than situation in code 5-7) and confirmation unknown (code 9: unknown whether or not microscopically confirmed, death certificate only). This data item recorded the best method used to confirm the presence of pancreatic cancer. The codes were in priority order; code 1 had the highest priority. Always code the procedure with the lower numeric value when presence of cancer was confirmed with multiple diagnostic methods. If at any time during the course of disease the patient had a diagnostic confirmation with a higher priority, the code would be changed to a lower code.
For the Race/Ethnicity, we reclassified the patients into 5 groups: “Caucasian” (code 1), “African American” (code 2), “Asian” (code 4-6, 8, 10-17 and 96), “Others” (The rest code, except for the code for unknown) and “Unknown” (code, 99).
Patients were classified as married and unmarried. Unmarried patients included single (“Single” and “Unmarried or Domestic Partner”), separated/divorced (“Separated” and “Divorced”) and widowed.
The primary site was defined by the following International Classification of Diseases for Oncology (ICD-O-2) codes: C25.0-C25.9. Head of pancreas, (C25.0), body of pancreas (C25.1), tail of pancreas (C25.2), pancreatic duct (C25.3) and others including islets of Langerhans (C25.4), other specified parts of pancreas (C25.7), overlapping lesion of pancreas (C25.8) and pancreas, NOS (C25.9).
Grade and differentiated was defined by the following ICD-O-2 codes; well differentiated (Code 1), moderate differentiated (Code 2), poorly differentiated (Code 3), undifferentiated (Code 4) and unknown (Code 9).
Since the AJCC 7th TNM staging system was released in 2010 and if we used this staging system, there would be no 5 year survival due to insufficient follow up, so we picked up the AJCC 6th TNM staging systems. Meanwhile, since the AJCC 6th TNM staging system was released in 2004, we restricted our study from 2004-2012 and we furthered divided the years of diagnosis into two groups: 2004-2007 and 2008-2012.
Tumor size (≤2 cm, 2-4 cm, 4+ cm) was determined based on Collaborative Stage.
For the insurance status, individuals in the “Any Medicaid”, “Insured” and “Insured/No specifics” groups were clustered together as “Insured group”. Patients were therefore divided into “insured group” and “uninsured group”. While patients whose insurance status unknown and blanks were classified as “Unknown SEER variables, RX Summ-radiation and RX summ-surg prim site and Radiation sequence with surgery were used to define treatment types: “Both” for patients who had both surgery and radiation no matter what the sequence was; “Surgery” for patients who only had surgery, “Radiation” for patients who only had radiation, “None” for patients who did not have surgery nor radiation therapy, and “Unknown”.
Patient Population
The study population was based on the SEER cancer registry. We restricted eligibility to adults (aged 18 years or older) who were diagnosed with pancreatic adenocarcinoma (ICD-O-3 histology codes: 8000, 8010, 8020-8022, 8140, 8141, 8211, 8230, 8500, 8521, 8050, 8260, 8441, 8450, 8453, 8470-8473, 8480, 8481, 8503) from 2004 to 2012. We excluded cases without follow-up records, as well as lacking documentation on diagnostic confirmation. Patients with multiple tumors while pancreatic cancer was not the first tumor were also excluded.
Statistical Methods
The patients' demographic and tumor characteristics were summarized with descriptive statistics. Comparisons of categorical variables among different groups of patients were performed using the Chi square test, and continuous variables were compared using Student's t test. The primary endpoint of this study was cause specific-survival (CSS), which was calculated from the date of diagnosis to the date of cancer specific death. Deaths attributed to pancreatic cancer were treated as events and deaths from other causes were considered as censored observations. Survival function estimation and comparison among different variables were performed using Kaplan-Meier estimates and the log-rank test. The multivariate Cox proportional hazard model was used to evaluate the hazard ratio (HR) and the 95 % confidence interval (CI) for all the known prognostic factors. We used log-rank test to analyze the potential relating factors to no microscopic confirmation. All of statistical analyses were performed using the Intercooled Stata 13.0 (Stata Corporation, College Station, TX). Statistical significance was set at two-sided P < 0.05.
Ethnic issues
This study was deemed exempt from institutional review board approval by Sun Yat-sen University Cancer Center; informed consent was waived.
Results
Patient baseline characteristics
The study identified 52,759 pancreatic adenocarcinoma patients (Table 1). Of these patients, 26,267 (49.79%) were male and 26,492 (50.21%) were female. Only 18,278 patients had the record of histologic grade including 2,262 (12.38%) well differentiated, 8,115 (44.40%) moderate differentiated, 7,502 (41.04%) poorly differentiated and 399 (2.18%) undifferentiated. Totally 6,206 of the patients (11.76%) didn't have pathologic diagnosis, including 4,476 (8.48%) radiography diagnosis, 1,113 (2.11%) clinical diagnosis, 368 (0.70%) direct visualization diagnosis and 249 (0.47%) laboratory test/marker diagnosis.
Comparison of basic features for pancreatic adenocarcinoma patients with different diagnosis.
| Pathologic diagnosis N(%) | No pathologic diagnosis N(%) | P | ||||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | Group E | Group F | Group G | ||
| Sex | ||||||||
| Male | 17,727 (67.49) | 5,996 (22.83) | 26 (0.10) | 98 (0.37) | 163 (0.62) | 1,822 (6.94) | 435 (1.66) | <0.001 |
| Female | 16,950 (63.98) | 5,828 (22.00) | 26 (0.10) | 151 (0.57) | 205 (0.77) | 2,654 (10.02) | 678 (2.56) | |
| Age | ||||||||
| Mean±SD | 66.19±11.67 | 68.52±11.82 | 69.75±13.26 | 77.52±11.88 | 75.71±12.30 | 76.85±12.23 | 77.61±11.83 | <0.001 |
| Year of diagnosis | ||||||||
| 2004-2007 | 14,282 (65.08) | 4,660 (21.23) | 30 (0.14) | 128 (0.58) | 244 (1.11) | 2,099 (9.56) | 502 (2.29) | <0.001 |
| 2008-2012 | 20,395 (66.19) | 7,164 (23.25) | 22 (0.07) | 121 (0.39) | 124 (0.40) | 2,377 (7.71) | 611 (1.98) | |
| Race/Ethnicity | ||||||||
| Caucasian | 27,506 (65.53) | 9,489 (22.61) | 43 (0.10) | 188 (0.45) | 287 (0.68) | 3,588 (8.55) | 875 (2.08) | <0.001 |
| African-American | 4,336 (67.37) | 1,341 (20.84) | 8 (0.12) | 23 (0.36) | 41 (0.64) | 542 (8.42) | 145 (2.25) | |
| Asian | 2,324 (64.74) | 850 (23.68) | 1 (0.03) | 34 (0.95) | 32 (0.89) | 278 (7.74) | 71 (1.98) | |
| Marital status | ||||||||
| Married | 20,059 (69.32) | 6,515 (22.51) | 24 (0.08) | 89 (0.31) | 149 (0.51) | 1,720 (5.94) | 382 (1.32) | <0.001 |
| Separated /divorced | 3,785 (66.38) | 1,293 (22.68) | 3 (0.05) | 24 (0.42) | 37 (0.65) | 442 (7.75) | 118 (2.07) | |
| Single | 43,98 (66.45) | 1,463 (22.10) | 5 (0.08) | 32 (0.48) | 43 (0.65) | 544 (8.22) | 134 (2.02) | |
| Widowed | 5,105 (53.87) | 2,115 (22.32) | 15 (0.16) | 95 (1.00) | 129 (1.36) | 1,592 (16.80) | 426 (4.50) | |
| Insurance | ||||||||
| Insured | 22,627 (66.45) | 7,949 (23.34) | 20 (0.06) | 139 (0.41) | 155 (0.46) | 2,525 (7.42) | 637 (1.87) | <0.001 |
| Uninsured | 792 (68.04) | 272 (23.37) | 1 (0.09) | 4 (0.34) | 4 (0.34) | 79 (6.79) | 12 (1.03) | |
| Site | ||||||||
| Head | 17,970 (64.68) | 6,595 (23.74) | 15 (0.05) | 114 (0.41) | 255 (0.92) | 2,302 (8.29) | 533 (1.92) | <0.001 |
| Body | 3,763 (62.43) | 1,731 (28.72) | 5 (0.08) | 16 (0.27) | 11 (0.18) | 449 (7.45) | 53 (0.88) | |
| Tail | 4,431 (74.43) | 1,013 (17.02) | 5 (0.08) | 17 (0.29) | 6 (0.10) | 439 (7.37) | 42 (0.71) | |
| Duct | 253 (77.61) | 50 (15.34) | 0 (0) | 2 (0.61) | 4 (1.23) | 14 (4.29) | 3 (0.92) | |
| Tumor size (mm) | ||||||||
| Mean ±sd | 40.72±28.92 | 41.27±31.48 | 41.83±18.40 | 37.29±20.64 | 36.58±16.04 | 41.96±28.40 | 40.39±51.18 | <0.001 |
| TNM stage | ||||||||
| I | 1,991 (55.52) | 897 (25.01) | 4 (0.11) | 26 (0.73) | 41 (1.14) | 497 (13.86) | 130 (3.63) | <0.001 |
| II | 10,136 (74.31) | 2,784 (20.41) | 5 (0.04) | 38 (0.28) | 94 (0.69) | 467 (3.42) | 116 (0.85) | |
| III | 2,883 (57.44) | 1,730 (34.47) | 5 (0.10) | 17 (0.34) | 39 (0.78) | 293 (5.84) | 52 (1.04) | |
| IV | 17,151 (70.14) | 4,975 (20.35) | 18 (0.07) | 79 (0.32) | 47 (0.19) | 1,966 (8.04) | 215 (0.88) | |
| Treatment | ||||||||
| Surgery and radiotherapy | 4,067 (94.08) | 237 (5.48) | 0 (0) | 1 (0.02) | 5 (0.12) | 9 (0.21) | 4 (0.09) | <0.001 |
| Surgery | 6,203 (96.70) | 204 (3.18) | 2 (0.03) | 0 (0) | 2 (0.03) | 1 (0.02) | 3 (0.05) | |
| Radiotherapy | 3,242 (56.48) | 2,154 (37.53) | 5 (0.09) | 20 (0.35) | 34 (0.59) | 231 (4.02) | 54 (0.94) | |
| None | 21,096 (58.42) | 9,205 (25.49) | 45 (0.12) | 228 (0.63) | 326 (0.90) | 4,174 (11.56) | 1,036 (2.87) | |
| Source of report | ||||||||
| Hospital inpatient /outpatient or clinic | 33,683 (66.46) | 11,523 (22.74) | 46 (0.09) | 229 (0.45) | 347 (0.68) | 3,935 (7.76) | 919 (1.81) | <0.001 |
| Laboratory only | 147 (77.37) | 35 (18.42) | 0 (0) | 1 (0.53) | 0 (0) | 4 (2.11) | 3 (1.58) | |
| Nursing /convalescent home/hospice | 35 (37.23) | 5 (5.32) | 1 (1.06) | 0 (0) | 1 (1.06) | 33 (35.11) | 19 (20.21) | |
| Other hospital outpatient unit or surgery center | 211 (54.95) | 131 (34.11) | 0 (0) | 1 (0.26) | 3 (0.78) | 30 (7.81) | 8 (2.08) | |
| Physician's office /private medical practitioner | 447 (37.53) | 93 (7.81) | 5 (0.42) | 18 (1.51) | 15 (1.26) | 451 (37.87) | 162 (13.60) | |
| Radiation treatment or medical oncology center | 154 (70.64) | 37 (16.97) | 0 (0) | 0 (0) | 2 (0.92) | 23 (10.55) | 2 (0.92) | |
Group A: Positive histology; Group B: Positive exfoliative cytology; Group C: Positive microscopic confirmation, method not specified; Group D: Positive laboratory test/marker study; Group E: Direct visualization without microscopic confirmation; Group F: Radiography without microscopic confirm; Group G: Clinical diagnosis only.
The median age of the entire cohort patients was 69 years old. Both the mean and median age of patients in the pathologic diagnosis group were significantly younger than those in no pathologic diagnosis group. More male patients had pathologic diagnosis than female (90.41% vs 86.08%). Married patients had the highest percentage of pathologic diagnosis, while widowed patients had the lowest (91.91% vs. 76.34%, P<0.001). The proportion of patients with pathologic diagnosis increased from 85.48% in 2004 to 91.25% in 2012. The pathologic diagnosis rate of tumors varied in different locations, 92.94% in pancreatic duct, 91.53% in tail, 91.22% in body, 88.47% in head and 84.64% in other locations.
The mean tumor size was slightly larger in the no pathologic diagnosis group than in the pathologic diagnosis group, 41.56 mm vs 40.89 mm. The percentage of patients with pathologic diagnosis from stage I to IV and stage unknown diseases was 80.65%, 94.76%, 92.01%, 90.56% and 65.55%, respectively. Over 99% of the patients who received treatments had pathologic diagnosis. The percentage of patients with pathologic diagnosis and received radiation was 94.09%. For patients without pathologic diagnosis, 339 (5.46%) received radiation only, 19 (0.31%) received both radiation and surgery, 6 (0.10%) received surgery only and 5,764 (92.88%) received no therapy. Patients who were reported from nursing/convalescent home/hospice or physician's office/private medical practitioner had very low percentage of pathologic diagnosis, only 43.62% and 45.76% respectively, compared with 89.29% from the hospital inpatient/outpatient or clinic.
Factors related to no pathologic diagnosis
We found that gender, age, location, time of diagnosis, TNM stage, insurance status, marital status, and tumor size as well as the reporting system all contributed to the pathologic diagnosis using the log-rank test (Table 2). Age was the most important factor. Older patients were less likely to have pathologic diagnosis. Stage I patients had high percentage of no pathologic diagnosis. Further analysis showed that the median age of patients in stage I was 73 years old, older than those in stage II to IV (68 in stage II, 66 in stage III and 67 in stage IV).
Factors related to no pathologic diagnosis.
| Odd ratio | P | 95% CI | |
|---|---|---|---|
| Gender | 0.93 | 0.016 | 0.87-0.99 |
| Age | 0.3 | <0.001 | 0.29-0.32 |
| Year of diagnosis | 1.11 | 0.013 | 1.02-1.21 |
| Ethnicity | 0.98 | 0.25 | 0.94-1.02 |
| Insurance | 0.93 | <0.001 | 0.89-0.97 |
| Marital status | 0.82 | <0.001 | 0.80-0.84 |
| Location | 1.04 | <0.001 | 1.02-1.06 |
| Size | 0.89 | <0.001 | 0.86-0.92 |
| TNM | 0.77 | <0.001 | 0.75-0.79 |
| Report system | 0.73 | <0.001 | 0.71-0.75 |
Abbreviations: CI: Confidence Interval; TNM: Tumor-Node-Metastasis.
Survival
In this study, 42,888 deaths (81.29 %) were observed including 37,702 (80.99%) in the pathologic diagnosis group and 5,186 (83.56%) in the no pathologic diagnosis group. The median CSS for the whole population was 6.65 months. The 1,2,3,4 and 5 year CSS was 30.8%, 14.1%, 8.9%, 7.0% and 6.0% respectively. Since most of the events happened in the first two years, in Table 3 we listed the median CSS, 1-year and 2-year CSS for patients with different clinicopathologic factors. The median CSS in patients with and without pathologic diagnosis was 7.36 months and 3.73 months (Figure 1), P<0.001. The survival of patients in these two groups crossed over after 40 months. The 1,2,3,4 and 5 year CSS was 33.1%, 15.0%, 9.3%, 7.1% and 6.0% for patients with pathologic diagnosis and 19.2%, 10.3%, 8.2%, 7.6% and 7.1% for those without pathologic diagnosis.
Multivariate analysis
Variables showing a trend for association with survival (P < 0.05) were selected in the cox proportional hazards model. Type of reporting source was not independent prognostic factor. Age, year of diagnosis, marital status, insurance status, tumor size, location, TNM stage, histologic grade, methods for diagnostic confirmation and therapy were all independent prognostic factors in the multivariable analysis (Table 4). The HR for pathologic diagnosis was 0.92 (95% CI: 0.89-0.95), P<0.001.
Survival difference between patients with and without pathologic diagnosis.
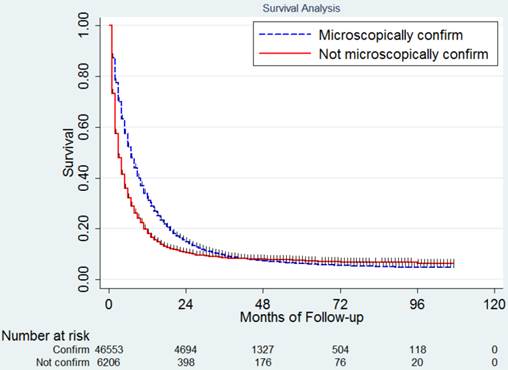
Survival analysis.
| Median CSS (Months) | 1-year CSS (95% CI) | 2-year CSS (95% CI) | P | |
|---|---|---|---|---|
| Sex | ||||
| Male | 6.88 | 31.5 (30.9-32.1) | 14.4 (13.9-14.9) | 0.9333 |
| Female | 6.81 | 31.5 (30.9-32.1) | 14.4 (14.0-14.9) | |
| Age | ||||
| 18-69 | 8.25 | 36.4 (35.8-37.0) | 17.1 (16.6-17.6) | <0.001 |
| >69 | 5.43 | 25.7 (25.1-26.2) | 11.2 (10.8-11.7) | |
| Year of diagnosis | ||||
| 2004-2007 | 6.4 | 29.1 (28.5-29.7) | 12.9 (12.4-13.4) | <0.001 |
| 2008-2012 | 7.24 | 33.3 (32.7-33.9) | 15.7 (15.2-16.2) | |
| Race/Ethnicity | ||||
| Caucasian | 6.71 | 30.9 (30.5-31.4) | 14.0 (13.7-14.4) | <0.001 |
| African-American | 6.25 | 28.9 (27.7-30.0) | 13.5 (12.6-14.4) | |
| Asian | 6.6 | 31.4 (29.8-33.0) | 15.5 (14.2-16.9) | |
| Insurance | ||||
| Insured | 7.27 | 33.2 (32.7-33.8) | 15.5 (15.1-16.0) | <0.001 |
| Uninsured | 5.81 | 30.4 (27.6-33.4) | 16.3 (13.8-18.9) | |
| Marital status | ||||
| Married | 7.78 | 34.6 (34.0-35.2) | 15.9 (15.4-16.4) | <0.001 |
| Widowed | 4.97 | 23.6 (22.7-24.5) | 10.2 (9.5-10.9) | |
| Single | 6.36 | 30.3 (29.1-31.4) | 14.8 (13.8-15.8) | |
| Divorced/separated | 6.48 | 30.0 (28.7-31.2) | 13.5 (12.5-14.5) | |
| Diagnostic confirmation | ||||
| Positive histology | 7.72 | 35.2 (34.6-35.7) | 16.9 (16.5-17.3) | <0.001 |
| Exfoliative cytology | 6.53 | 27.0 (26.2-27.9) | 9.1 (8.5-9.7) | |
| Method not specified | 5.7 | 22.0 (11.1-35.2) | 12.6 (4.4-25.3) | |
| Laboratory test/marker | 4.01 | 15.5 (11.2-20.5) | 7.2 (4.3-11.1) | |
| Direct visualization | 5.51 | 26.9 (22.3-31.7) | 12.8 (9.4-16.8) | |
| Radiography | 3.54 | 18.1 (16.9-19.3) | 9.5 (8.6-10.5) | |
| Clinical diagnosis | 3.92 | 22.0 (19.5-24.6) | 13.0 (10.9-15.3) | |
| Site | ||||
| Head of pancreas | 8.29 | 37.2 (36.6-37.8) | 17.4 (16.9-17.9) | <0.001 |
| Body of pancreas | 6.55 | 27.7 (26.5-28.9) | 11.3 (10.4-12.2) | |
| Tail of pancreas | 5.41 | 25.9 (24.7-27.1) | 13.0 (12.1-14.0) | |
| Pancreatic duct | 11.63 | 48.3 (42.5-53.8) | 25.1 (20.1-30.4) | |
| Tumor size | ||||
| ≤2cm | 11.66 | 49.0 (47.4-50.6) | 29.1 (27.6-30.7) | <0.001 |
| 2-4cm | 8.62 | 37.9 (37.2-38.6) | 17.5 (16.9-18.1) | |
| ≥4cm | 6.13 | 27.3 (26.5-28.0) | 11.5 (11.0-12.1) | |
| Grade | ||||
| Well differentiated | 15.75 | 59.2 (57.0-61.2) | 36.5 (34.3-38.7) | <0.001 |
| Moderately differentiated | 13.04 | 53.1 (51.9-54.2) | 28.5 (27.4-29.6) | |
| Poorly differentiated | 7.87 | 35.3 (34.1-36.4) | 16.2 (15.3-17.2) | |
| Undifferentiated | 6.11 | 28.8 (24.3-33.5) | 13.0 (9.6-16.9) | |
| TNM Stage | ||||
| I | 12.99 | 52.4 (50.7-54.2) | 35.7 (33.9-37.4) | <0.001 |
| II | 13.02 | 53.4 (52.5-54.2) | 27.7 (26.8-28.5) | |
| III | 9.24 | 38.0 (36.5-39.4) | 12.3 (11.3-13.3) | |
| IV | 4.31 | 15.8 (15.3-16.3) | 4.5 (4.2-4.8) | |
| Therapy | ||||
| Both | 20.08 | 74.4 (73.0-75.7) | 42.0 (40.4-43.6) | <0.001 |
| Radiation | 21.84 | 78.9 (77.5-80.2) | 46.1 (44.3-47.8) | |
| Surgery | 18.69 | 65.3 (64.0-66.5) | 41.7 (40.3-43.1) | |
| None | 4.7 | 19.0 (18.5-19.4) | 6.5 (6.2-6.8) | |
| Source of report | ||||
| Hospital or clinic | 6.94 | 31.9 (31.4-32.3) | 14.7 (14.3-15.0) | <0.001 |
| Laboratory only | 5.57 | 23.7 (17.6-30.4) | 11.3 (6.7-17.2) | |
| Nursing/convalescent home/hospice | 3.23 | 10.5 (7.3-14.3) | 2.5 (1.1-4.9) | |
| Other hospital center | 7.2 | 33.1 (28.2-38.0) | 15.4 (11.5-19.8) | |
| Private practitioner | 3.27 | 10.3 (9.1-11.6) | 2.6 (2.0-3.3) | |
| Radiation or medical oncology center | 10.06 | 44.3 (37.4-51.0) | 24.4 (18.2-31.1) | |
Abbreviations: CI: confidence interval; CSS: Cause specific survival; TNM: Tumor-Node-Metastasis.
Multivariable analysis for survival in the whole population.
| Hazard ratio | 95% CI | P | |
|---|---|---|---|
| Age | 1.21 | 1.19-1.24 | <0.001 |
| Year of diagnosis | 0.95 | 0.93-0.98 | 0.001 |
| Race/Ethnicity | 0.98 | 0.97-0.99 | 0.016 |
| Insurance | 1.02 | 1.01-1.04 | 0.003 |
| Marital status | 1.02 | 1.01-1.03 | <0.001 |
| Diagnostic confirmation | 0.92 | 0.89-0.95 | <0.001 |
| Grade | 1.03 | 1.02-1.04 | <0.001 |
| Tumor size | 1.04 | 1.03-1.05 | <0.001 |
| TNM Stage | 1.12 | 1.11-1.13 | <0.001 |
| Site | 1.01 | 1.00-1.02 | 0.001 |
| Therapy | 0.68 | 0.67-0.69 | <0.001 |
| Source of report | 1 | 0.99-1.02 | 0.79 |
Abbreviations: CI: confidence interval; TNM: Tumor-Node-Metastasis.
Discussion
This is the first study to demonstrate the status of pathologic diagnosis for pancreatic adenocarcinoma patients. Amazingly, we found 11.76% of patients were diagnosed as pancreatic cancer without pathologic confirm in the SEER database. Factors related to no pathologic diagnosis including older age, reported from nursing/convalescent home/hospice or physician's office/private medical practitioner, early year of diagnosis, larger tumor size, locating in pancreatic head, unmarried patients, uninsured and stage I disease. Age was the most important factor contributing to no pathologic diagnosis. The peak incidence of pancreatic cancer occurs in the seventh and eighth decades of life (1). The median age was 80 for those without pathologic diagnosis and 67 for those with. The median age for patients in the previous reports about biopsy was about 53-61 years old (13, 14). Elderly patients were at higher multi-morbidity risks from many aging-related diseases (23) and may therefore could not bear biopsy or surgery. New diagnosis techniques for elderly patients are wanted.
Interestingly, stage I patients had high percentage of no pathologic diagnosis. Further analysis showed that the median age of patients in stage I was older than those in stage II to IV. So age may still be the main reason leading to no pathologic diagnosis in stage I patients.
Unmarried patients had a higher rate of no pathologic diagnosis. Previous studies showed that spouse might provide social support and encourage the patients to seek medical treatment (24, 25). Similarly, the insurance status would also affect the diagnosis and treatment of pancreatic cancer (26, 27).
Compared with tumors in other sites of pancreas, tumors in pancreatic head were harder to get histological evidence. Tumors in pancreatic head was easily misdiagnosed and usually found to be advanced when tumor size was too large. It was therefore not feasible for surgical resection or biopsy (28). Another probable reason was that tumors in pancreatic head might cause obstruction and then induced progressive jaundice (22, 29). The poor performance status might prevent patients from biopsy.
Good news is that with the time changes, the rate of no pathologic diagnosis decreased gradually, from 14.52% in 2004 to 8.75% in 2012. The improvement of pathologic diagnosis with the time changed might be largely due to the technique of EUS guided FNA which could safely and accurately establish a cytological diagnosis in patients with both early-stage and advanced pancreatic cancer (12, 13, 30). This indicated the importance of diagnostic methods. To increase the pathologic diagnosis rate, more safe and effective diagnostic methods are needed.
The median survival was significantly higher for patients with pathologic diagnosis than those without, however, the survival curves crossed over after 40 months. The purpose of getting pathologic diagnosis was to guide treatment. Although a pathologic diagnosis was not required before surgery, it was basically necessary before administration of neoadjuvant therapy to locally advanced, unresectable or metastatic diseases. In our data, we found that 65.19% of patients with pathologic diagnosis did not receive surgery nor radiation, while, 92.88% of patients without pathologic diagnosis did not receive these two treatments. Since there was no information of chemotherapy in the SEER database, we could not calculate the number of patients who received chemotherapy. However, the effect of treatment might mainly improve early results but make little change to long term survival. Pancreatic cancer patients might still suffer from recurrence or metastasis even after radical resection or radiation (31, 32).
We found that about 50% of the patients without pathologic diagnosis had unknown stage while only 8.83% patients with pathologic diagnosis had unknown stage. For patients without pathologic diagnosis, there was always a concern whether they were malignant diseases, even if they were, what their histologic subtypes were. Chronic pancreatitis, other benign conditions and malignant diseases were all possible differential diagnosis for patients suspected of having pancreatic adenocarcinoma cancer (33-35). These diseases had a better prognosis than pancreatic adenocarcinoma (18, 36-38). Speer AG et al. retrospectively analyzed the long term survival of pancreatic cancer patients and found that among 763 patients, 20 patients survived to 5 years. Moreover, they found that of the 20 patients, 10 did not have histological confirmation of carcinoma and were presumably false-positive diagnoses (39). Sinn M et al. analyzed patients with long term survival from the CONKO-001 study. They found 39 (11.0%) patients with an overall survival ≥5 years and available tumor specimens. Histological re-evaluation confirmed adenocarcinoma in 38 patients and 1 patient turned out to be high-grade neuroendocrine tumor (38). Long-term survival could be achieved in pancreatic adenocarcinoma patients but there was risk of misdiagnosis. Especially for those without pathologic confirm, the risk of false-positive diagnoses could not be excluded.
Of note, the little effect of therapy on long term survival and risk of misdiagnosis for those without pathologic diagnosis might be responsible for the crossover of survival curve after 40 months.
In the multivariable analysis, both age and pathologic confirm diagnosis were independent prognostic factors for pancreatic adenocarcinoma patients. While age is the most important factor that leads to the no pathologic diagnosis, we therefore urge the new techniques to improve the pathologic diagnosis for elderly patients. By this, we can improve the prognosis of pancreatic adenocarcinoma patients.
Potential limitations of our study should be taken into consideration. Firstly, data related to chemotherapy were not available in SEER database. Secondly, not all the patients enrolled for analysis had the full information of all the clinical features. For example the information of insurance was available after 2007. Finally, the genetic difference among patients were also out of our reach. Patients with a long term survival might have a relatively indolent biology behavior compared with those with short term survival.
In conclusion, using the SEER database, we revealed that about 11% of the pancreatic cancer patients had no pathologic diagnosis. The most important factor contributing to no pathologic diagnosis was age. The multivariate analysis showed that pathologic confirm was an independent prognostic factor for pancreatic adenocarcinoma patients. We therefore encourage patients who are suspected the diagnosis of pancreatic cancer to get a pathologic evidence. New techniques for biopsy are needed, especially for elderly patients.
Acknowledgements
This work was supported by the third outstanding young talents training plan of Sun Yat-sen University cancer center, Medical Scientific Research of Guangdong province (No. B201416), Scientific and Technological projects Guangdong Esophageal Cancer Institute (No. Q201408), Science and Technology Planning Project of Guangdong Province (No. 2013A022100023), National High Technology Research and Development Program of China (863 Program), China (No. 2015AA020103), National Natural Science Foundation of China (No.81372570, 81572392), Natural Science Foundation of Guangdong Province (No.2014A030312015), Science and Technology Program of Guangzhou (No. 15570006, 158100066).
We would like to thank the staff members of the National Cancer Institute and their colleagues across the United States and at Information Management Services, Inc., who have been involved with the Surveillance, Epidemiology and End Results (SEER) Program.
Conflict of Interest
All authors declared that there is no conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29
2. Faris JE, Blaszkowsky LS, McDermott S. et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18:543-8
3. Howard TJ, Krug JE, Yu J. et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338-45 discussion 45-6
4. Sohn TA, Yeo CJ, Cameron JL. et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-79
5. StatBite. U.S. pancreatic cancer rates. J Natl Cancer Inst. 2010;102:1822
6. Worni M, Guller U, White RR. et al. Modest improvement in overall survival for patients with metastatic pancreatic cancer: a trend analysis using the surveillance, epidemiology, and end results registry from 1988 to 2008. Pancreas. 2013;42:1157-63
7. Chen W, Zheng R, Zeng H, Zhang S. The updated incidences and mortalities of major cancers in China, 2011. Chin J Cancer. 2015;34:53
8. Arnold LD, Patel AV, Yan Y. et al. Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity? Cancer Epidemiol Biomarkers Prev. 2009;18:2397-405
9. Eheman C, Henley SJ, Ballard-Barbash R. et al. Annual Report to the Nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338-66
10. Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62:118-28
11. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758-65
12. Matsubara J, Okusaka T, Morizane C, Ikeda M, Ueno H. Ultrasound-guided percutaneous pancreatic tumor biopsy in pancreatic cancer: a comparison with metastatic liver tumor biopsy, including sensitivity, specificity, and complications. J Gastroenterol. 2008;43:225-32
13. Micames C, Jowell PS, White R. et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-5
14. Okasha HH, Naga MI, Esmat S. et al. Endoscopic Ultrasound-Guided Fine Needle Aspiration versus Percutaneous Ultrasound-Guided Fine Needle Aspiration in Diagnosis of Focal Pancreatic Masses. Endosc Ultrasound. 2013;2:190-3
15. Brugge WR, De Witt J, Klapman JB. et al. Techniques for cytologic sampling of pancreatic and bile duct lesions: The Papanicolaou Society of Cytopathology Guidelines. Cytojournal. 2014;11:2
16. Layfield LJ, Dodd L, Factor R, Schmidt RL. Malignancy risk associated with diagnostic categories defined by the Papanicolaou Society of Cytopathology pancreaticobiliary guidelines. Cancer Cytopathol. 2014;122:420-7
17. Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc. 2007;65:832-41
18. Strasberg SM, Middleton WD, Teefey SA, McNevin MS, Drebin JA. Management of diagnostic dilemmas of the pancreas by ultrasonographically guided laparoscopic biopsy. Surgery. 1999;126:736-41 discussion 41-3
19. Adsay V, Mino-Kenudson M, Furukawa T. et al. Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Ann Surg. 2015
20. Zalatnai A. [Pathologic diagnosis of pancreatic cancer-facts, pitfalls, challenges]. Orv Hetil. 2001;142:1885-90
21. Zhu HM, Li YX, Wang LS. et al. [Value of peritoneoscopy via natural orifice transluminal endoscopic surgery in the diagnosis of peritoneal carcinomatosis]. Zhonghua Yi Xue Za Zhi. 2011;91:1895-8
22. Kimura H, Matsubayashi H, Sasaki K. et al. Factors affecting the yield of endoscopic transpapillary bile duct biopsy for the diagnosis of pancreatic head cancer. Pancreatology. 2013;13:524-9
23. Akushevich I, Kravchenko J, Ukraintseva S, Arbeev K, Kulminski A, Yashin AI. Morbidity risks among older adults with pre-existing age-related diseases. Exp Gerontol. 2013;48:1395-401
24. Aizer AA, Paly JJ, Zietman AL. et al. Multidisciplinary care and pursuit of active surveillance in low-risk prostate cancer. J Clin Oncol. 2012;30:3071-6
25. Cohen SD, Sharma T, Acquaviva K, Peterson RA, Patel SS, Kimmel PL. Social support and chronic kidney disease: an update. Adv Chronic Kidney Dis. 2007;14:335-44
26. DaCosta Byfield S, Nash Smyth E, Mytelka D, Bowman L, Teitelbaum A. Healthcare costs, treatment patterns, and resource utilization among pancreatic cancer patients in a managed care population. J Med Econ. 2013;16:1379-86
27. Standop J, Kuhn Y, Glowka TR. et al. Association of socio-economic status and stage of pancreatic cancer at time of surgery in a German setting. Hepatogastroenterology. 2012;59:2614-7
28. Hua YP, Liang LJ, Peng BG, Li SQ, Huang JF. Pancreatic head carcinoma: clinical analysis of 189 cases. Hepatobiliary Pancreat Dis Int. 2009;8:79-84
29. Bestari MB, Ang TL, Abdurachman SA. Endoscopic ultrasound in the diagnosis of occult pancreatic head cancer. Acta Med Indones. 2009;41:144-7
30. Raut CP, Grau AM, Staerkel GA. et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg. 2003;7:118-26 discussion 27-8
31. Motoi F, Rikiyama T, Katayose Y, Egawa S, Unno M. Retrospective evaluation of the influence of postoperative tumor marker status on survival and patterns of recurrence after surgery for pancreatic cancer based on RECIST guidelines. Ann Surg Oncol. 2011;18:371-9
32. Strasberg SM, Linehan DC, Hawkins WG. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. J Am Coll Surg. 2007;204:244-9
33. Menges M, Lerch MM, Zeitz M. The double duct sign in patients with malignant and benign pancreatic lesions. Gastrointest Endosc. 2000;52:74-7
34. Kalady MF, Peterson B, Baillie J. et al. Pancreatic duct strictures: identifying risk of malignancy. Ann Surg Oncol. 2004;11:581-8
35. Kajiwara M, Kojima M, Konishi M. et al. Autoimmune pancreatitis with multifocal lesions. J Hepatobiliary Pancreat Surg. 2008;15:449-52
36. NIH state-of-the-science statement on endoscopic retrograde cholangiopancreatography (ERCP) for diagnosis and therapy. NIH Consens State Sci Statements. 2002;19:1-26
37. Buechter M, Klein CG, Kloeters C, Gerken G, Canbay A, Kahraman A. Diagnostic Dilemma in a Patient with Jaundice: How to Differentiate between Autoimmune Pancreatitis, Primary Sclerosing Cholangitis and Pancreas Carcinoma. Case Rep Gastroenterol. 2012;6:211-6
38. Sinn M, Striefler JK, Sinn BV. et al. Does long-term survival in patients with pancreatic cancer really exist? Results from the CONKO-001 study. J Surg Oncol. 2013;108:398-402
39. Speer AG, Thursfield VJ, Torn-Broers Y, Jefford M. Pancreatic cancer: surgical management and outcomes after 6 years of follow-up. Med J Aust. 2012;196:511-5
Author contact
![]() Corresponding author: Dajun Yang, M.D. Ph.D., Department of Experimental Research, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China; Phone: +86-20-87342285; Fax: +86-20-87342285; E-mail: yangdjorg.cn or Ruihua Xu, M.D. Ph.D., Department of Medical Oncology, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China. Tel: +86-20-8734 3468 Fax: +86-20-8734 3468 E-mail: xurhorg.cn.
Corresponding author: Dajun Yang, M.D. Ph.D., Department of Experimental Research, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China; Phone: +86-20-87342285; Fax: +86-20-87342285; E-mail: yangdjorg.cn or Ruihua Xu, M.D. Ph.D., Department of Medical Oncology, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China. Tel: +86-20-8734 3468 Fax: +86-20-8734 3468 E-mail: xurhorg.cn.

 Global reach, higher impact
Global reach, higher impact