Impact Factor
ISSN: 1837-9664
J Cancer 2016; 7(9):1066-1073. doi:10.7150/jca.13547 This issue Cite
Research Paper
A Retrospective Comparison of Taxane and Fluorouracil-based Chemoradiotherapy in Patients with Inoperable Esophageal Squamous Cell Carcinoma
1. Department of Radiation Oncology, Zhejiang Cancer Hospital, Hangzhou China;
2. Key Laboratory of Radiation Oncology, Zhejiang Cancer Hospital, Hangzhou 310022, China.
# All authors contributed equally to this work.
Received 2015-8-13; Accepted 2016-3-15; Published 2016-5-25
Abstract
Purpose: To retrospectively compare taxane-based with fluorouracil-based chemoradiotherapy in terms of toxicity profiles, efficacy and survival in patients with inoperable esophageal cancer.
Methods and Materials: We analyzed retrospectively 179 consecutive patients who were unresectable or medically unfit for surgery between March 2009 and November 2014. Eight-three patients were included in the taxane group and 96 cases were in the fluorouracil group.
Results: The overall response rate (ORR) in the taxane group was higher than fluorouracil group, but was not significantly different (71.6% vs. 63.5%, respectively, P=0.255). In total, 53.0% (44/83) of the patients in the taxane group had progressive disease versus 54.2% (52/96) in the fluorouracil group (not significantly different (P=0.758)). There was no significant difference in overall response rate, progression free survival and overall survival, as well as treatment-related death. In terms of non-hematological toxicity, patients in the taxane group experienced a lower incidence of ≥ grade 3 esophageal perforation or fistula (4.8% vs. 13.5%, P=0.047) and pneumonia (4.8% vs. 9.7%, P=0.242). Regarding hematological toxicity, thrombocytopenia in the taxane group was significantly lower (4.8% vs. 13.5%, P=0.047), but there was a trend towards a higher rate of ≥ grade 3 leukopenia (34.9% vs.26.0%, P=0.196).
Conclusions: Chemoradiation with taxane-based regimens is well tolerated, with potentially promising efficacy, and could become a good alternative treatment in a first line setting for patients with inoperable esophageal squamous cell carcinoma.
Keywords: esophageal cancer, chemoradiotherapy, taxane, esophageal squamous cell carcinoma
Introduction
Esophageal cancer is one of most common cancers and ranks as the sixth most common cause of cancer-related mortality1. The 5-year survival rate is poor, approximately 17% in the period 1996-20042. The Dutch CROSS study improved the survival to a 5-yr rate of 44% and proposed neoadjuvant chemoradiation followed by surgery as a standard regimen in patients with resectable locally advanced lesions. In esophageal cancer patients who are unresectable or medically unfit for surgery, such as T4 tumors or M1 lymph-node metastasis, definitive concurrent chemoradiation (dCRT) using cisplatin with 5-fluorouracil (5-FU) is the standard treatment, regardless of squamous cell esophageal cancer or adenocarcinoma3. However, the tumor control was far from satisfactory and the 5-year survival rate was low.
Taxanes, which are mitotic inhibitors, including paclitaxel (PTX) and docetaxel (DTX), have shown radiosensitizing potential in some tumor cell lines4-6 and a good response to chemoradiation with taxane and platinum in the first-and second-line setting7-27 in patients with esophageal cancer. Taxane-combination chemoradiotherapy as a first-line definitive treatment resulted in an overall response rate (ORR) of 50% and was even higher in some publications7,9,10,17. The median progression-free survival (PFS) and overall survival (OS) were reported greater than one year and approximately two years, respectively, with a relatively well tolerated toxic profiles7,9,10,17. These studies demonstrated the feasibility of definitive chemoradiotherapy with taxane and platinum in esophageal carcinoma. Given the encouraging results, comparisons of taxane-based chemotherapy with fluorouracil-based therapy have been reported in several studies, in term of toxicity, response and survival28-33. Some of them favored the taxane-based regimen because of its lower toxicity29,30,33 or excellent efficacy28. However, Adelstein et al. found increased toxicity and no promising outcomes in the paclitaxel-based treatment group34. Furthermore, most of the studies mentioned above had small sample sizes, especially those describing patients with esophageal squamous cell carcinoma (ESCC; approximately 50 cases) 28,29,31.
Considering the controversial results and small-scale studies of the previous reports, we conducted this larger-sized sample study that aimed to compare the taxane-based regimen with a PF regimen in combination with radiotherapy in terms of toxicity profiles, efficacy and survival in patients with unresectable esophageal cancer.
Methods and Materials
Study population
Overall, 179 consecutive patients who were unresectable or medically unfit for surgery from the Zhejiang Cancer Hospital between March 2009 and November 2014 were analyzed retrospectively. The eligibility criteria were as follows: (1) histologically or cytologically confirmed ESCC; (2) clinical stages of T3-4N0M0, T1-4N1M0 or T1-4N0-1M1 (lymph node metastasis) were included; (3) unresectable or inoperable to receive surgical treatment; (3) two-drug chemotherapy, including taxane-based and fluorouracil-based regimens, was delivered in the first-line setting.
Pretreatment evaluation
Pretreatment evaluation comprised taking a sufficient history; physical examination; routine hematological and biochemical tests; electrocardiogram; pulmonary-function tests; endoscopy with biopsy; electrolaryngoscope; endoscopic ultrasonography (EUS); computed tomography (CT) of the esophagus and upper abdomen; and external ultrasonography of the neck and supraclavicular region with fine-needle aspiration of involvement-suspected lymph nodes. Emission computed tomography was performed as deemed necessary for tumor staging, as well as bronchoscopy, brain magnetic resonance imaging (MRI) and positron emission tomography. The clinical TNM staging was based on the International Union against Cancer guidelines (UICC 6th).
Radiation therapy
All patients were subjected to intensity-modulated radiation therapy (IMRT) or three-dimensional conformal radiation therapy (3DCRT). The target volume was delineated on multiple CT slices. The gross tumor volume (GTV) was defined as primary esophageal cancer lesions and lymph node metastatic sites. The clinical target volume (CTV) was defined as the primary tumor plus a 3-4 cm expansion superiorly and inferiorly along the length of the esophagus and circumferential CTV margin of 0.8-1.0cm. The nodal CTV should be defined by a 0.5 to 1.0 cm expansion from the nodal GTV. CTV should also include coverage of elective nodal regions, such as the bilateral supraclavicular region, superior mediastinum and subcarinal. The planning target volume (PTV) was expanded by 0.5cm on the base of the CTV in all directions. The maximum dose to the spinal cord was limited to 45 Gy at any point. The volume of both lungs that received more than 20 Gy (V20) was limited to within 28% and the heart received 40 Gy (V40) < 50%. The median radiation dose for the PTV was 56 Gy (range: 14.4Gy-66Gy).
Chemotherapy
Patients were administered concomitantly or sequentially with radiotherapy. In the taxane group, paclitaxel and docetaxel were used. The regimen consisted of combination taxane and platinum dosed either per 3 weeks or per week, according to provider discretion. Intravenous taxane (PTX: 50 mg/m2/day or DTX: 25 mg/m2/day on day 1, 5-6weeks) and platinum (carboplatin: area under the curve (AUC)=2 or cisplatin: 25 mg/m2/day on day 1, 5-6 weeks) were given weekly in combination with concurrent radiation. When the 3-week regimen was used, taxane (PTX 150 mg/m2 or DTX 75 mg/m2 on day 1) and platinum (carboplatin: AUC=5 on day 1, cisplatin: 75 mg/m2 on days 1 or nedaplatin: 75 mg/m2 on days 1) was administered for two cycles at three-week intervals. In the fluorouracil-based group, all the patients were treated with a 3-week schedule. The regimens consisted of platinum (cisplatin: 75 mg/m2 or nedaplatin: 75 mg/m2 on days 1) and fluorouracil as well as its analogs (600 mg/m2 of 5-fluorouracil as continuous infusion for 72 h on days 1-3, Tegafur 1000 mg/day on days 1-3, FT207 1000 mg/day on days 1-3) for two cycles at three week intervals. In both groups, two cycles of consolidation chemotherapy were given with previous chemotherapy regimens. Some patients received induction chemotherapy. Chemotherapy was delayed for toxicities until recovery to normal levels, and/or the dose was reduced by 80% for grade ≥3 toxicities. Radiation was interrupted in cases of ≥3 grade non-hematological toxicity until the side effects were relieved.
Clinical evaluation and follow-up
Patients were necessarily evaluated during the treatment, an then routinely followed every 3 months for 2 years, every 6 months for 3 years, and then annually. Treatment efficacy was evaluated on the base of clinical examination, blood tests, ultrasound, esophagogram and CT scans. Response criteria were defined according to RACIST criteria. Patients who were lost to regular medical follow-up records before death were followed up by telephone. Complete response (CR) and partial response (PR) were considered as response. The date and site of progression (first failure) were investigated through follow-up. Worst grade during treatment was retrospectively scored according to the Common Terminology Criteria for Adverse Events v4.0 (CTCAE4), including hematological and non-hematological toxicities. Toxicity ≥ grade 3 was analyzed. Follow-up data were updated in May 2015.
Statistical analysis
Progression-free survival (PFS) was calculated from the start of therapy to the first event (i.e., locoregional progression, distant metastasis or death). Overall survival (OS) was calculated from the start of therapy to death. Statistical analysis was carried out using IBM SPSS 22.0. Chi-squared or Fisher's exact tests were performed to compare proportions. Difference between groups were tested using the Mann-Whitney U test for tumor length, age and radiation dose. Logistic regression was performed for multivariate analysis of progression, and factors with P value < 0.2 in the univariate analysis were included. The Kaplan-Meier method was performed for PFS and OS, using a log-rank test. A P-value of 0.05 or less was considered statistically significant (two-tailed).
Results
Patient and characteristics
Between March 2009 and November 2014, a consecutive series of 179 esophageal cancer patients were eligible for our analysis. Among the population, who were aged 42-76 years (median 60 years), 70% were male and most patients (83.8%) were diagnosed with stage III/IV lesions. Eight-three patients were included in the taxane group and 96 in the fluorouracil group. Table 1 shows the baseline characteristics of the two groups. There was no significant difference in patient and tumor characteristics. The median radiation dose was 56 Gy in the taxane and fluorouracil group (P=0.509). Among the patients in the taxane group, 97.5% completed the planned radiation, which was equivalent to the other group (97.9%). The remaining patients stopped radiotherapy because of esophageal perforation. The taxane-based regimens in the 83 patients comprised 55 (66.3%) Paclitaxel/platinum and 28 (33.7%) docetaxel/platinum, while the fluorouracil group comprised 42 (43.8%) 5-FU, 49 (51.0%) tegafur and 5 (5.2%) FT-207. Fifty-six patients (67.5%) in taxane group and 65 (67.7%) in fluorouracil group underwent concurrent chemoradiotherapy.
Patient characteristics
| Characteristic | Taxane group (n=83) | fluorouracil group (n=96) | P value | |
|---|---|---|---|---|
| Male | 70 (84.3) | 86 (89.6) | 0.296 | |
| Age (years), median (range) | 61 (45-76) | 59 (42-75) | 0.891 | |
| KPS ≥90 | 81 (97.6) | 89(92.7) | 0.179 | |
| Liquid or pappy diet | 62 (74.7) | 76 (79.2) | 0.478 | |
| Tumor length(cm), median (range) | 5 (1.5-16) | 5 (2-13) | 0.367 | |
| Tumor site | ||||
| Cervical | 2 (2.4) | 6 (6.3) | 0.162 (Upper vs. Mid vs. Distal vs. others) | |
| Upper | 18 (21.7) | 35 (36.5) | ||
| Mid | 39 (47) | 35 (36.5) | ||
| Distal | 20 (24.1) | 18 (18.8) | ||
| Two sites | 4 (4.8) | 2 (2.1) | ||
| CT1 | 1 (1.2) | 4 (4.2) | 0.163 (cT3 vs. cT4 vs other) | |
| CT2 | 17 (20.5) | 24 (25.0) | ||
| CT3 | 54 (65.1) | 49(51.0) | ||
| CT4 | 11 (13.3) | 19(19.8) | ||
| CN1 | 70 (84.3) | 84 (87.5) | 0.543 | |
| CM1 | 42 (50.6) | 46 (47.9) | 0.72 | |
| Clinical stage | ||||
| II | 11 (13.3) | 18 (18.8) | 0.607 | |
| III | 30 (36.1) | 32 (33.3) | ||
| IV | 42 (50.6) | 46 (47.9) | ||
| Ulcerative lesions | 34 (41.0) | 40 (41.7) | 0.924 | |
| Cardiovascular and pulmonary comorbidity | 16 (19.3) | 25 (26.0) | 0.283 | |
| Dose to PTV (Gy), median (range, Gy) | 56 (14.4-63) | 56 (44-66) | 0.509 | |
| Completion of radiation (%) | 81 (97.6) | 94 (97.9) | 1 | |
| Concurrent chemoradiotherapy | 56 (67.5) | 65 (67.7) | 0.973 |
PTV: planning target volume
Assessment of treatment response
One hundred and seventy-seven (98.9%) of the 179 patients were assessable for response. The other two patients with deep ulcerative tumors, both in the taxane group, were not evaluated because of esophageal perforation. Esophageal perforations both occurred after one cycle of weekly carboplatin and paclitaxel and concurrent eight fractions (14.4Gy) of radiation. The two patients received no more anti-tumor treatment thereafter. The data of the 177 response-evaluable patients are listed in Table 2. Our data showed that the CR and PR rate in taxane and fluorouracil groups was 8.6% vs. 3.1% and 63.0% vs. 60.4%, respectively. The ORR in the taxane group was higher than fluorouracil group, but was not significantly different (71.6% vs. 63.5%, respectively, P =0.255).
Progression and survival
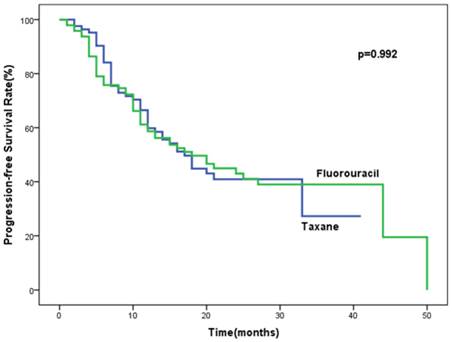
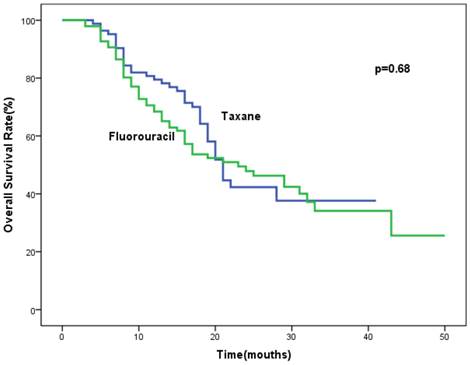
Follow-up data were updated in May 2015. The median follow-up time was 28 months (range, 11-74 months). The median progression-free survival was 17 months (95%CI, 12.8-21.2 months) for patients in the taxane group and 18 months (95%CI, 10.4-25.6 months) for patients of fluorouracil group. There was no significant difference in PFS between the taxane and fluorouracil groups (P = 0.992). Data concerning progression is shown in Table 3. Among the population, 53.0% (44/83) versus 54.2% (52/96) in the taxane and fluorouracil group had progressive disease, P=0.758. Locoregional relapse (LR), distant organ metastases (DM) and both locoregional and distant metastases in the taxane and fluorouracil groups accounted for 63.6%, 29.5%, 6.8% and 61.5%, 26.9% and 11.5%, respectively. The most frequent sites of progression were locoregional failure in both groups. No significant difference in median overall survival (21 months, 95%CI, 19.2-22.8 months vs. 23 months, 95%CI, 13.8-32.2 months) were found between the taxane and fluorouracil groups (P=0.68). The final result by multivariate analysis indicated that weight loss during radiation (OR = 2.0, 95%CI, 1.1-3.9, P = 0.032) and stage IV (lymph node metastasis) were the independent risk factors for progression (OR = 2.0, 95%CI, 1.5-5.5, P = 0.001). Liquid or pappy diet due to large tumor (OR = 2.1, 95%CI, 0.97-4.3, P = 0.06) and tumor length ≥ 5cm (OR = 1.7, 95%CI, 0.9-3.3, P = 0.093) tended to be correlated with progression, with marginal significance. The progresssion was not associated with chemotherapy regimens or age.
Treatment toxicity
The both regimens were generally well tolerated. The most common ≥ grade 3 toxicity was hematological toxic effect (leukopenia, neutropenia, anemia and thrombocytopenia) and non-hematological toxicity (nausea/vomiting, mucositis, esophageal perforation/fistula and pneumonia) (Table 4). Hematological toxicity of grade 3 or above occurred in 42.2% of taxane group and 43.8% of fluorouracil group (P=0.831). Compared with the fluorouracil group, the taxane group experienced a lower incidence of grade 3/4 thrombocytopenia (4.8% vs. 13.5%, P=0.047), but a trend towards a higher rate of ≥ grade 3 leukopenia (34.9% vs. 26.0%, P=0.196).
Response to treatment
| Response | Taxane group(n=81) | fluorouracil group (n=96) | P value |
|---|---|---|---|
| Complete response (CR) | 7 (8.6%) | 3 (3.1%) | |
| Partial response (PR) | 51 (63.0%) | 58 (60.4%) | |
| Stable disease (SD) | 21 (25.9) | 28 (29.2%) | |
| Progressive disease (PD) | 2 (2.5%) | 7 (7.3%) | |
| Overall response rate (%) | 71.6 | 63.5 | 0.255 |
Progression data
| Relapse | Taxane group(n=83) | fluorouraci group(n=96) | P value |
|---|---|---|---|
| Local relapse (LR) | 28 (63.6%) | 32 (61.5%) | |
| Distant metastasis (DM) | 13 (29.5%) | 14 (26.9%) | |
| LR+DM | 3 (6.8%) | 6 (11.5%) | |
| Total | 44 (53.0%) | 52 (54.2%) | 0.758 |
Treatment-related toxicities (≥ grade 3)
| Event | Taxane group (n=83) | fluorouraci group (n=96) | P value |
|---|---|---|---|
| Hematological toxicity | 35(42.2) | 42 (43.8) | 0.831 |
| Leukopenia | 29 (34.9) | 25 (26.0) | 0.196 |
| Neutropenia | 21 (25.3) | 22 (22.9) | 0.71 |
| Anemia | 4 (4.8) | 8 (8.3) | 0.349 |
| Thrombocytopenia | 4 (4.8) | 13 (13.5) | 0.047 |
| Non-hematological toxicity | |||
| Nauseau/vomiting | 5(6.0) | 3 (3.1) | 0.475 |
| Mucositis | 16 (19.3) | 22 (22.9) | 0.553 |
| Irradiation-induced skin injury | 2 (2.4) | 3 (3.3) | 1 |
| Irradiation-induced trachitis | 1 (1.2) | 0 (0) | 0.464 |
| Esophageal perforation | 4 (4.8) | 13 (13.5) | 0.047 |
| Bleeding | 2 (2.4) | 3 (3.3) | 1 |
| Pneumonia | 4 (4.8) | 9 (9.7) | 0.242 |
| Treatment-related death | 1 (1.2) | 3 (3.1) | 0.625 |
For non-hematological toxicities, the incidence of esophageal perforation or fistula in the taxane group was significantly lower than fluorouracil group (13.5% vs. 4.8%, P=0.047). Among the 17 patients with perforation or fistula, 11 (64.7%) patients were deep ulcerative type. Eleven patients were diagnosed with T3 lesions, four with T4 tumors and one with T2 tumor. Membrane-covered stents were placed in these patients, and seven patients received no further anti-tumor treatment because of poor physical condition or infection. Treatment-related death was not significantly different between the two groups (1.2% vs. 3.1%, P=0.625). One patient died of abdominal bleeding in the taxane group, and three patients died as a result of pulmonary infection and multiple organ failure caused by esophageal perforation and tracheo-esophageal fistula in another group. The incidence of pneumonia in the taxane group was lower, but not significant (4.8% vs. 9.7%, P=0.242). In addition, no grade 3 or above treatment-related neuropathy was observed in this study.
Analysis of progression-free survival (PFS) in the studied population.
Analysis of overall survival (OS) in the studied population.
Discussion
Many studies, including phase I/II trials and the retrospective analysis mentioned above, have demonstrated that taxane-based regimens were active, with a satisfactory outcome and manageable toxicity. Given the chemoradiation with PF regimens used worldwide as a standard treatment, the aim of our work was to compare the taxane-based with the PF regimen in combination with radiotherapy regarding toxicity profiles, efficacy and survival, in a larger simple size. In the present study, there were no significant difference in overall response rate and PFS and OS, as well as treatment-related death, between the two regimens. Both regimens were tolerated in terms of non-hematological and hematological toxicity. However, compared with the fluorouracil group, we found a lower incidence of ≥ grade 3 esophageal perforation or fistula (P=0.047) and pneumonia (P=0.242) in the taxane group. Regarding hematological toxicity, thrombocytopenia in the taxane group was significantly lower (P=0.047); The results of this study suggested that definitive chemoradiotherapy according to the taxane-based regimen is superior to the fluorouracil-based regimen in a first line setting of patients with inoperative ESCC in terms of toxicity.
The response rate in the two groups both exceeded 50%, with an ORR of 71.6% in taxane group and the 63.5% in fluorouracil group. This result was consistent with previous reports7,9,10,17. Shim et al.10 treated patients with advanced ESCC (clinical T2-4, N0-1, M0-M1 lymph node (LN) disease) using docetaxel at a dose of 20 mg/m2 and cisplatin at a dose of 25 mg/m2 at weeks 1, 2, 3, 5, 6 and 7 with concurrent radiotherapy (GTV, 54Gy/27F). That study showed an ORR of 85.8%, which was higher than the ORR of 71.6% reported in present work. Li et al.17 evaluated the feasibility of combination chemoradiotherapy (54-60Gy) using docetaxel (60 mg/m2) and cisplatin (80 mg/m2) in patients with ESCC and achieved an extremely high response rate of 98.3%, with a ORR of 71.2%. The median OS in these phase II studies were 26.7 months and 22.6 months, respectively, which were slightly longer than the 21 months in the current work. These encouraging results suggested that chemoradiotherapy using taxane and platinum was a promising treatment in previously untreated advanced ESCC in term of response and survival.
Comparisons of taxane-based therapy and PF regimens have been conducted in several publications28-34 in first-(neoadjuvant or definitive) (see Table 5) or second line settings. Considering the higher efficacy or more favorable overall toxicity, taxane-based therapy has been suggested superior to the fluorouracil-based regimen. The median OS in docetaxel/cisplatin group in the study of Zhao et al28 was significantly longer than the PF group (43.2 vs. 22.3 months, p<0.05). Nevertheless, the median OS between the taxane and fluorouracil groups in our work was similar (21 vs. 23 months, P=0.68), which was supported by the result (13.8 vs. 16.1 months, P=0.879) from Honing et al.30. Thus, more randomized controlled clinical trials with larger sample sizes are needed.
Progression after first-line treatment of esophageal cancer is a major failure causing a poor prognosis. The first site of progression includes local relapse (LR), distant metastasis (DM), and both LR and DM. Approximately 50% of patients treated with definitive chemoradiotherapy according to a fluorouracil-based regimen developed locoregional recurrence, and the pattern of locoregional recurrence accounted for the largest proportion of recurrence35-37. Button et al36 found that most (96%) locoregional relapse occurred within the RT field, and large field margins (CTV, 2-cm margin in the SI direction, 1-cm in the radial direction) seem to have not prevented the progression. However, a similar result was reported by Welsh et al37, in spite of the larger field (CTV, 3-cm margin in the SI direction, 1-cm in the radial direction). The radiation dose was 50.4Gy and 50Gy in the two studies, respectively. Thus, the extensive RT fields or higher doses (RTOG 850138 and INT 0123 trial39) do not seem to increase survival or locoregional control in the fluorouracil-based setting. Therefore, it is necessary to identify a novel radiation sensitizer, such as taxane, to overcome tumor radioresistance and reduce locoregional failure risk. However, our results indicated that the progerssion seems asssociated with lack of food intake and advanced tumor stage, not the chemotherapy regimens. Whether taxanes could reduce treatment failure still needs more reserch.
Non-hematological toxicities ≥ grade 3 occurred more frequently among patients in the fluorouracil-based group in our work, whereas the incidence of hematological and non-hematological toxicity ≥ grade 3 in the paclitaxel/carboplatin group was significantly lower than 5-FU/cisplatinum (P=0.001) in Honing et al30. Overall, irradiation-induced esophagitis was the most common non-hematological toxicity in our study as well as others28,34,40. Esophageal perforation or fistula was more frequent in fluorouracil administration than with taxane (13.5% vs. 4.8%, P=0.047) among the patients with T3 or T4 lesions in our work. A similar finding was reported by Shim et al10, two patients (5.7%) who developed tracheo-esophageal fistula were both T4 diseases. Fistula formation was reported 9-18 % of patients with T4 lesions during or after dCRT using fluorouracil regimen41-43. An explanation for why we observed a higher incidence of perforation or fistula in the fluorouracil group might be that fluorouracil increased the risk of mucositis, leading to a higher probability of perforation. Mucositis in our work was higher in fluorouracil group (22.9% vs. 19.3%, P= 0.553), which was in line with the result (18% vs. 13%) from Adelstein et al34. Most patients who developed a perforation or fistula had deep invasively ulcerative lesions (T3 or T4), like the two patients in the taxane group who were not evaluated regarding response. Such patients may be more like to develop perforation or fistula when fluorouracil-based treatment is used. These results provide further support for taxane-based regimens in esophageal cancer.
Comparisons of taxane-based therapy and PF regimens in first line setting
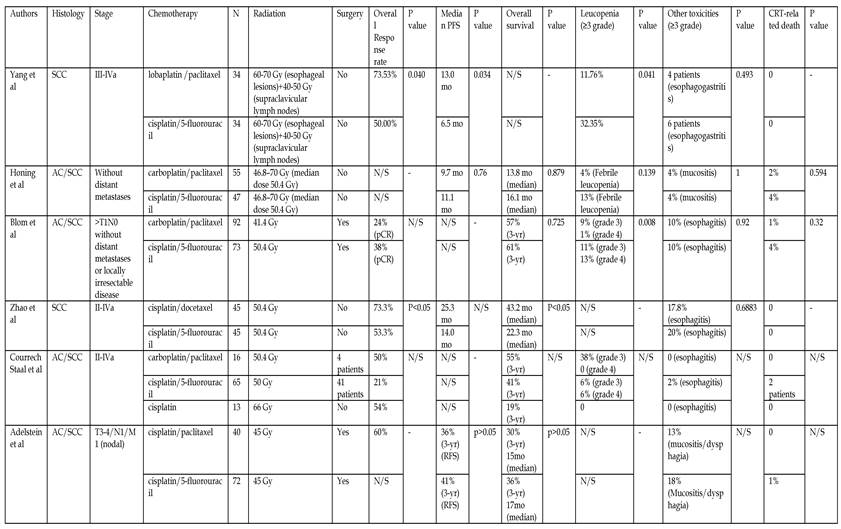
PFS: progression-free survival; RFS: recurrence-free survival; SCC: squamous cell cancer; AC: adenocarcinoma; N/S: not stated; CRT: chemoradiotherapy
This study was subject to some limitations. Our sample size is larger than other studies, but a bias still exists because of the retrospective design. Another drawback is that approximately 30% of the patients in this study were not concurrently treated, as definitive concurrent chemoradiation was the standard treatment. These limitations could make the conclusions less definitive.
In conclusion, our study found that chemoradiation with taxane-based regimens were effective and well tolerated with a lower incidence of severe hematological and non-hematological toxicities including esophageal perforation/fistula. There was no significantly difference in OS, PFS and treatment-related death between groups. The results of this study suggest that definitive chemoradiotherapy using taxane-based regimen could become a good alternative treatment in a first line setting for patients with inoperable ESCC.
Acknowledgements
This work was supported by the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents (to Yaping Xu).
Abbreviations
ESCC: squamous cell esophageal cancer; ORR: overall response rate; dCRT: definitive concurrent chemoradiation; PF: cisplatin/5-fluorouracil; PTX: paclitaxel; DTX: docetaxel; PFS: progression-free survival; OS: overall survival; EUS: endoscopic ultrasonography; CT: computed tomography; MRI: magnetic resonance imaging; IMRT: intensity-modulated radiation therapy; 3DCRT: three-dimensional conformal radiation therapy; GTV: gross tumor volume; CTV: clinical target volume; PTV: planning target volume; CR: complete response; PR: partial response; CTCAE 4.0: Common Terminology Criteria for Adverse Events v4.0; SD: stable disease; PD: progressive disease; LR: local relapse; DM: distant metastasis.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Parkin DM, Bray F. Evaluation of data quality in the cancer registry: principles and methods Part II. Completeness. European journal of cancer (Oxford, England: 1990). 2009;45:756-764
2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: a cancer journal for clinicians. 2009;59:225-249
3. Higuchi K, Koizumi W, Tanabe S. et al. Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointestinal cancer research: GCR. 2009;3:153-161
4. Tishler RB, Schiff PB, Geard CR, Hall EJ. Taxol: a novel radiation sensitizer. International journal of radiation oncology, biology, physics. 1992;22:613-617
5. Leonard CE, Chan DC, Chou TC, Kumar R, Bunn PA. Paclitaxel enhances in vitro radiosensitivity of squamous carcinoma cell lines of the head and neck. Cancer research. 1996;56:5198-5204
6. Mason KA, Hunter NR, Milas M, Abbruzzese JL, Milas L. Docetaxel enhances tumor radioresponse in vivo. Clinical cancer research: an official journal of the American Association for Cancer Research. 1997;3:2431-2438
7. Day F, Leong T, Ngan S. et al. Phase I trial of docetaxel, cisplatin and concurrent radical radiotherapy in locally advanced oesophageal cancer. British journal of cancer. 2011;104:265-271
8. Cao W, Xu C, Lou G. et al. A phase II study of paclitaxel and nedaplatin as first-line chemotherapy in patients with advanced esophageal cancer. Japanese journal of clinical oncology. 2009;39:582-587
9. Tu L, Sun L, Xu Y. et al. Paclitaxel and cisplatin combined with intensity-modulated radiotherapy for upper esophageal carcinoma. Radiation oncology (London, England). 2013;8:75
10. Shim HJ, Kim DE, Hwang JE. et al. A phase II study of concurrent chemoradiotherapy with weekly docetaxel and cisplatin in advanced oesophageal cancer. Cancer chemotherapy and pharmacology. 2012;70:683-690
11. El-Rayes BF, Shields A, Zalupski M. et al. A phase II study of carboplatin and paclitaxel in esophageal cancer. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2004;15:960-965
12. He YF, Ji CS, Hu B. et al. A phase II study of paclitaxel and nedaplatin as front-line chemotherapy in Chinese patients with metastatic esophageal squamous cell carcinoma. World journal of gastroenterology: WJG. 2013;19:5910-5916
13. Kim JY, Do YR, Park KU. et al. A multi-center phase II study of docetaxel plus cisplatin as first-line therapy in patients with metastatic squamous cell esophageal cancer. Cancer chemotherapy and pharmacology. 2010;66:31-36
14. Gong Y, Ren L, Zhou L. et al. Phase II evaluation of nedaplatin and paclitaxel in patients with metastatic esophageal carcinoma. Cancer chemotherapy and pharmacology. 2009;64:327-333
15. Polee MB, Eskens FA, van der Burg ME. et al. Phase II study of bi-weekly administration of paclitaxel and cisplatin in patients with advanced oesophageal cancer. British journal of cancer. 2002;86:669-673
16. Shi Y, Qin R, Wang ZK, Dai GH. Nanoparticle albumin-bound paclitaxel combined with cisplatin as the first-line treatment for metastatic esophageal squamous cell carcinoma. OncoTargets and therapy. 2013;6:585-591
17. Li QQ, Liu MZ, Hu YH, Liu H, He ZY, Lin HX. Definitive concomitant chemoradiotherapy with docetaxel and cisplatin in squamous esophageal carcinoma. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus / I.S.D.E. 2010;23:253-259
18. Zhang X, Shen L, Li J, Li Y, Li J, Jin M. A phase II trial of paclitaxel and cisplatin in patients with advanced squamous-cell carcinoma of the esophagus. American journal of clinical oncology. 2008;31:29-33
19. Laack E, Andritzky B, DüRK H. et al. Docetaxel and cisplatin as first-line treatment for patients with metastatic esophageal cancer: a pilot study. Oncology Research and Treatment. 2005;28:647-650
20. Du J, Hu C, Zhang Y, Hu B, Wang F, Zhang Y. A retrospective study of paclitaxel combining nedaplatin chemotherapy for esophageal cancer. Anti-cancer drugs. 2015;26:101-105
21. Nakajima Y, Suzuki T, Haruki S. et al. A pilot trial of docetaxel and nedaplatin in cisplatin-pretreated relapsed or refractory esophageal squamous cell cancer. Hepato-gastroenterology. 2008;55:1631-1635
22. Jin J, Xu X, Wang F. et al. Second-line combination chemotherapy with docetaxel and nedaplatin for Cisplatin-pretreated refractory metastatic/recurrent esophageal squamous cell carcinoma. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2009;4:1017-1021
23. Shim HJ, Cho SH, Hwang JE. et al. Phase II study of docetaxel and cisplatin chemotherapy in 5-fluorouracil/cisplatin pretreated esophageal cancer. American journal of clinical oncology. 2010;33:624-628
24. Matsumoto H, Hirabayashi Y, Kubota H. et al. A combined therapy with docetaxel and nedaplatin for relapsed and metastatic esophageal carcinoma. Anticancer research. 2012;32:1827-1831
25. Huang J, Zhou Y, Zhang H. et al. A phase II study of biweekly paclitaxel and cisplatin chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma: ERCC1 expression predicts response to chemotherapy. Medical oncology (Northwood, London, England). 2013;30:343
26. Akutsu Y, Shuto K, Kono T. et al. A phase 1/11 study of second-line chemotherapy with fractionated docetaxel and nedaplatin for 5-FU/cisplatin-resistant esophageal squamous cell carcinoma. Hepato-gastroenterology. 2012;59:2095-2098
27. Song Z, Zhang Y. Second-line docetaxel-based chemotherapy after failure of fluorouracil-based first-line treatment for advanced esophageal squamous cell carcinoma. OncoTargets and therapy. 2014;7:1875-1881
28. Zhao T, Chen H, Zhang T. Docetaxel and cisplatin concurrent with radiotherapy versus 5-fluorouracil and cisplatin concurrent with radiotherapy in treatment for locally advanced oesophageal squamous cell carcinoma: a randomized clinical study. Medical oncology (Northwood, London, England). 2012;29:3017-3023
29. Yang JS, Wang T, Qiu MQ, Li QL. Comparison of efficacy and toxicity profiles between paclitaxel/lobapoatin- and cisplatin/5-fluorouracil-based concurrent chemoradiotherapy in advanced inoperable esophageal cancer. Internal medicine journal. 2015;45:757-761
30. Honing J, Smit JK, Muijs CT. et al. A comparison of carboplatin and paclitaxel with cisplatinum and 5-fluorouracil in definitive chemoradiation in esophageal cancer patients. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2014;25:638-643
31. Fujita Y, Hiramatsu M, Kawai M, Sumiyoshi K, Nishimura H, Tanigawa N. Evaluation of combined docetaxel and nedaplatin chemotherapy for recurrent esophageal cancer compared with conventional chemotherapy using cisplatin and 5-fluorouracil: a retrospective study. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus / I.S.D.E. 2008;21:496-501
32. Courrech Staal EF, Aleman BM, van Velthuysen ML. et al. Chemoradiation for esophageal cancer: institutional experience with three different regimens. American journal of clinical oncology. 2011;34:343-349
33. Blom RL, Sosef MN, Nap M. et al. Comparison of two neoadjuvant chemoradiotherapy regimens in patients with potentially curable esophageal carcinoma. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus / I.S.D.E. 2014;27:380-387
34. Adelstein DJ, Rice TW, Rybicki LA. et al. Does paclitaxel improve the chemoradiotherapy of locoregionally advanced esophageal cancer? A nonrandomized comparison with fluorouracil-based therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000;18:2032-2039
35. Denham JW, Steigler A, Kilmurray J. et al. Relapse patterns after chemo-radiation for carcinoma of the oesophagus. Clinical oncology (Royal College of Radiologists (Great Britain)). 2003;15:98-108
36. Button MR, Morgan CA, Croydon ES, Roberts SA, Crosby TD. Study to determine adequate margins in radiotherapy planning for esophageal carcinoma by detailing patterns of recurrence after definitive chemoradiotherapy. International journal of radiation oncology, biology, physics. 2009;73:818-823
37. Welsh J, Settle SH, Amini A. et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118:2632-2640
38. Cooper JS, Guo MD, Herskovic A. et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA: the journal of the American Medical Association. 1999;281:1623-1627
39. Minsky BD, Pajak TF, Ginsberg RJ. et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20:1167-1174
40. Haj Mohammad N, Hulshof MC, Bergman JJ. et al. Acute toxicity of definitive chemoradiation in patients with inoperable or irresectable esophageal carcinoma. BMC cancer. 2014;14:56
41. Itoh Y, Fuwa N, Matsumoto A, Asano A, Morita K. Outcomes of radiotherapy for inoperable locally advanced (T4) esophageal cancer-retrospective analysis. Radiation medicine. 2001;19:231-235
42. Kaneko K, Ito H, Konishi K. et al. Definitive chemoradiotherapy for patients with malignant stricture due to T3 or T4 squamous cell carcinoma of the oesophagus. British journal of cancer. 2003;88:18-24
43. Ohtsu A, Boku N, Muro K. et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17:2915-2921
Author contact
![]() Corresponding author: Yaping Xu, MD, 1. Department of Radiation Oncology, Zhejiang Cancer Hospital, Hangzhou China; 2. Key Laboratory of Radiation Oncology, Zhejiang Cancer Hospital, Hangzhou 310022, China, 38 Guangji Road, Hangzhou, Zhejiang 310022, P.R. China; Telephone: +8613857101269; Fax: +86057188368079; Email: xuyporg.cn
Corresponding author: Yaping Xu, MD, 1. Department of Radiation Oncology, Zhejiang Cancer Hospital, Hangzhou China; 2. Key Laboratory of Radiation Oncology, Zhejiang Cancer Hospital, Hangzhou 310022, China, 38 Guangji Road, Hangzhou, Zhejiang 310022, P.R. China; Telephone: +8613857101269; Fax: +86057188368079; Email: xuyporg.cn

 Global reach, higher impact
Global reach, higher impact