Impact Factor
ISSN: 1837-9664
J Cancer 2016; 7(11):1487-1496. doi:10.7150/jca.15515 This issue Cite
Research Paper
Magnetofection Based on Superparamagnetic Iron Oxide Nanoparticles Weakens Glioma Stem Cell Proliferation and Invasion by Mediating High Expression of MicroRNA-374a
1. Department of Neurosurgery, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai 200040, China;
2. Department of Neurobiology, School of Basic Medical Sciences and Research Center of Aging Medicine, Shanghai Medical College, Fudan University, Shanghai 200032, China;
3. Laboratoire PROTEE, Bâtiment R, Université du Sud Toulon-Var, 83957 LA GARDE Cedex, France.
4. Shanghai Geriatric Institute of Chinese Medicine, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200031, China.
5. Shanghai Tenth People's Hospital, Medical School, Tongji University, Shanghai 200072, China.
* These authors contributed equally to this work.
Received 2016-3-12; Accepted 2016-6-3; Published 2016-7-7
Abstract
Glioma stem cells belong to a special subpopulation of glioma cells that are characterized by strong proliferation, invasion and drug resistance capabilities. Magnetic nanoparticles are nanoscale biological materials with magnetic properties. In this study, CD133+ primary glioma stem cells were isolated from patients and cultured. Then, magnetic nanoparticles were used to mediate the transfection and expression of a microRNA-374a overexpression plasmid in the glioma stem cells. Transmission electron microscopy detected the presence of significant magnetic nanoparticle substances within the CD133+ glioma stem cells after transfection. The qRT-PCR and Northern blot results showed that the magnetic nanoparticles could be used to achieve the transfection of the microRNA-374a overexpression plasmid into glioma stem cells and the efficient expression of mature microRNA-374a. The MTT and flow cytometry results showed that the proliferation inhibition rate was significantly higher in cells from the microRNA-374a transfection group than in cells from the microRNA-mut transfection group; additionally, the former cells presented significant cell cycle arrest. The Transwell experiments confirmed that the overexpression of microRNA-374a could significantly reduce the invasiveness of CD133+ glioma stem cells. Moreover, the high expression of microRNA-374a mediated by the magnetic nanoparticles effectively reduced the tumourigenicity of CD133+ glioma stem cells in nude mice. The luciferase assays revealed that mature microRNA-374a fragments could bind to the 3'UTR of Neuritin (NRN1), thereby interfering with Neuritin mRNA expression. The qRT-PCR and Western blotting results showed that the overexpression of microRNA-374a significantly reduced the expression of genes such as NRN1, CCND1, CDK4 and Ki67 in glioma stem cells. Thus, magnetic nanoparticles can efficiently mediate the transfection and expression of microRNA expression plasmids in mammalian cells. The overexpression of microRNA-374a can effectively silence NRN1 expression, thereby inhibiting the proliferation, invasion and in vivo tumourigenicity of human glioma stem cells.
Keywords: Glioma stem cells (GSCs), Magnetofection based on superparamagnetic iron oxide nanoparticles (SPIONs), microRNA-374a (miR-374a), Neuritin (NRN1), Proliferation and invasion.
Introduction
An increasing number of studies have confirmed the presence of cancer stem cells (CSCs) in many types of tumour tissues and have shown that CSCs have important significance for tumourigenesis and recurrence [1-4]. CSCs belong to a special subpopulation and account for a very small proportion of the entire tumour cell population (approximately 0.1%-1%); however, CSCs not only possess characteristics associated with normal stem cells, but also have strong potential for self-proliferation, invasion and resistance to chemotherapeutic agents [1-4]. A number of studies have reported the isolation and enrichment of glioma stem cells (GSCs) from human glioma tissues [1-4]. Glioma stem cell subpopulations with high expression of CD133 or CD44 can be isolated using flow cytometry-based sorting techniques. Human GSCs may be continuously cultured and passaged in vitro in a 'neurosphere' state by utilizing a serum-free culture system [1-4]. In-depth studies have found that this population of cells possesses stem cell characteristics including high expression levels of several important stem cell markers (i.e., SSEA-1/CD-15, L1CAM, A2B5, CD90 and CD44); moreover, GSCs exhibit tumour cell characteristics such as proliferation, invasion and tumourigenicity [1-4]. Therefore, CD133+ or CD44+ glioma cells are considered to be a representative group of GSCs [1-4]. MicroRNA molecules are small non-coding RNA molecules that are approximately 21-23 nucleotides in length, do not contain open reading frames, and do not encode any proteins [5-7]. MicroRNAs can bind to specific sequences in target genes to induce Dicer-mediated cleavage and finally silence target gene expression [5-9]. MicroRNAs are ubiquitous in eukaryotic organisms and play a very important role in regulating growth and development, morphogenesis, disease occurrence and development, and cell fate [5-14]. Nanoparticles refer to microscopic nanoscale particles [15-18]. Nanoparticles are very small and can easily pass through cell membranes, penetrate into organelles and spread throughout an entire organ or the body along the synapses and axons of neurons, blood vessels and lymphatic vessels [15-18]. Currently, it is possible to utilize various materials (i.e., emulsoid, polymers, ceramics, metals, lipids and carbon) to manufacture particulate materials 50-100 nm in size through specific processes [15-18]. The magnetofection of superparamagnetic iron oxide nanoparticles (SPIONs) generates a special type of magnetic nanoparticle. The SPION particle diameter is approximately 20 ± 5 nm, the Zeta point is 15 ± 5 mV, and the appearance is an irregular, round-shaped shell structure [15-18]. After binding to plasmid DNA and siRNA, SPIONS can transfect nucleic acids into mammalian cells and improve nucleic acid accumulation within the cells and in specific areas of the external magnetic field through magnetic attraction, thereby enhancing gene expression [15-18]. Wang et al. developed a SPION-based stable nuclear transporter system for nucleic acid expression vectors. They used polyethylenimine to modify the surface of spherical Fe3O4 nanoparticles so that the modified Fe3O4 nanoparticles had higher affinities for plasmid DNAs encoding dsRFP and GFP and could mediate the simultaneous high expression of green (GFP) or red (dsRed) fluorescent proteins in porcine kidney cells [15]. Lo et al. utilized polyethylenimine (PEI) and chondroitin-conjugated copolymers (CPS) to modify the SPION surfaces and thus accomplished the SPION-mediated transfection and expression of a microRNA-128 expression plasmid into cells [16]. Delyagina et al. added biotin-streptavidin to the surface of PEI-SPIONs to increase their affinity for plasmid DNA, enabling them to efficiently mediate intracellular nucleic acid transfection even in the absence of an external magnetic force [17]. Thus, SPION is a novel nanomaterial with a great potential to efficiently mediate nucleic acid transfection and expression in cells.
Based on the above information, in this study we constructed a plasmid DNA for miR-374a overexpression and utilized SPION to mediate the transfection and expression of the miR-374a overexpression plasmid in CD133+ GSCs. Then, we want to determine whether overexpression miR-374a could specifically regulate expression of Neuritin protein-coding gene NRN1 and inhibit cell proliferation, invasion and tumourigenicity of CD133+ GSCs.
Materials and methods
Isolation and incubation of primary CD133+ human GSCs
Primary CD133+ human GSCs were isolated from tumour tissues surgically resected from 4 glioma patients in the Department of Neurosurgery at the Shanghai Huashan Hospital (The median age of these populations was 45 years (45.5±7.0); poorly differentiated variant star glioma (stage III)). Weight of approximation 300mg glioma tissues were digested with 0.25% trypsin under sterile conditions. Approximation 1x106 cells were collected via centrifugation of the cell suspension, followed by the addition of 0.5 mL of ice-cold sterile PBS (HyClone). Five microliters of an anti-human CD133-FITC antibody (eBioscience) was added into approximation 1x106 cells at the same time to a final concentration of 0.01 mg/mL and mixed well. The cells were incubated at 4 °C in the dark for 30 minutes. After completion of the reaction, the cells were washed twice with ice-cold PBS and then CD133+ human primary GSCs were sorted and enriched using flow cytometry (BD FACS Aria, BD Bioscience, CA, USA). After Sorting, approximation 2x104 CD133+ cells could be isolated. The concentration of the cells was adjusted to 1000 cells/mL, and the cells were seeded into non-adherent spherical clusters. The cells were incubated with DMEM:F12 (HyClone) medium containing 10 ng/mL basic fibroblast growth factor (bFGF), 10 ng/mL epidermal growth factor (EGF), 5 μg/mL insulin and 0.5% bovine serum albumin (BSA) (all from Sigma-Aldrich). The cells were cultured to the third generation and then used for the subsequent experiments.
SPION-induced microRNA plasmid transfection into cells
SPION was purchased from NOVOBIO Biotechnology Co., Ltd. (Shanghai, China). According to the procedures in the manual and the methods described in previous studies [15-18], 5 μl of 0.2 mM SPIO and 5 μl of 10 μM of the pRNAT-CMV32-miR-374a or pRNAT-CMV32-miR-mut plasmid were mixed thoroughly by vortexing for 10 s; then, the mixtures were incubated at room temperature for 20 min. The 10-μl SPIO-microRNA plasmid DNA mixture was mixed with 90 μl of DMEM serum-free medium and then added to 1x104/mL cells, followed by 72 h of incubation at 37 °C with 5% CO2.
Luciferase reporter assay
All cells were seeded into 24-well cell culture plates at a density of 3 × 104 cells/well. The Lipofectamine 2000 Reagent was used to transfect the cells in the respective groups with 400 ng of pRNAT-CMV32-miR-374a, pRNAT-CMV32-miR-mut or pRNAT-CMV32 and 20 ng of pGL6-NRN1-3UTR, pGL6-NRN1-mut or pGL6 (Beyotime Biotechnology Co., Ltd., Zhejiang, China). At 48 h after transfection, the dual-luciferase reporter assay system (Beyotime Biotechnology Co., Ltd., Zhejiang, China) was used to detect the luciferase activity in each group.
RNA extraction and analysis by quantitative real-time PCR (qRT-PCR)
The total RNA was extracted from the cells in each group according the TRIzol reagent manual (Invitrogen). The total RNA was treated with DNase I (Sigma-Aldrich), quantified and subjected to reverse transcription using the ReverTra Ace-α First Strand cDNA Synthesis Kit (TOYOBO) to generate cDNA. The qRT-PCR was conducted in the RealPlex4 real-time PCR detection system (Eppendorf Co., Germany) using the SyBR Green Real-Time PCR Master Mix (TOYOBO) as the fluorescent dye for nucleic acid amplification. The qRT-PCR included 40 amplification cycles consisting of denaturation at 95 °C for 15 s, annealing at 58 °C for 30 s, and extension at 72 °C for 42 s. We used the 2-ΔΔCt calculation method to determine the relative expression of the genes as follows: ΔCt=Ct_genes-Ct_18sRNA and ΔΔCt = ΔCt_all_groups-ΔCt_blankcontrol_group. The mRNA expression levels were normalized according to the expression level of the 18s rRNA gene. The primers used for the amplification of each gene were described in previous studies [8, 9].
Northern blotting
Based on the methods described in previous studies [8, 9], total RNA was extracted from the cells in all groups utilizing the TRIzol extraction kit. After quantification, 20 μg of high quality total RNA was selected for 7.5 M urea 12% polyacrylamide (PAA) denaturing gel electrophoresis. After completion of the electrophoresis, the RNAs were transferred to a Hybond N+ nylon membrane (Amersham, Freiburg, Germany). The membrane was cross-linked under 1200 mjoule/cm2 UV light for 30 s and hybridized with the MiR-374a antisense DNA probe to detect the expression of miR-374a. After hybridization and membrane washing, the membrane was exposed to Kodak XAR-5 film for 20-40 h (Sigma-Aldrich Chemical). As a positive control, all of the membranes were hybridized with the human U6 snRNA probe (5'-GCAGGGGCCATGCTAATCTTCTCTGTATCG-3'). The U6 snRNA probe exposure time was maintained for 15-30 min.
Western blotting
Based on the methods described in previous studies [8, 9], total proteins from the cells in each group were subjected to 12% SDS PAGE denaturing gel electrophoresis and transferred to PVDF membranes (Millipore) after the completion of electrophoresis. After blocking and membrane washing, primary antibodies (rabbit polyclonal antibody against human CCND1 (1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit polyclonal antibody against human CDK4 (1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit polyclonal antibody against human Neuritin (1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit polyclonal antibody against human GAPDH (1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA)) were added, followed by incubation at 37 °C for 45 min. Secondary antibodies (peroxidase-linked goat anti-rabbit IgG (1 : 1000; Santa Cruz)) were added after sufficiently washing the membrane, followed by incubation at 37 °C for 45 min. The membrane was washed 4 times with TBST (Tris-buffered saline and Tween 20) at room temperature with 14 minutes per wash. The proteins in the membrane were detected with ECL enhanced chemiluminescence (ECL kit, Pierce Biotechnology). Densitomertic analysis was performed by Quantity One Software (Bio-Rad, Hercules, CA, USA).
CCK-8 assay
The number of cells in each group was adjusted, and 2×103 cells/mL were seeded into 96-well cell culture plates. At 72 h after transfection, the cells in each group were treated with 20 µL of CCK-8 solution (Sigma-Aldrich Chemical) and incubated at 37 °C for 3 h. The cell culture plates were placed in the plate reader and the absorbance values were recorded at the 450 nm wavelength. The cell proliferation inhibition rate (%) was calculated as follows: (1- OD value of the experimental group of cells/ OD value of the control group of cells) × 100%.
Propidium iodide (PI) staining and flow cytometry (FCM) assay
Based on the methods described in previous studies [8, 9], 5×105 cells/mL were collected and were added to 1 mL of 70% ice-cold ethanol for 48 h for fixation. The cells were centrifuged for 5 min at 4 °C at 1500 r/min. The cell pellets were collected and resuspended in the PI staining solution (Sigma Chemicals) for 30 minutes at 4 °C in the dark. The distribution of the cell cycle was analysed for the cells in each group via flow cytometry (BD FACSAria). The data analysis was conducted using the CellQuest software.
Transwell migration assay
A total of 200 μL of serum-free cell culture medium containing 2×103/mL cells was seeded into the top chamber of a Transwell chamber with an 8.0-μm pore size. A total of 600 μl of complete medium containing 10% FBS was inoculated into the lower chamber of the Transwell chamber. The cells were cultured at 37 °C with 5% CO2 for 48 h. The cells adhering to the membrane surface were fixed with 4% paraformaldehyde at room temperature for 30 min and stained with 4,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich Chemical) for 10 min. Three non-overlapping fields under the microscope were chosen to calculate the total number of cells.
In vivo xenograft experiments
Based on the methods described in previous studies [8, 9], the cells in each group were transfected with plasmids. A total of 1×105 cells/mL in logarithmic growth phase was used to subcutaneously inoculate BALB/Cnu/nu mice (4 mice per group; 6-8-week-old male BALB/Cnu/nu mice were provided by the Experimental Animal Research Center of Fudan University). After 8 weeks of observation, the mice were sacrificed, and the tumours were dissected. The tumours were weighed, and the tumour volume was calculated according to the formula: Tumour volume (mm3) = (ab2)/2 (a: the longest axis (mm), b: the shortest axis (mm)).
Histopathology assay
All fresh tissues were fixed for 30 minutes at room temperature in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA), followed by gradient ethanol dehydration, paraffin embedding, sectioning (with a thickness of 6 μm), and deparaffinization in xylene. The tissue sections were stained with haematoxylin-eosin (H & E, Sigma-Aldrich, St. Louis, MO, USA), clarified in xylene (Sigma-Aldrich, St. Louis, USA), and mounted in neutral resin (Sigma-Aldrich, St. Louis, MO, USA).
Immunohistochemical staining assay
All fresh tissues were fixed in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) at room temperature for 30 minutes, followed by gradient ethanol dehydration, paraffin embedding, sectioning (with a thickness of 6 μm), and deparaffinization in xylene. The tissue sections were blocked in the immunohistochemistry blocking solution (Beyotime Biotechnology Co., Ltd., Zhejiang, China) at 37 °C for 30 min. The blocking solution was discarded, and the samples were washed with the immunohistochemistry washing fluid (Beyotime Biotechnology Co., Ltd., Zhejiang, China) 3 times for 5 min at room temperature. The primary antibody was added, followed by 45 minutes of incubation at 37 °C. The antibody was discarded, and the samples were washed 3 times with the immunohistochemistry washing fluid (Beyotime Biotechnology Co., Ltd., Zhejiang, China) for 5 min at room temperature. The secondary antibodies (peroxidase-linked goat anti-rabbit IgG (1 : 1000; Santa Cruz)) was added, followed by 45 minutes of incubation at 37 °C. The antibody was discarded, and the samples were washed with the immunohistochemistry washing fluid (Beyotime Biotechnology Co., Ltd., Zhejiang, China) 3 times for 5 min at room temperature. Finally, neutral resin (Sigma-Aldrich, St. Louis, MO, USA) or immunofluorescence mounting medium (Sigma-Aldrich, St. Louis, MO, USA) was added to the samples to mount the sections. Three randomly vision (200 x) of each tissue section was observed and analyzed by IPP software. The color range was divided into 4 grades: + color range <1/4 vision; ++ color range 1/4 to 1/2 vision; +++ color range 1/2-3/4 vision; ++++ color range>3/4 vision. The degree of coloration was divided into weak (+), moderate (++), strong (+++). The color range and the color degree were converted into the color rendering index = color degree x color range. + is 1, ++ is 2, +++ is 3, ++++ is 4.
Transmission electron microscopy (TEM) analysis
The samples were fixed and embedded according to the procedures described in previous studies. Tissue samples were first fixed with 1% glutaraldehyde (Sigma-Aldrich, St. Louis, MO, USA) for 4 hours and then fixed with 1% osmium tetroxide (Sigma-Aldrich, St. Louis, MO, USA) for 1 hour, followed by acetone dehydration and resin 12 embedment (Ted Pella, USA). Ultrathin sections (with a cross-sectional thickness of 70 nm) were placed on a copper mesh, stained with 1% uranyl acetate (Sigma-Aldrich, St. Louis, MO, USA) and 1% lead citrate (Sigma-Aldrich, St. Louis, MO, USA), and observed and imaged using a JEM-1230 transmission electron microscope (JEOL, Japan).
Statistical analysis
Each experiment was performed at least three times. The data are shown as the mean±SE where applicable, and differences were evaluated using Student's t-test. A probability < 0.05 was considered statistically significant.
Results
CD133+ human GSCs have low expression of miR-374a
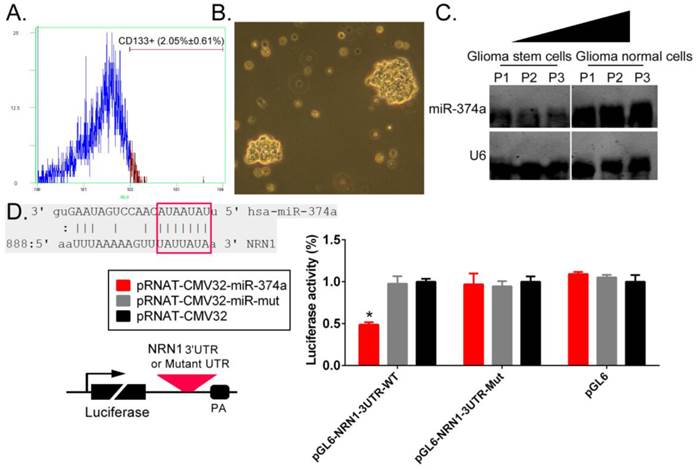
Flow cytometry techniques were used to isolate CD133+ human GSCs from primary glioma tissues (Figure 1A). In suspension cultures, we observed under the microscope that GSCs exhibited clone-like growth, with tight and unclear gaps between cells; additionally, the clones had good refraction and were plump in appearance (Figure 1B). The Northern blotting test results showed that the hybridization signal intensity of endogenous miR-374a expression were significantly lower in CD133+ human GSCs than in CD133- regular glioma cells. A bioinformatic comparative analysis revealed that the mature miR-374a molecule had 7 nucleotides that were fully complementary to specific regions in the NRN1 3' untranslated region (3'UTR). The luciferase reporter assay showed that NIH-3T3 cells cotransfected with the miR-374a overexpression plasmid and the luciferase reporter carrying the NRN1 gene 3'UTR sequence exhibited a significant decrease in the luciferase reporter activity (Figure 1D). The experimental results showed that NRN1 might be one of the target genes of miR-374a.
NRN1 is a target gene of miR-374a. (A) Flow cytometry techniques were used to isolate CD133+ human GSCs from glioma tissues. (B) CD133+ human GSCs morphology (original magnification, x200). (C) Northern blotting results showed that the hybridization signal intensity of mature miR-374a was significantly lower in CD133+ human GSCs than CD133- glioma cells. (D) The sequence alignment and the result of luciferase reporter assay. *p<0.05 vs. pRNAT-CMV32, t test, n = 3.
Overexpression of miR-374a significantly inhibits the proliferation and invasion of CD133+ human GSCs. (A) Transmission electron microscopy detected multiple electron clouds of SPION in miR-374a transfection GSCs. Blue arrows indicate SPION. (B) The CCK-8 experiment results suggested that the proliferation inhibition rate of SPION-mediated miR-374a transfection groupwas significantly increased. **p<0.01 vs. Untransfected, #p>0.05 vs. Untransfected, t test, n = 3. (C) Flow cytometry demonstrated that the SPION-mediated high expression of miR-374a effectively causes cell cycle arrest in GSCs. *p<0.05 vs. Untransfected, #p>0.05 vs. Untransfected, t test, n=3. (D) The Transwell experiments showed that the invasion cell number of CD133+ human GSCs in the SPION-mediated miR-374a transfection group was significantly less than the control group. *p<0.05 vs. Untransfected, #p>0.05 vs. Untransfected, t test, n=3.
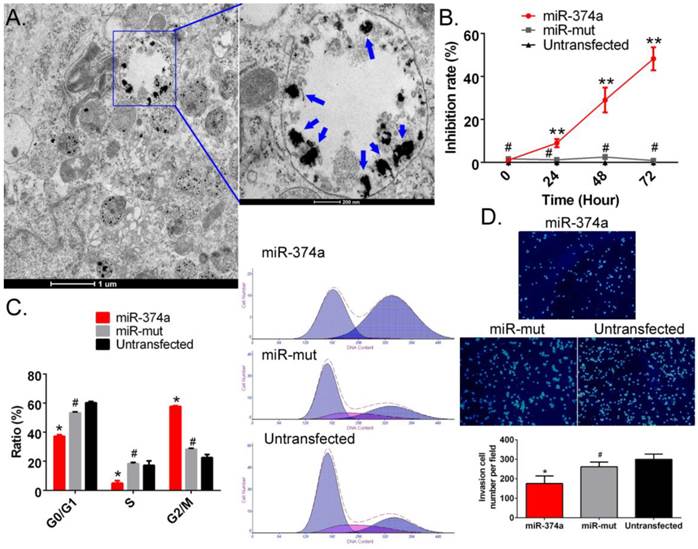
SPION-mediated promotion of high expression of exogenous miR-374a in human GSCs inhibits the proliferation and invasion of human GSCs
First, SPION and the miR-374a overexpression plasmid were crosslinked and added to CD133+ human GSCs. After 72 hours, TEM showed the presence of multiple circular dense electron clouds with a size of 80 nm-100 nm in either the cytoplasm or the nucleus of cells treated with the nanoparticles (Figure 2A). The dense signals observed as black dots were the SPIONs. Additionally, the CCK-8 cell proliferation assay results indicated that the SPION-mediated high expression level of miR-374a in CD133+ human GSCs effectively enhanced the in vitro cell proliferation inhibition rate. GSCs in both the negative control group (miR-mut transfection group) and the blank control group (Untransfected) had a very low in vitro proliferation inhibition rate (Figure 2B). Moreover, flow cytometry showed that the number of cells in the S phase of the cell cycle was significantly reduced and the number of cells in the G2/M phase was significantly increased after SPION-mediated transfection of miR-374a into human GSCs (Figure 2C). In contrast, the numbers of cells in S phase and G2/M phase were not significantly different in the miR-mut transfection group and the blank control group. Our results suggest that the SPION-mediated high expression of exogenous miR-374a effectively causes cell cycle arrest in human GSCs. The Transwell experiments also showed that the number of cells passing through the Transwell chamber was much lower in the group with magnetic nanoparticle-mediated transfection of miR-374a (175.667 ± 22.376) than in the SPION-mediated miR-mut transfection group (261.667 ± 14.146) and the blank control group (299.333 ± 15.899) (Figure 2D). These results suggest that SPION can effectively mediate the expression of the exogenous miR-374a overexpression plasmid in GSCs and that high expression of miR-374a can inhibit the in vitro proliferation and invasion of CD133+ human GSCs.
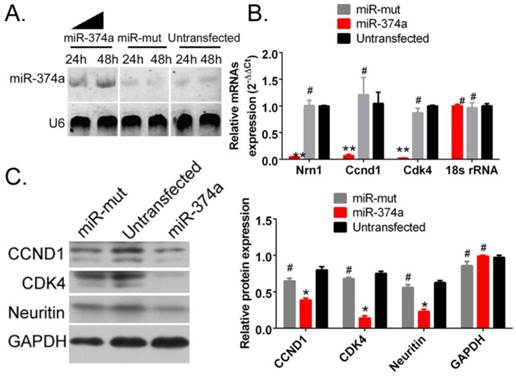
SPION-mediated promotion of the high expression of exogenous miR-374a in human GSCs inhibits the expression of Neuritin and cell cycle regulatory proteins
The probe hybridization signal detecting mature miR-374a by Northern blotting was significantly stronger in human GSCs following SPION-mediated transfection of the miR-374a overexpression plasmid than GSCs in the miR-mut plasmid transfection group and the untransfected group (Figure 3A). These results suggest that SPION can effectively mediate the transfection and expression of the miR-374a overexpression plasmid in GSCs. The qRT-PCR test results showed that the NRN1 gene and the cell cycle regulatory factors Ccnd1 and Cdk4 exhibited significantly reduced mRNA expression levels in CD133+ human GSCs in the SPION-mediated miR-374a transfection group compared to the miR-mut transfection group and the untransfected group (Figure 3B). Moreover, the Western blotting results showed that the Neuritin, CCND1 and CDK4 protein expression levels were significantly lower in the CD133+ human GSCs in the SPION-mediated miR-374a transfection group compared to those in the miR-mut transfection group and the untransfected group (Figure 3C). The experimental results show that the SPION-mediated promotion of the high expression of exogenous miR-374a in human GSCs inhibits the expression of Neuritin and cell cycle regulatory proteins
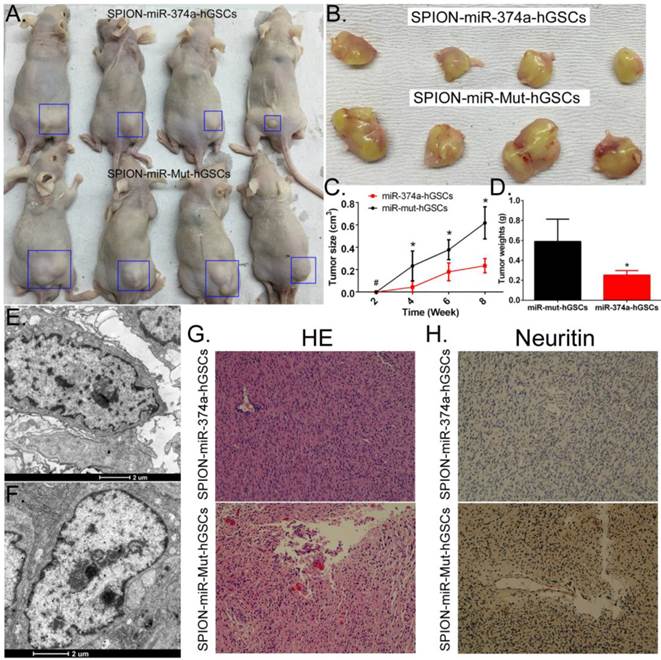
Overexpression of miR-374a inhibits the tumourigenicity of CD133+ human GSCs in nude mice
Human GSCs that received SPION-mediated transfection of the miR-374a overexpression plasmid and the miR-mut overexpression plasmid were subcutaneously inoculated into BABL/Cnu/nu mice (Figure 4A, B). The tumour volumes were observed and calculated every two weeks. The statistical results indicated that human GSCs overexpressing miR-374a had a significantly reduced tumourigenic speed, tumour growth rate, and tumour size than the tumours in the miR-mut transfection group (Figure 4C). Tumours formed by the human GSCs overexpressing miR-374a had a significantly reduced weight compared to the tumours in the miR-mut transfection group (Figure 4D). Eight weeks after cell inoculation, the animals were sacrificed to collect the tumours. The tumour weight was calculated followed by a histopathological examination. TEM showed the presence of multiple circular high-density electron clouds with a size of 80 nm-100 nm in tumours formed by either group of cells (Figure 4E, F) that presumably corresponded to the SPION substance. Although the two groups of cells were both capable of forming tumour tissues similar to the human glioma from which the primary cells were derived, HE staining showed that the tumours formed by GSCs overexpressing miR-374a had a lower level of tumour cell malignancy than the miR-mut transfection group (Figure 4G). The immunohistochemical staining results also showed that the Neuritin protein level was significantly lower in the tumours formed by the GSCs in the miR-374a overexpression group than in the miR-mut transfection group (Figure 4H). The above experiments confirmed that miR-374a overexpression suppressed the growth and tumour-forming ability of CD133+ human GSCs in nude mice.
Overexpression of miR-374a significantly inhibits the expression of Neuritin and cell cycle regulatory proteins in CD133+ human GSCs. (A) The result of Northern blotting. (B) The qRT-PCR results showed that the NRN1, Ccnd1, and Cdk4 mRNA expression levels in SPION-mediated miR-374a transfection group were also significantly lower than the control group. **p<0.01 vs. Untransfected, #p>0.05 vs. Untransfected, t test, n=3. (C) Western blotting results showed that the Neuritin, CCND1 and CDK4 protein expression levels in SPION-mediated miR-374a transfection group were significantly lower than the control group. *p<0.05 vs. Untransfected, #p>0.05 vs. Untransfected, t test, n=3.
Overexpression of miR-374a significantly inhibits the tumourigenicity of CD133+ human GSCs. (A) CD133+ human GSCs overexpressing miR-374a or miR-mut formed tumours in nude mice. Blue boxes indicate the location of the tumour. (B) Glioma tumour tissues in nude mice. (C) CD133+ human GSCs overexpressing miR-374a had significantly reduced tumourigenic speed, tumour growth rate, and tumour size. *p<0.05 vs. miR-mut-hGSCs, # p>0.05 vs. miR-mut-hGSCs, t test, n = 4. (D) Tumours formed by the CD133+ human GSCs overexpressing miR-374a had significantly reduced weights compared to the tumours in the miR-mut transfection group. *p<0.05 vs. miR-mut transfection human GSCs, t test, n = 4. (E) and (F) TEM showed the presence of multiple circular high-density electron clouds of SPION in tumours formed by SPION-mediated miR-374a transfection GSCs or SPION-mediated miR-mut transfection GSCs. (G) HE staining showed glioma cell morphology (original magnification, x200). (H) Immunohistochemical staining showed that the expression level of the Neuritin protein was significantly lower in tumours formed by GSCs of the miR-374a overexpression group than those formed by the miR-mut transfection group (original magnification, x200).
Discussion
Neurotrophic factor (NT) plays a key role in nervous system development [19-21]. Nerve growth factor (NGF) is a soluble neurotrophic peptide that promotes not only the growth of neurons but also the development of synapses and sympathetic neurons [19-21]. The NT family includes three structurally similar members: brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5) [19-21]. Neuritin is a new member of the NT family encoded by the Nrn1 gene that can activate rat hippocampal neurons [19-21]. Moreover, its high expression in the central nervous system can promote neuronal proliferation, axon growth and synapse maturation while inhibiting neuronal apoptosis [19-21]. Neuritin can regulate nerve growth and axon development and maturation as part of the extracellular matrix, suggesting that it is involved in the regulation of nervous system plasticity [19-21]. Additionally, the expression of Neuritin in the optic nerve has been shown to be induced by photic stimulation [19-21]. It is possible that Neuritin is a downstream factor of BDNF that is regulated by BDNF to play a role in NT functions [19-21]. Previous studies noted that Neuritin was highly expressed in human gliomas (especially the U251 cell line), suggesting that Neuritin played a significant role in promoting glioma proliferation and was positively correlated with the degree of malignancy of tumours [19-21]. Moreover, some studies have indicated that Neuritin also acts as an angiogenic factor in addition to its NT function and can efficiently promote tumour angiogenesis [19-21]. Neuritin has also been found to be highly expressed in breast cancer, Kaposi's sarcoma and other tissues in addition to the nervous system and is closely related to apoptosis and tumourigenesis [19-21].
Nanoparticles refer to nano-scale microscopic particles. When a substance has a scale of less than 100 nm in one or more dimensions, this particulate substance can be referred to as a nanoparticle. Because nanoparticles are very small, they not only easily pass the cell membrane and penetrate into the organelle but also spread throughout the entire organ or the body along neuronal synapses, axons and blood and lymphatic vessels. Moreover, nanoparticles can selectively deposit in specific cells or specific organelles in a cell. The special affinity of nanoparticles for tissues and cells makes it possible for nanoparticles to be used as effective drug delivery carriers. Currently, many nanoparticles are man-made particulate materials in the 50-100 nm scale level that adopt the morphologies of emulsoids, polymers, ceramic particles, metallic particles, liposomes, and carbon particles. Nanoparticles are widely used in the development of drug release systems and drug delivery systems. Nanoparticles have important scientific and practical value and function as a bridge between macroscopic matter and microscopic substances such as atoms and molecules.
In previous studies, liposomes were often used as the carrier to mediate the transfection of plasmid DNA into cells by taking into account the structure of the phospholipid bilayer of eukaryotic cell membranes. Despite many years of application, the transfection efficiency of liposomes is not very high. With increasing amounts of nanomaterial research, a number of studies have reported that nanoparticles can be used to mediate the delivery of drugs or nucleic acids into cells and can improve the transfection efficiency. In this study, we used a special class of nanomaterials (SPION) to mediate the transfection of nucleic acid into cells. Our results showed that SPION could successfully deliver the plasmid expressing miR-374a into the nuclei of GSCs and induce the normal transcription and expression of miR-374a. The high expression level of miR-374a effectively silenced Neuritin gene expression, thereby inhibiting the in vitro and in vivo proliferation and invasion of GSCs and ultimately significantly reducing GSC tumourigenicity.
In summary, SPION is a substance with a great potential to mediate the transfection of nucleic acids into mammalian cells. SPION can effectively mediate miR-374a expression in human GSCs and inhibit the proliferation, invasiveness and tumourigenicity of human GSCs.
Acknowledgements
This work was supported by grant from Shanghai Natural Science Foundation (No. 16ZR1434000), and National Natural Science Foundation of China (No. 81202811, 81571197), and Project funded by China Postdoctoral Science Foundation (No. 2014M550250, 2015T80455). We declared no potential conflicts of interest.
Competing Interests
The authors declare no conflict of interest.
References
1. Balasubramaniyan V, Vaillant B, Wang S, Gumin J, Butalid ME, Sai K. et al. Aberrant mesenchymal differentiation of glioma stem-like cells: implications for therapeutic targeting. Oncotarget. 2015;6:31007-17
2. Zheng J, Li XD, Wang P, Liu XB, Xue YX, Hu Y. et al. CRNDE affects the malignant biological characteristics of human glioma stem cells by negatively regulating miR-186. Oncotarget. 2015;6:25339-55
3. Yin J, Park G, Kim TH, Hong JH, Kim YJ, Jin X. et al. Pigment Epithelium-Derived Factor (PEDF) Expression Induced by EGFRvIII Promotes Self-renewal and Tumor Progression of Glioma Stem Cells. PLoS biology. 2015;13:e1002152
4. Hjelmeland AB, Wu Q, Wickman S, Eyler C, Heddleston J, Shi Q. et al. Targeting A20 decreases glioma stem cell survival and tumor growth. PLoS biology. 2010;8:e1000319
5. Cheng CJ, Bahal R, Babar IA, Pincus Z, Barrera F, Liu C. et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107-10
6. Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC. et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100-4
7. He S, Su H, Liu C, Skogerbo G, He H, He D. et al. MicroRNA-encoding long non-coding RNAs. BMC genomics. 2008;9:236
8. Cheng W, Liu T, Wan X, Gao Y, Wang H. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. The FEBS journal. 2012;279:2047-59
9. Gao Y, Liu T, Huang Y. MicroRNA-134 suppresses endometrial cancer stem cells by targeting POGLUT1 and Notch pathway proteins. FEBS letters. 2015;589:207-14
10. Liu T, Hou L, Huang Y. EZH2-specific microRNA-98 inhibits human ovarian cancer stem cell proliferation via regulating the pRb-E2F pathway. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:7239-47
11. Liu T, Huang Y, Liu J, Zhao Y, Jiang L, Huang Q. et al. MicroRNA-122 influences the development of sperm abnormalities from human induced pluripotent stem cells by regulating TNP2 expression. Stem cells and development. 2013;22:1839-50
12. Liu T, Shen D, Xing S, Chen J, Yu Z, Wang J. et al. Attenuation of exogenous angiotensin II stress-induced damage and apoptosis in human vascular endothelial cells via microRNA-155 expression. International journal of molecular medicine. 2013;31:188-96
13. Liu T, Tang H, Lang Y, Liu M, Li X. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer letters. 2009;273:233-42
14. Zhang L, Liu T, Huang Y, Liu J. microRNA-182 inhibits the proliferation and invasion of human lung adenocarcinoma cells through its effect on human cortical actin-associated protein. Int J Mol Med. 2011;28:381-8
15. Wang Y, Cui H, Li K, Sun C, Du W, Cui J. et al. A magnetic nanoparticle-based multiple-gene delivery system for transfection of porcine kidney cells. PloS one. 2014;9:e102886
16. Lo YL, Chou HL, Liao ZX, Huang SJ, Ke JH, Liu YS. et al. Chondroitin sulfate-polyethylenimine copolymer-coated superparamagnetic iron oxide nanoparticles as an efficient magneto-gene carrier for microRNA-encoding plasmid DNA delivery. Nanoscale. 2015;7:8554-65
17. Delyagina E, Schade A, Scharfenberg D, Skorska A, Lux C, Li W. et al. Improved transfection in human mesenchymal stem cells: effective intracellular release of pDNA by magnetic polyplexes. Nanomedicine. 2014;9:999-1017
18. Wang Y, Cui H, Sun C, Du W, Cui J, Zhao X. Study on performance of magnetic fluorescent nanoparticles as gene carrier and location in pig kidney cells. Nanoscale research letters. 2013;8:127
19. Schiffer D, Annovazzi L, Caldera V, Mellai M. On the origin and growth of gliomas. Anticancer research. 2010;30:1977-98
20. Feng YA, Liu TE, Wu Y. microRNA-182 inhibits the proliferation and migration of glioma cells through the induction of neuritin expression. Oncology letters. 2015;10:1197-203
21. Grimm SA, Chamberlain MC. Brainstem glioma: a review. Current neurology and neuroscience reports. 2013;13:346
Author contact
![]() Corresponding author: Te Liu Ph.D., Shanghai Tenth People's Hospital, Medical School, Tongji University, Shanghai, China, 200072, Phone: 86-21-66300588; Fax: 86-21-66300588; E-Mail: teliu79com.
Corresponding author: Te Liu Ph.D., Shanghai Tenth People's Hospital, Medical School, Tongji University, Shanghai, China, 200072, Phone: 86-21-66300588; Fax: 86-21-66300588; E-Mail: teliu79com.

 Global reach, higher impact
Global reach, higher impact