Impact Factor
ISSN: 1837-9664
J Cancer 2016; 7(11):1571-1578. doi:10.7150/jca.15309 This issue Cite
Review
Effects of Animal Venoms and Toxins on Hallmarks of Cancer
1. Department of Pharmacology, Phramongkutklao College of Medicine, Bangkok 10400, Thailand.
2. Monash Venom Group, Department of Pharmacology, Biomedical Discovery Institute, Monash University, Clayton, VIC 3800, Australia.
3. Department of Biochemistry & Molecular Biology, Biomedical Discovery Institute, Monash University, Clayton, VIC 3800, Australia.
4. Division of Medical Oncology, Department of Medicine, Phramongkutklao Hospital, Bangkok 10400, Thailand.
Received 2016-2-18; Accepted 2016-5-18; Published 2016-7-15
Abstract
Animal venoms are a cocktail of proteins and peptides, targeting vital physiological processes. Venoms have evolved to assist in the capture and digestion of prey. Key venom components often include neurotoxins, myotoxins, cardiotoxins, hematoxins and catalytic enzymes. The pharmacological activities of venom components have been investigated as a source of potential therapeutic agents. Interestingly, a number of animal toxins display profound anticancer effects. These include toxins purified from snake, bee and scorpion venoms effecting cancer cell proliferation, migration, invasion, apoptotic activity and neovascularization. Indeed, the mechanism behind the anticancer effect of certain toxins is similar to that of agents currently used in chemotherapy. For example, Lebein is a snake venom disintegrin which generates anti-angiogenic effects by inhibiting vascular endothelial growth factors (VEGF). In this review article, we highlight the biological activities of animal toxins on the multiple steps of tumour formation or hallmarks of cancer. We also discuss recent progress in the discovery of lead compounds for anticancer drug development from venom components.
Keywords: anticancer, apoptosis, animal venoms, cytotoxicity, Hallmarks of cancer.
Introduction
Humans have been using natural substances for their therapeutic properties since the beginning of civilization. Toxins from venomous animals are selective and potent to vital physiological processes in prey animals. As a result, venoms are a veritable mixture of biologically active components, including neurotoxins (i.e. toxins which target the transmission of nerve impulses), myotoxins (i.e. toxins which attack and break down muscle tissue), enzymes and substances which induce pain (e.g. ion channel toxins). Therefore, it is not surprising that research focussed on identifying lead compounds for the development of new therapeutics has long included a focus on animal venoms. Despite this intensive focus, successful transition of venom components to therapeutic agents has been only moderately successful. Examples of therapeutics developed from venoms include captopril (Bothrops jararaca), exenatide (Heloderma suspectum) and ziconotide (Conus magus). Advanced cancer is a deadly aggressive disease and remains a significant cause of mortality worldwide [1]. Cancer cells are capable of growing (Figure 1) and metastasizing to other regions causing a number of devastating outcomes [2]. Certain snake and arthropod venoms have highly selective toxicity on a variety of cancer cells (Table 1) and have also been used as additional treatments in cancer therapy. For example, the administration of crotoxin (a neurotoxin isolated from Crotalus durissus terrificus venom) to advanced cancer patients was reported to minimize the consumption of analgesics indicating an analgesic effect of this neurotoxin [3]. Furthermore, sweet bee venom is applied in pharmacopuncture for patients with chemotherapy-induced peripheral neuropathy [4]. In fact, many venom components such as phospholipase A2, L-amino acid oxidase, disintegrins, melittin, polybia-MPI and chlorotoxin have profound effects on cancer development. Therefore, the use of animal toxins with the capacity to exert anticancer effects through multiple mechanisms (Table 2) may be an alternative tool in clinical approaches. This review article examines recent progress in the search for lead compounds for anticancer drug development from the venoms of reptiles and/or arthropods and also shows that each venom component is capable of inhibiting the multiple steps involved in cancer progression or hallmarks of cancer by mechanisms involving cell proliferation, interruption in cell cycle or apoptosis and neovascularization (Figure 2).
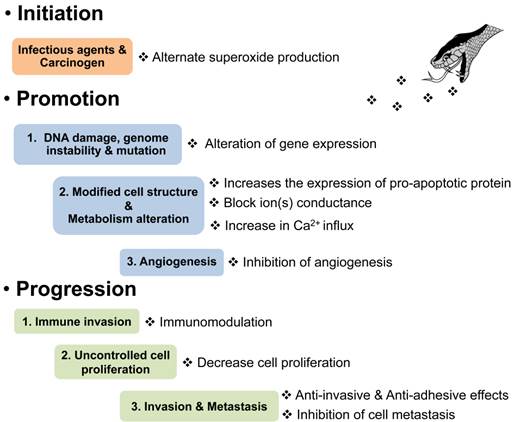
Effects of animal venoms or toxins on the pathophysiology of cancer development. ❖is indicated venom or toxin.
Anticancer mechanisms of venom peptides from snake, bee, wasp and scorpion on several types of cancer cells.
| Toxins/Venoms | Toxin name | Anticancer Mechanisms | Type of Cancer cells |
|---|---|---|---|
| svPLA2s | CC-PLA2-1 & CC-PLA2-2 | Inhibition of cell adhesion and migration to fibrinogen and fibronectin | Brain microvascular endothelial cells (HBMECs) [11] |
| MVL-PLA2 | Anti-angiogenic effects Increase microtubule dynamicity | microvascular endothelial cells (HMEC-1) [13] | |
| Cytotoxins or Cardiotoxins | CTX-I & CTX-II | Apoptotic effect via lysosomal pathway | Breast cancer cell (MCF-7) |
| Hepatocellular carcinoma (HepG2) | |||
| Prostate cancer (DU145) | |||
| Human promyelocytic leukemia cell (HL-60) [18] | |||
| Cardiotoxin III | Inhibition of the HGF-induced invasion and migration via HGF/c-Met-dependent PI3K/Akt, ERK1/2 and NF-κB signalling pathway | Human breast cancer (MDA-MB-231) cells [19] | |
| Metalloproteinases | Jararhagin | Inhibition of cancer cell proliferation | B16F10 murine melanoma cells [22] |
| Increase in apoptosis | |||
| Increase in caspase-3 | |||
| svLAAOs | OH-LAAO | Apoptosis by oxidative reaction of H2O2 causing alteration of gene expression | Prostate adenocarcinoma (PC-3 cells) [29] |
| Rusvinoxidase | Apoptosis with DNA fragmentation via the activation of caspase-8 and caspase-7 | MCF-7 [30] | |
| Disintegrins | Obtustatin | Specific binding to α1β1 integrin causing the inhibition of tumour cells adhesion and migration | S-180 sarcoma [76] |
| Rhodostomin | Anti-inflammatory effect by blocking the adhesion of activated neutrophils to fibrinogen and attenuating superoxide production | Neutrophils [45] | |
| Lebein | Inhibition of VEGF-induced neovascularization | Human colon cancer cells [43] | |
| Bee venom | Melittin | Apoptosis via mitochondrial pathway | Human gastric cancer cell (SGC-7901) [77] |
| Activation of L-type Ca2+ channel | Human MG63 osteosarcoma cells [50] | ||
| Inhibition of inflammatory mediators involved in the MAPK signalling pathway | Lewis lung cancer cell (VEGF-A-hm LLC ) [53] | ||
| Wasp venom | Polybia-MPI | Pore formation | Prostate and bladder cancer cells [59] |
| Scorpion venom | Chlorotoxin | Inhibition of Cl- conductance that reduces cancer progression | Pancreatic cancer cells (PANC-1) [66] |
| Inhibition of cancer cells migration and invasion via binding activity to matrix metalloproteinase-2 (MMP-2) | |||
| Bengalin | Activated caspase 9, caspase 3 and induced poly (ADP-ribose) polymerase (PARP) cleavage | Human leukemic cells (U937) and chronic myelogenous leukemia (K562) [75] |
Comparison of the mechanism(s) behind the anticancer effect(s) of certain animal venoms and toxins.
| Animals | Species | Name(s)/Venom components or product | Cellular mechanism(s) | Targeted Hallmarks of cancer | |
|---|---|---|---|---|---|
| Bee | Apis mellifera | Melittin, pore-forming peptide | Blocks VEGFR2 and the cyclooxygenase-2 (COX-2) mediated MAPK (mitogen-activated protein kinase) signaling pathway [53] | Tumour-promoting inflammation | |
| bv-sPLA2 | Blockade of epidermal growth factor (EGF)-induced signalling [78] | Sustaining proliferative signalling | |||
| Immune activation [49] | |||||
| Avoiding immune destruction | |||||
| Apamin, neurotoxin | Inhibition of vascular smooth muscle cell proliferation and migration by the regulation of Cyclin Dependent Kinase (CDK) [56] | Evading growth suppressors | |||
| Propolis, Honey bee Product | Inhibits human telomerase [57] | Inhibits human telomerase | |||
| Scorpion | Heterometrus begalensis | Bengalin | Activation of poly (ADP-ribose) polymerase (PARP) cleavage [75] | Genome instability & mutation | |
| Leiurus quinquestriatus | Chlorotoxin | Binding activity to matrix metalloproteinase-2 (MMP-2) [66] | Resisting cell death | ||
| Snake | Crotalus durissus terificu | Crotoxin, svPLA2 | EGFR inhibitor [14] | Sustaining proliferative signalling | |
| Macrovipera lebetina | Lebein, Disintegrin | Inhibition of VEGF-induced neovascularization [43] | Inducing angiogenesis | ||
| Naja naja atra | Factor inhibiting glycolysis | Inhibition of glycolysis [79] | Deregulation cellular energetic | ||
| Naja naja atra | Cardiotoxin III, Cytotoxin | Inhibition of the HGF-induced invasion and migration [19] | Activating invasion & metastasis | ||
| Trimeresurus stejnegeri | TSV-DM, Metalloproteinase | Inhibition cancer cell proliferation [23] | Evading growth suppressors | ||
| Bungarus fasciatus | BF-CT, Cytotoxic protein | Initiation of cell cycle arrest through cyclin D and CDK down regulation [80] | Evading growth suppressors |
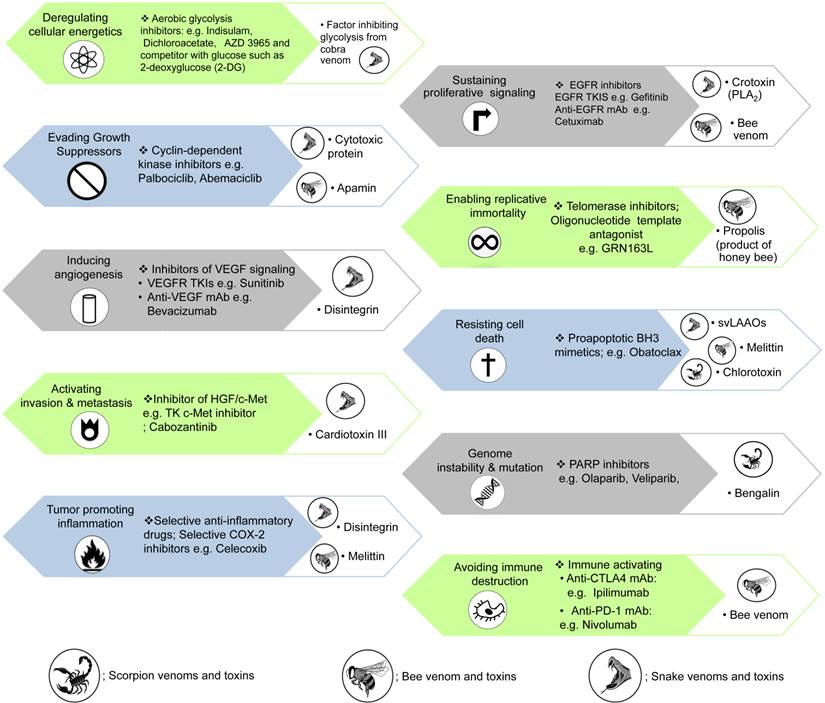
How currently available chemotherapeutic agents [2] and animal venoms/toxins act on the hallmarks of cancer.
Hallmarks of cancer
Hallmarks of cancer (Figure 2) are established to describe the biological capabilities acquired during the various steps involved in the development of cancers. The hallmarks of cancer originally included 1) sustaining proliferative signalling, 2) evading growth suppressors, 3) resisting cell death, 4) enabling replicative immortality, 5) inducing angiogenesis and 6) activating invasion and metastasis [5]. In 2011, two emerging hallmarks were proposed to be involved in cellular metabolism and avoiding immune destruction. Additionally, genome instability and mutation as well as inflammation that induces tumours were also labelled as enabling characteristics in the core and emerging hallmarks of cancer [2]. Currently, many studies and clinical trials have demonstrated agents that interrupt each of the biological capabilities necessary for tumour progression (Figure 2). Indeed, modern chemotherapy remains problematic due to a lack of cytotoxic specificity and decline in the patient quality of life from serious adverse effects, e.g. bone marrow suppression, mucositis and chemotherapy-induced peripheral neuropathy. Many animal venoms and toxins contain largely cytotoxic components affecting several kinds of tumour cells [6].
Venom Phospholipase A2s (PLA2s) and Cytotoxins
PLA2 (EC 3.1.1.4) enzymes are the most common phospholipase found in animal venoms especially in snake and bee venoms. Snake venom PLA2s (svPLA2s) contribute to a number of pharmacological outcomes including neurotoxicity, myotoxicity, anticoagulation, vascular relaxation and hypotension [7-9]. The biological activities of PLA2 may be produced either as a consequence of enzymatic activity of PLA2 or as a consequence of direct interaction of the PLA2 enzyme with its ligand without any catalytic activation [10]. The hydrolytic activity of the PLA2 enzyme at the phospholipid membrane induces the release of lysophospholipids (LysoPL) and fatty acids (FAs) resulting in the pharmacological effects within cells such as membrane damage, disruption of membrane-bound protein and functional disturbances of the cellular cascade [10]. These mechanisms can exert antitumour effects. Previous studies have shown that purified svPLA2s contain antitumour and anti-angiogenic properties [6]. CC-PLA2-1 and CC-PLA2-2, two secreted PLA2s purified from Cerastes cerastes venom, caused dose-dependent inhibition of human brain microvascular endothelial cell adhesion and migration [11]. 'MVL-PLA2', an acidic Asp49 PLA2 from Macrovipera lebetina venom exhibited anti-integrin activity by the inhibition of adhesion and migration of human tumour and microvascular endothelial cells [12, 13]. Crotoxin was reported to display anti-proliferative activity through the regulation of epidermal growth factor receptor (EGFR) on the squamous carcinoma cells [14]. Abolition of enzymatic activity by 4-BPB (p-bromophenacyl-bromide) failed to attenuate the antitumour effects, indicating the antitumour effect is independent of the catalytic property of venom PLA2 [12, 15]. Many studies have shown that the enzymatic activity of venom PLA2 is not responsible for the cytotoxic and myotoxic activities, and other mechanisms such as interaction with a specific receptor may be involved [10]. The antitumour effects of an acidic PLA2 from the venom of Bothrops jararacussu, BthA-I-PLA2, was thought to induce apoptosis in breast adenocarcinoma, human leukemia T and Erlich ascetic tumour cell lines [16]. Additionally, cytotoxins or cardiotoxins from elapid venoms also induce membrane damage and necrotic cell death. These effects are mediated via the direct interaction of these toxins with cell membrane phospholipids resulting in membrane pore formation [17]. Two cobra venom cytotoxins, CTX-I and CTX-II, possess significant anticancer activity mediated via a lysosomal pathway and entry of cathepsin to cytosol with a minimum effect on normal cells [18]. Moreover, cardiotoxin III, isolated from Naja naja atra venom also shows anti-invasive and antimetastatic activities via the suppression of hepatocyte growth factor (HGF) induced c-Met phosphorylation in human breast cancer cells [19].
Snake Venom Metalloproteinases (SVMPs)
Metalloproteinases are major components in venoms of snakes of the families Crotilidae and Viperidae [20]. SVMPs cause coagulation factor activation, inhibition of platelet aggregation as well as hemorrhagic and fibrinolytic activities [20]. Anticancer activities of SVMPs involve pro-inflammatory effect and apoptotic activity. A purified SVMP from Bothrops jararaca, Jararhagin, interrupted cancer cell adhesion and exhibited a potent cytotoxic effect on melanoma cells [21] via an increase in caspase-3 and anti-proliferative activities [22]. A metalloproteinase from Trimeresurus stejnegeri venom, TSV-DM, inhibited cancer cell proliferation and induced morphological changes on ECV304 cells [23].
Snake Venom L-Amino Acid Oxidases (svLAAOs)
LAAOs are flavorenzymes found in several organisms. These enzymes catalytically deaminate L-amino acid to the corresponding α-keto acid with the concomitant release of ammonia (NH3) and hydrogen peroxide (H2O2) [24]. Snakes of families Viperidae, Crotalidae and Elapidae contain 1-9% LAAOs in their venoms [25]. Most svLAAOs contribute to a number of pharmacologic effects including platelet aggregation, antimicrobial activity, edema, hemolytic or hemorrhage effects, cytotoxicity and apoptosis [25, 26]. Previous research has postulated that LAAOs initiate cell death or apoptotic activity by oxidative reaction of H2O2 causing alteration of gene expression [27]. Accumulated oxidative stress from H2O2 in the cell surface then triggers apoptosis, which can be abolished by antioxidants, catalase or specific H2O2 scavengers [25, 28] resulting in the inhibition of svLAAO-induced apoptosis. Mechanisms behind svLAAO-induced apoptosis of cancer cells may involve the inhibition of metastasis in the host cells by preventing platelet aggregation and activation of phagocytic cells including the interruption of tumour cell adhesion [6, 81]. 'OH-LAAO', a purified L-amino acid oxidase from king cobra (Ophiophagus hannah) venom displays a specific antitumour activity on human breast adenocarcinoma cell lines by inducing cell apoptosis via both intrinsic and extrinsic pathways, which can be indicated by the enhancement of caspase-8 and -9 activities. OH-LAAO also induced the alteration of gene expression in 178 genes for which at least 27 genes may be involved in the cytotoxic and apoptotic effects [27]. The immunohistochemical staining on the PC-3 tumour xenograft section showed a marked increase of apoptotic cells among OH-LAAO-treated nude mice [29]. Rusvinoxidase, a novel LAAO isolated from the venom of Daboia russelii russelii, also caused changes in cell morphology, membrane integrity, shrinkage of cells and apoptosis with DNA fragmentation on human breast cancer (MCF-7) cells through the activation of caspase-8 that subsequently activated caspase-7 [30].
Disintegrins from snake venoms
Integrins are heterodimeric transmembrane proteins associated by non-covalent bond of α- and β-subunits (e.g. αIIbβ3, α5β1, α6β1 and αvβ5). Integrins play many important roles to promote major mechanisms of tumour development including cell-extracellular matrix interaction, cytoskeleton organization, signalling transduction, epithelial cell adhesion, growth, proliferation, invasion and migration [31]. Disintegrins (MW 5-10 kDa) are a family of nontoxic and non-enzymatic RGD-containing peptides (Arg-Gly-Asp sequence) in the venoms of vipers, crotalids, elapids, and colubrids [32]. Peptides containing the RGD domain (disintegrins and metalloproteinases) were proven to have antitumour effects involving angiogenesis and cancer metastatic dissemination both in vitro and in vivo [33]. A number of purified disintegrins have been demonstrated to have potential interaction with integrins and to abolish cancer development [34]. Mechanisms behind antitumour activity of disintegrin include inhibition of cell adhesion, attenuation of cancer cell migration, invasion and antimetastatic activity [34, 35]. Jerdostatin, a purified disintegrin from Protobothrops jerdonii venom [36], and obtustatin, a disintegrin from Vipera lebetina obtuse, were found to bind to α1β1 causing blockages of adhesion and cell migration in in vitro studies including inhibition of angiogenesis in the in vivo experiments [34, 37]. Contortrostatin is a disintegrin from Agkistrodon contortrix contortrix was reported to display a potential binding activity on many types of integrins, e.g. αvβ3, α5β1, αIIbβ3 and αvβ5 [34] causing the interruption of tumour growth in vitro and blockage of metastasis and neovascularization in vivo [38, 39]. In addition, recombinant contortrostatin initiated and displayed antitumour and antiangiogenic activities on a breast carcinoma model following administration by liposomal encapsulation [40]. The purified disintegrin from a Korean snake (Agkistrodon halys brevicaudus) venom, 'salmosin', inhibited cell proliferation and also induced cell cycle arrest resulting in active apoptosis [41]. A previous animal study showed that salmosin was able to inhibit tumour-induced angiogenesis without toxic activity on normal function of pre-existing blood vessels [42]. Lebein, a heterodimeric disintegrin isolated from Macrovipera lebetina venom was recently reported to have anti-angiogenic activity through the inhibition of Vascular Endothelial Growth Factor (VEGF)-induced neovascularization [43]. Major limitations in translating the possible use of disintegrins into the clinic are their instability and limited bioavailability. However, administration of salmosin by integrating the salmosin gene complex into cationic liposomes has produced promising results [44]. This approach has not only shown that salmosin can exert antiangiogenic activity but also demonstrates the feasibility of this approach in other anticancer gene therapies [44]. Furthermore, anti-inflammatory effects are likely to be important mechanisms behind the anticancer effects of disintegrin. This effect has been investigated where purified disintegrin, 'rhodostomin', from the venom of Calloselasma rhodostoma displayed anti-inflammatory effects by blocking the adhesion of activated neutrophils to fibrinogen and decreasing superoxide production [45].
Bee and wasp venoms
Phospholipases, hyaluronidases and mastoparans are found in bee and wasp venoms [46]. Bee venom induces membrane lysis and inhibits tumour cell proliferation, and also promotes cancer cell apoptosis by an increase in reactive oxygen species (ROS) and a rise in intracellular Ca2+. Previous studies showed that bee venom also evoked the attenuation of mitochondrial membrane potential, which activates the release of cytochrome C and caspase-3, resulting in DNA fragmentation [47, 48]. Moreover, bee venom secretory PLA2 (bv-sPLA2) and phosphatidylinositol-(3,4)-bisphosphate synergistically inhibited cell proliferation by a mechanism involving the maturation of immunostimulatory human monocyte-derived dendritic cells [49]. Melittin, a pore-forming peptide from Apis mellifera (Western honey bee) venom exerts neoplastic activity on many types of cancer cell lines. In human osteosarcoma cells, melittin (> 0.075 µM) induced apoptosis by activating the L-type Ca2+ channel leading to an increase in intracellular Ca2+ which was fully abolished by pre-treatment with a Ca2+ chelating agent, BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetra-acetic acid) [50]. Anti-proliferative effects of melittin proportionally involve the inhibition of calmodulin binding activity, which appears to be a major intracellular target [51]. Many in vivo studies have demonstrated that administration of melittin is capable of minimizing tumour size suggesting the presence of antitumour activity in this peptide [52, 53]. Melittin significantly inhibited tumour cell metastasis and tumour cell growth in nude mice by reducing cell motility and migration by the suppression of the Rac1-dependent pathway [54]. These data suggest that bee venom and melittin could be of potential use in cancer treatment. However, reports have indicated that administration of melittin in high doses may cause hepatic injury and haemolysis [51]. Recently, vascular endothelial growth factor-melittin fusion protein (VEGF165-melittin fusion protein) displayed inhibitory effects on the development of human hepatocellular carcinoma cell lines and inhibited tumour growth in vivo [55]. In addition, apamin, a neurotoxin from bee venom inhibited vascular smooth muscle cell proliferation and migration by the regulation of Cyclin Dependent Kinase (CDK) [56]. Besides melittin, apamin and bee venom, 'propolis', a product of honey bees also produces inhibitory effects on human telomerase leading to failed leukemic cell growth [57]. Furthermore, the venom of the Brazilian wasp (Polybia paulista) displayed cytotoxic, genotoxic and mutagenic activities on human cancer cells [58]. These activities replicate the effect of phospholipases, mastoparan and hyaluronidase on cell membrane function and permeability causing the interaction between DNA and other toxic compounds [58]. An antimicrobial agent purified from Brazilian wasp venom, 'polybia-MPI' or MPI including its modified analogue 'MRI-1', significantly inhibited tumour growth in vitro and in vivo via pore formation [59, 60].
Scorpion venoms including purified or synthetic toxins from scorpion venoms
Envenoming by scorpions causes a broad range of symptoms, from local pain to deadly symptoms [61]. However, the scorpion's body parts and venoms have been used as traditional medicine for many diseases. Scorpion venoms contain mucopolysaccarides, free amino acids, phospholipases, hyaluronidases, amines and nucleotides [61]. The non-disulfide-bridged scorpion venom peptide has become a crucial substance to initiate pharmacologic activities including antibacterial, antifungal, antimalarial, antiviral and anticancer activities [62, 63]. Scorpion venom and toxins exhibit antitumour activities both in cell lines and animal models [46]. Specific interaction with ion channels seems to be the major mechanism behind anticancer effects of scorpion toxins. Chlorotoxin (ClTx), from the venom of Leiurus quinquestriatus, and its synthetic compound, 'TM601', inhibited cancer progression by the attenuation of Cl- conductance and angiogenesis [61]. ClTx blocked migration of glioma cells [64] and inhibited aggressive metastatic breast cancer cells [65]. In pancreatic cancer cells (PANC-1), ClTx inhibited cell migration and invasion by binding activity to the matrix metalloproteinase-2 (MMP-2) [66]. Additionally, ClTx promoted efficacy and increased bioavailability of certain clinical chemotherapies, e.g., doxorubicin [67] and cisplatin [68]. Moreover, gene encoded peptides such as Bm-12 [69] and Gst-BmKCT [70] also reduced Cl- conductance and metastasis of glioma cells in both in vitro and in vivo studies. The potassium channel blocking peptide Margatoxin (MgTx), from the venom of Centruroides margaritatus blocked human lung adenocarcinoma cell proliferation and decreased tumour volume in mice [71]. The calcium-activated potassium channel (BKca-channel) blockers, 'Iberiotoxin' (IbTx) and 'Charybdotoxin' (ChTx) were also reported to inhibit the proliferation and migration of glioma [72] and melanoma cells [73], respectively. Furthermore, anticancer effects of Heterometrus bengalensis venom and its purified substance 'Bengalin' were reported to decrease cancer cell proliferation and enhance apoptosis in leukemic cell lines [74] by the activation of caspase-9, caspase-3 and induced poly (ADP-ribose) polymerase (PARP) cleavage [75].
Conclusion
The clinical use of many chemotherapeutic agents can induce a number of significant adverse effects leading to unsatisfactory clinical outcomes. In addition, it has been reported that application of specifically targeted cancer therapy may promote cancer development by residual functional capabilities and lead to the adaptation or resistance of tumour cells to chemotherapy. As a result of these, anticancer agents from natural resources appear to be promising tools for cancer therapy. This review demonstrates that animal venom peptides and certain animal products exert antitumour effects by interfering with multiple processes or hallmarks of tumour development. Venom peptides, especially svLAAOs, disintegrins or melittin are anticipated to prevent adaptive resistance caused by specific inhibition of targeted therapy, potentially involving mutation or remodelling of cancer growth. Although a number of venom components have been shown to have a promising effect on tumour development, further pre-clinical and clinical studies are required to prove the efficacy and safety of these anticancer peptides. We envisage that the natural potential of these toxins can be exploited to produce clinically useful agents, as well as diagnostic tools for cancer assessment.
Acknowledgements
The authors wish to acknowledge the Office of Research Development, Phramongkutklao Collage of Medicine & Phramongkutklao Hospital (ORD, PCM & PMK) for a Publication grant.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359-86
2. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
3. Cura JE, Blanzaco DP, Brisson C, Cura MA, Cabrol R, Larrateguy L. et al. Phase I and pharmacokinetics study of crotoxin (cytotoxic PLA2, NSC-624244) in patients with advanced cancer. Clin Cancer Res. 2002;8:1033-41
4. Yoon J, Jeon J-H, Lee Y-W, Cho C-K, Kwon K-R, Shin J-E. et al. Sweet bee venom pharmacopuncture for chemotherapy-induced peripheral neuropathy. J Acupunct Meridian Stud. 2012;5:156-65
5. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70
6. Calderon LA, Sobrinho JC, Zaqueo KD, de Moura AA, Grabner AN, Mazzi MV. et al. Antitumoral activity of snake venom proteins: new trends in cancer therapy. BioMed Res Int. 2014;2014:203639
7. Kuruppu S, Smith AI, Isbister GK, Hodgson WC. Neurotoxins from Australo-Papuan elapids: a biochemical and pharmacological perspective. Crit Rev Toxicol. 2008;38:73-86
8. Chaisakul J, Isbister GK, Tare M, Parkington HC, Hodgson WC. Hypotensive and vascular relaxant effects of phospholipase A2 toxins from Papuan taipan (Oxyuranus scutellatus) venom. Eur J Pharmacol. 2014;723:227-33
9. Hart AJ, Smith AI, Reeve S, Hodgson WC. Isolation and characterisation of acanmyotoxin-2 and acanmyotoxin-3, myotoxins from the venom of the death adder Acanthophis sp. Seram. Biochem Pharmacol. 2005;70:1807-13
10. Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42:827-40
11. Kessentini-Zouari R, Jebali J, Taboubi S, Srairi-Abid N, Morjen M, Kallech-Ziri O. et al. CC-PLA2-1 and CC-PLA2-2, two Cerastes cerastes venom-derived phospholipases A2, inhibit angiogenesis both in vitro and in vivo. Lab Invest. 2010;90:510-9
12. Bazaa A, Luis J, Srairi-Abid N, Kallech-Ziri O, Kessentini-Zouari R, Defilles C. et al. MVL-PLA2, a phospholipase A2 from Macrovipera lebetina transmediterranea venom, inhibits tumor cells adhesion and migration. Matrix Biol. 2009;28:188-93
13. Bazaa A, Pasquier E, Defilles C, Limam I, Kessentini-Zouari R, Kallech-Ziri O. et al. MVL-PLA2, a snake venom phospholipase A2, inhibits angiogenesis through an increase in microtubule dynamics and disorganization of focal adhesions. PloS One. 2010;5:e10124
14. Donato NJ, Martin CA, Perez M, Newman RA, Vidal JC, Etcheverry M. Regulation of epidermal growth factor receptor activity by crotoxin, a snake venom phospholipase A2 toxin. A novel growth inhibitory mechanism. Biochem Pharmacol. 1996;51:1535-43
15. Murakami T, Kamikado N, Fujimoto R, Hamaguchi K, Nakamura H, Chijiwa T. et al. A [Lys(4)(9)]phospholipase A2 from Protobothrops flavoviridis venom induces caspase-independent apoptotic cell death accompanied by rapid plasma-membrane rupture in human leukemia cells. Biosci Biotechnol Biochem. 2011;75:864-70
16. Roberto PG, Kashima S, Marcussi S, Pereira JO, Astolfi-Filho S, Nomizo A. et al. Cloning and identification of a complete cDNA coding for a bactericidal and antitumoral acidic phospholipase A2 from Bothrops jararacussu venom. Protein J. 2004;23:273-85
17. Dubovskii PV, Konshina AG, Efremov RG. Cobra cardiotoxins: membrane interactions and pharmacological potential. Curr Med Chem. 2014;21:270-87
18. Ebrahim K, Shirazi FH, Mirakabadi AZ, Vatanpour H. Cobra venom cytotoxins; apoptotic or necrotic agents? Toxicon. 2015;108:134-40
19. Tsai PC, Chu CL, Chiu CC, Chang LS, Lin SR. Cardiotoxin III suppresses hepatocyte growth factor-stimulated migration and invasion of MDA-MB-231 cells. Cell Biochem Funct. 2014;32:485-95
20. Markland FS Jr, Swenson S. Snake venom metalloproteinases. Toxicon. 2013;62:3-18
21. Correa MC Jr, Maria DA, Moura-da-Silva AM, Pizzocaro KF, Ruiz IR. Inhibition of melanoma cells tumorigenicity by the snake venom toxin jararhagin. Toxicon. 2002;40:739-48
22. Maria DA, da Silva MG, Correia Junior MC, Ruiz IR. Antiproliferative effect of the jararhagin toxin on B16F10 murine melanoma. BMC Complement Altern Med. 2014;14:446
23. Wan SG, Jin Y, Lee WH, Zhang Y. A snake venom metalloproteinase that inhibited cell proliferation and induced morphological changes of ECV304 cells. Toxicon. 2006;47:480-9
24. Ullah A, Souza TA, Abrego JR, Betzel C, Murakami MT, Arni RK. Structural insights into selectivity and cofactor binding in snake venom L-amino acid oxidases. Biochem Biophys Res Commun. 2012;421:124-8
25. Guo C, Liu S, Yao Y, Zhang Q, Sun MZ. Past decade study of snake venom L-amino acid oxidase. Toxicon. 2012;60:302-11
26. Zhang L, Cui L. A cytotoxin isolated from Agkistrodon acutus snake venom induces apoptosis via Fas pathway in A549 cells. Toxicol In Vitro. 2007;21:1095-103
27. Fung SY, Lee ML, Tan NH. Molecular mechanism of cell death induced by king cobra (Ophiophagus hannah) venom L-amino acid oxidase. Toxicon. 2015;96:38-45
28. Torii S, Yamane K, Mashima T, Haga N, Yamamoto K, Fox JW. et al. Molecular cloning and functional analysis of apoxin I, a snake venom-derived apoptosis-inducing factor with L-amino acid oxidase activity. Biochemistry. 2000;39:3197-205
29. Lee ML, Fung SY, Chung I, Pailoor J, Cheah SH, Tan NH. King cobra (Ophiophagus hannah) venom L-amino acid oxidase induces apoptosis in PC-3 cells and suppresses PC-3 solid tumor growth in a tumor xenograft mouse model. Int J Med Sci. 2014;11:593-601
30. Mukherjee AK, Saviola AJ, Burns PD, Mackessy SP. Apoptosis induction in human breast cancer (MCF-7) cells by a novel venom L-amino acid oxidase (Rusvinoxidase) is independent of its enzymatic activity and is accompanied by caspase-7 activation and reactive oxygen species production. Apoptosis. 2015;20:1358-72
31. Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269-80
32. McLane MA, Sanchez EE, Wong A, Paquette-Straub C, Perez JC. Disintegrins. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:327-55
33. Selistre-de-Araujo HS, Pontes CL, Montenegro CF, Martin AC. Snake venom disintegrins and cell migration. Toxins. 2010;2:2606-21
34. Arruda Macedo JK, Fox JW, de Souza Castro M. Disintegrins from snake venoms and their applications in cancer research and therapy. Curr Protein Pept Sci. 2015;16:532-48
35. Eble JA, Niland S, Dennes A, Schmidt-Hederich A, Bruckner P, Brunner G. Rhodocetin antagonizes stromal tumor invasion in vitro and other α2β1 integrin-mediated cell functions. Matrix Biol. 2002;21:547-58
36. Juarez P, Bolas G, de Rezende FF, Calvete JJ, Eble JA. Recombinant expression in human cells of active integrin α1β1-blocking RTS-disintegrin jerdostatin. Toxicon. 2010;56:1052-8
37. Marcinkiewicz C, Weinreb PH, Calvete JJ, Kisiel DG, Mousa SA, Tuszynski GP. et al. Obtustatin: a potent selective inhibitor of α1β1 integrin in vitro and angiogenesis in vivo. Cancer Res. 2003;63:2020-3
38. Swenson S, Costa F, Ernst W, Fujii G, Markland FS. Contortrostatin, a snake venom disintegrin with anti-angiogenic and anti-tumor activity. Pathophysiol Haemost Thromb. 2005;34:169-76
39. Golubkov V, Hawes D, Markland FS. Anti-angiogenic activity of contortrostatin, a disintegrin from Agkistrodon contortrix contortrix snake venom. Angiogenesis. 2003;6:213-24
40. Minea R, Swenson S, Costa F, Chen TC, Markland FS. Development of a novel recombinant disintegrin, contortrostatin, as an effective anti-tumor and anti-angiogenic agent. Pathophysiol Haemost Thromb. 2005;34:177-83
41. Kang IC, Kim DS, Jang Y, Chung KH. Suppressive mechanism of salmosin, a novel disintegrin in B16 melanoma cell metastasis. Biochem Biophys Res Commun. 2000;275:169-73
42. Kang IC, Lee YD, Kim DS. A novel disintegrin salmosin inhibits tumor angiogenesis. Cancer Res. 1999;59:3754-60
43. Zakraoui O, Marcinkiewicz C, Aloui Z, Othman H, Grepin R, Haoues M. et al. Lebein, a snake venom disintegrin, suppresses human colon cancer cells proliferation and tumor-induced angiogenesis through cell cycle arrest, apoptosis induction and inhibition of VEGF expression. Mol Carcinog. 2016 doi: 10.1002/mc.22470
44. Kim SI, Kim KS, Kim HS, Kim DS, Jang Y, Chung KH. et al. Inhibitory effect of the salmosin gene transferred by cationic liposomes on the progression of B16BL6 tumors. Cancer Res. 2003;63:6458-62
45. Tseng YL, Peng HC, Huang TF. Rhodostomin, a disintegrin, inhibits adhesion of neutrophils to fibrinogen and attenuates superoxide production. J Biomed Sci. 2004;11:683-91
46. Heinen TE, da Veiga AB. Arthropod venoms and cancer. Toxicon. 2011;57:497-511
47. Ip SW, Wei HC, Lin JP, Kuo HM, Liu KC, Hsu SC. et al. Bee venom induced cell cycle arrest and apoptosis in human cervical epidermoid carcinoma Ca Ski cells. Anticancer Res. 2008;28:833-42
48. Ip SW, Liao SS, Lin SY, Lin JP, Yang JS, Lin ML. et al. The role of mitochondria in bee venom-induced apoptosis in human breast cancer MCF7 cells. In Vivo. 2008;22:237-45
49. Putz T, Ramoner R, Gander H, Rahm A, Bartsch G, Thurnher M. Antitumor action and immune activation through cooperation of bee venom secretory phospholipase A2 and phosphatidylinositol-(3,4)-bisphosphate. Cancer Immunol Immunother. 2006;55:1374-83
50. Chu ST, Cheng HH, Huang CJ, Chang HC, Chi CC, Su HH. et al. Phospholipase A2-independent Ca2+ entry and subsequent apoptosis induced by melittin in human MG63 osteosarcoma cells. Life Sci. 2007;80:364-9
51. Gajski G, Garaj-Vrhovac V. Melittin: a lytic peptide with anticancer properties. Environ Toxicol Pharmacol. 2013;36:697-705
52. Qian CY, Wang KL, Fang FF, Gu W, Huang F, Wang FZ. et al. Triple-controlled oncolytic adenovirus expressing melittin to exert inhibitory efficacy on hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:10403-11
53. Huh JE, Kang JW, Nam D, Baek YH, Choi DY, Park DS. et al. Melittin suppresses VEGF-A-induced tumor growth by blocking VEGFR-2 and the COX-2-mediated MAPK signaling pathway. J Nat Prod. 2012;75:1922-9
54. Liu S, Yu M, He Y, Xiao L, Wang F, Song C. et al. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology. 2008;47:1964-73
55. Wang D, Hu L, Su M, Wang J, Xu T. Preparation and functional characterization of human vascular endothelial growth factor-melittin fusion protein with analysis of the antitumor activity in vitro and in vivo. Int J Oncol. 2015;47:1160-8
56. Kim JY, Kim KH, Lee WR, An HJ, Lee SJ, Han SM. et al. Apamin inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration through suppressions of activated Akt and Erk signaling pathway. Vascul Pharmacol. 2015;70:8-14
57. Cogulu O, Biray C, Gunduz C, Karaca E, Aksoylar S, Sorkun K. et al. Effects of Manisa propolis on telomerase activity in leukemia cells obtained from the bone marrow of leukemia patients. Int J Food Sci Nutrition. 2009;60:601-5
58. Hoshina MM, Santos LD, Palma MS, Marin-Morales MA. Cytotoxic, genotoxic/antigenotoxic and mutagenic/antimutagenic effects of the venom of the wasp Polybia paulista. Toxicon. 2013;72:64-70
59. Wang KR, Zhang BZ, Zhang W, Yan JX, Li J, Wang R. Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides. 2008;29:963-8
60. Zhang W, Li J, Liu LW, Wang KR, Song JJ, Yan JX. et al. A novel analog of antimicrobial peptide Polybia-MPI, with thioamide bond substitution, exhibits increased therapeutic efficacy against cancer and diminished toxicity in mice. Peptides. 2010;31:1832-8
61. Ortiz E, Gurrola GB, Schwartz EF, Possani LD. Scorpion venom components as potential candidates for drug development. Toxicon. 2015;93:125-35
62. Almaaytah A, Albalas Q. Scorpion venom peptides with no disulfide bridges: a review. Peptides. 2014;51:35-45
63. Harrison PL, Abdel-Rahman MA, Miller K, Strong PN. Antimicrobial peptides from scorpion venoms. Toxicon. 2014;88:115-37
64. Lui VC, Lung SS, Pu JK, Hung KN, Leung GK. Invasion of human glioma cells is regulated by multiple chloride channels including ClC-3. Anticancer Res. 2010;30:4515-24
65. Qin C, He B, Dai W, Zhang H, Wang X, Wang J. et al. Inhibition of metastatic tumor growth and metastasis via targeting metastatic breast cancer by chlorotoxin-modified liposomes. Mol Pharm. 2014;11:3233-41
66. El-Ghlban S, Kasai T, Shigehiro T, Yin HX, Sekhar S, Ida M. et al. Chlorotoxin-Fc fusion inhibits release of MMP-2 from pancreatic cancer cells. Bio Med Res Int. 2014;2014:152659
67. Xiang Y, Liang L, Wang X, Wang J, Zhang X, Zhang Q. Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J Control Release. 2011;152:402-10
68. Graf N, Mokhtari TE, Papayannopoulos IA, Lippard SJ. Platinum(IV)-chlorotoxin (CTX) conjugates for targeting cancer cells. J Inorg Biochem. 2012;110:58-63
69. Wu JJ, Dai L, Lan ZD, Chi CW. The gene cloning and sequencing of Bm-12, a chlorotoxin-like peptide from the scorpion Buthus martensi Karsch. Toxicon. 2000;38:661-8
70. Fan S, Sun Z, Jiang D, Dai C, Ma Y, Zhao Z. et al. BmKCT toxin inhibits glioma proliferation and tumor metastasis. Cancer Lett. 2010;291:158-66
71. Jang SH, Choi SY, Ryu PD, Lee SY. Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur J Pharmacol. 2011;651:26-32
72. Weaver AK, Liu X, Sontheimer H. Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J Neurosci Res. 2004;78:224-34
73. Schwab A, Reinhardt J, Schneider SW, Gassner B, Schuricht B. K+ channel-dependent migration of fibroblasts and human melanoma cells. Cell Physiol Biochem. 1999;9:126-32
74. Das Gupta S, Halder B, Gomes A. Bengalin initiates autophagic cell death through ERK-MAPK pathway following suppression of apoptosis in human leukemic U937 cells. Life Sci. 2013;93:271-6
75. Gupta SD, Gomes A, Debnath A, Saha A. Apoptosis induction in human leukemic cells by a novel protein Bengalin, isolated from Indian black scorpion venom: through mitochondrial pathway and inhibition of heat shock proteins. Chem Biol Interact. 2010;183:293-303
76. Ghazaryan NA, Ghulikyan LA, Kishmiryan AV, Kirakosyan GR, Nazaryan OH, Ghevondyan TH. et al. Anti-tumor effect investigation of obtustatin and crude Macrovipera lebetina obtusa venom in S-180 sarcoma bearing mice. Eur J Pharmacol. 2015;764:340-5
77. Kong GM, Tao WH, Diao YL, Fang PH, Wang JJ, Bo P. et al. Melittin induces human gastric cancer cell apoptosis via activation of mitochondrial pathway. World J Gastroenterol. 2016;22:3186-95
78. Putz T, Ramoner R, Gander H, Rahm A, Bartsch G, Bernardo K. et al. Bee venom secretory phospholipase A2 and phosphatidylinositol-homologues cooperatively disrupt membrane integrity, abrogate signal transduction and inhibit proliferation of renal cancer cells. Cancer Immunol Immunother. 2007;56:627-40
79. Brisbois L, Rabinovitch-Mahler N, Gillo L. Isolation from cobra venom of a factor inhibiting glycolysis in Ehrlich ascites carcinoma cells. Experientia. 1968;24:673-4
80. Bhattacharya S, Das T, Biswas A, Gomes A, Dungdung SR. A cytotoxic protein (BF-CT1) purified from Bungarus fasciatus venom acts through apoptosis, modulation of PI3K/AKT, MAPKinase pathway and cell cycle regulation. Toxicon. 2013;74:138-50
81. Markland FS. Snake venoms and the hemostatic system. Toxicon. 1998;36:1749-800
Author contact
![]() Corresponding author: Tel.: ++6691-478-8197 E-mail : naiyaratac.th.
Corresponding author: Tel.: ++6691-478-8197 E-mail : naiyaratac.th.

 Global reach, higher impact
Global reach, higher impact