Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(2):199-206. doi:10.7150/jca.16406 This issue Cite
Research Paper
11R-P53 and GM-CSF Expressing Oncolytic Adenovirus Target Cancer Stem Cells with Enhanced Synergistic Activity
1. Department of Viral and Gene Therapy Laboratory, Shanghai Eastern Heptobiliary Surgery Hospital, Shanghai, 200438, China;
2. Ningbo NO.5 Hospital (Ningbo Cancer Hospital), Ningbo 315201, China.
* These authors contributed equally to this manuscript.
Received 2016-6-7; Accepted 2016-9-18; Published 2017-1-13
Abstract
Targeting cancer stem cells with oncolytic virus (OV) holds great potential for thorough elimination of cancer cells. Based on our previous studies, we here established 11R-P53 and mGM-CSF carrying oncolytic adenovirus (OAV) SG655-mGMP and investigated its therapeutic effect on hepatocellular carcinoma stem cells Hep3B-C and teratoma stem cells ECCG5. Firstly, the augmenting effect of 11R in our construct was tested and confirmed by examining the expression of EGFP with Fluorescence and FCM assays after transfecting Hep3B-C and ECCG5 cells with OVA SG7605-EGFP and SG7605-11R-EGFP. Secondly, the expressions of 11R-P53 and GM-CSF in Hep3B-C and ECCG5 cells after transfection with OAV SG655-mGMP were detected by Western blot and Elisa assays, respectively. Thirdly, the enhanced growth inhibitory and augmented apoptosis inducing effects of OAV SG655-mGMP on Hep3B-C and ECCG5 cells were tested with FCM assays by comparing with the control, wild type 5 adenovirus, 11R-P53 carrying OVA in vitro. Lastly, the in vivo therapeutic effect of OAV SG655-mGMP toward ECCG5 cell-formed xenografts was studied by measuring tumor volumes post different treatments with PBS, OAV SG655-11R-P53, OAV SG655-mGM-CSF and OAV SG655-mGMP. Treatment with OAV SG655-mGMP induced significant xenograft growth inhibition, inflammation factor AIF1 expression and immune cells infiltration. Therefore, our OAV SG655-mGMP provides a novel platform to arm OVs to target cancer stem cells.
Keywords: OAV, cancer stem cells, P53, GM-CSF, 11R.
Introduction
Cancer is a leading cause for death worldwide, with an overall proportion of 0.56% for the 5-year prevalence in China and a total of 1,658,370 new cases in the United States in 2015 [1, 2]. It was firstly introduced in leukemia that among cancer cells there was a small portion of cancer stem/stem like/initiating cells (CSCs), which so far have been reported in various malignancies [3-5]. CSCs, capable to go through self-renewal and asymmetric division, have been proposed as a dominant reason for chemical drug resistance, through quiescence transition, drug efflux system and other mechanisms [6-10]. Thus, the existence of CSCs generates great enthusiasm to treat malignances by targeting CSCs. However, the therapeutic efficacy of targeting CSCs has been unsatisfactory due to their robustness caused by heterogeneity, plasticity and the niche of tumor tissue [11]. New weapons are still in desperate need to target CSCs.
Oncolytic virus (OV) has gained much attention due to its advantages, include specific ability to selectively replicate in malignant cells and lyse targets via multiple mechanisms, such as induction of primary cell death, interaction with antiviral elements of tumor cells, initiation of innate and adaptive anti-tumor immunity and bystander effects [12-14]. With the approval of H100 (Shanghai Sunway Biotech) and T-VEC (talimogene laherparepvec, Amgen) by the Chinese cFDA and American FDA, OV was considered as a novel immunotherapy drug for cancer treatment [15-17], and this was further verified by the study indicating the improved efficacy of CAR-T cell therapy through arming with chemokine RANTES and cytokine IL-15 expressing OV [18]. Moreover, the potential of OV in targeting both cancer cells and CSCs has been formerly demonstrated and investigated [19-24]. However, the potentials of OV, when armed with novel factors, against CSCs are still of great interests for cancer treatment.
In our previous studies, we have successfully established and characterized hepatocellular carcinoma stem cells (Hep3B-C) and teratoma stem cells (ECCG5) [25, 26]. In this study, we investigated the effect of P53 and GM-CSF expressing oncolytic adenovirus (OVA) SG655-mGMP toward Hep3B-C and ECCG5 cells with coupled expression of P53 and penetrating peptide 11R in vitro and in vivo. Results showed that OAV SG655-mGMP exhibited synergistic effect of growth suppressive effect of 11R-P53 and apoptosis inducing effect of GM-CSF. Thus, this study provides a new method to target cancer stem cells for cancer immunotherapy.
Materials and Methods
Cell culture
293 cells were purchased from the ATCC (Manassas, VA, USA), and cultured in high glucose DMEM with 10% FBS. Hepatocellular carcinoma stem cells Hep3B-C and teratoma stem cells ECCG5 were derived and cultured according to the previous study [25, 26]. All cells were incubated under 37 °C, 5% CO2 conditions.
OAV construction
Plasmids were extracted and used to transfect well-growing state 293 cells by using Polyfect transfection kit (QIAGEN, China). About 6 days post transfection, lacunas were formed, and then replicative OAV SG655-mGMPC and SG655-mGMP were purified and collected. The 11R-P53 expressing OAV SG655-11R-P53 was constructed according to the previous study [27].
Western blot analysis
Protein lysates from cells were prepared in lysis buffer and centrifuged at 12, 000 rpm at 4 °C. Western blot was performed according to a previously described procedure [28]. The primary antibodies used were mouse anti-P53 (Beyotime, China) and mouse anti-GAPDH (Beyotime, China). The secondary antibody used was HRP-labeled goat anti-mouse IgG (H+L) (Beyotime, China). The expression of each band was quantitatively analyzed using the Image Lab TM Software (Bio-Rad, USA) and normalized to the expression of GAPDH in the same lane.
CCK-8 analysis
Hep3B-C and ECCG5 cells were collected and coated in 96-well plate with 1×104 cells per well. The transfection with OAV was performed in 24 hours at MOI=10, each group were replicated in 4 wells. 20μl CCK-8 agent was added to each well in 24 hours, 48 hours and 72 hours and incubated at 37 °C for two hours. And then the absorption of 450nm was tested. Detailed procedures were conducted by using CCK-8 kit (Beytime, China).
Flowcytometry
Hep3B-C and ECCG5 cells, transfected with different OAV, and tumor cells that digested from xenografts were collected and incubated with antibodies for 30mins on ice and in dark room. Then cells were washed with PBS and centrifuged at 4°C 1500 rpm. Thereafter, cells were re-suspended with PBS in 200μl volume and analyzed with the GUAVA system (Millipore, USA). Primary antibodies used were Annexin-FITC, CD11b-FITC, Gr-1-APC, NK1.1-APC, CD4-PE, F4/80-PE, CD11c-PE.
Enzyme-linked immunosorbent analysis
Suspensions from transfected Hep3B-C and ECCG5 cells were collected and diluted five-fold for GM-CSF secretion tests by Enzyme-linked Immunosorbent Analysis through using the LEGEND MAX™ Human GM-CSF ELISA Kit with Pre-coated Plates (BioLegend, USA). Assays were carried out according to the manufacturer's instructions.
In vivo analysis
All animal experiments were undertaken in accordance with the National Institute of Health guidelines for the care and use of laboratory animals, with the approval of the Scientific Investigation Board at the Second Military Medical University, Shanghai. ECCG5 cells were injected subcutaneously into C57BL/6 mice (5×106 cells per mouse, 6 per group, 4 groups). Twelve days post injection, when the diameter of xenografts reached 8mm approximately, treatment was started by intra-tumoral injection of OAV. Tumor volumes were measured every two days [volume= (W2 ×L)/2; W, width; L, length, in cubic millimeters]. Mice were euthanized 30 days post treatment, and the tumor tissues were collected for immunohistological analysis by using the avidin biotin-perox-dase complex (ABC) method with Anti-AIF1 antibody, or digested with 0.25% trypsin.
Results
Construction of 11R-P53 and GM-CSF expressing OAV SG655-mGMP
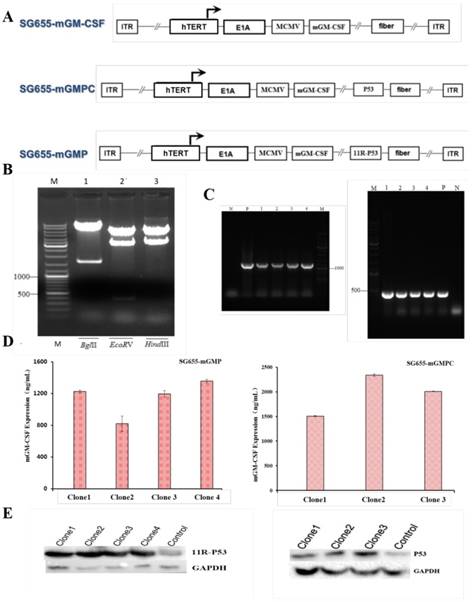
The 11R-P53 and GM-CSF expressing OAV SG655-mGMP was constructed based on our previous OVA SG7605-11R-p53, in which the E1A gene expressing cassatte was placed under hTERT promoter [27]. In OVA SG655-mGMP, the E1A gene and mGM-CSF expressing cassettes were placed under hTERT promoter and MCMV promoter respectively, the 11R-P53 expressing cassette was inserted into the E3 area, the control virus SG655-mGMPC was also constructed (Fig 1A). The construct was confirmed by restriction enzyme digestions with BglII, EcoRV and HindIII by showing expected fragments during agarose gel electrophoresis after DNA extraction (Fig 1B). Meanwhile, PCR assay was carried out to detect the expression of 11R-P53 and GM-CSF and showed efficient expression of 11R-P53 and GM-CSF from 293 cell clones after transfection with OAV SG655-mGMP and SG655-mGMPC (Fig 1C). The expression of 11R-P53 and GM-CSF was additionally confirmed by Western blot and Elisa assays (SG655-mGMP: 1226.01, 820.36, 1197.63, 1358.33 ng/mL for clone 1-4; SG655-mGMPC: 1506.68, 2341.83, 2008.64 ng/mL for clone 1-3), respectively (Fig 1D, E).
Construction of the OVA SG655-mGMP. A: Linearized depiction of plasmids for SG655-mGM-CSF, SG655-mGMPC and SG655-mGMP, the E1A gene and mGM-CSF expressing cassettes were placed under hTERT promoter and MCMV promoter respectively, the 11R-P53 expressing cassette was inserted in the E3 area in SG655-mGMP; B: The construct was confirmed by restriction enzyme digestions with BglII, EcoRV and HindIII, showing expected DNA fragments of 8465+1613bp, 7575+2114+389bp and 7476+2602bp during agarose gel electrophoresis; C: Agarose gel electrophoresis of PCR products showed expected bands of 1227bp and 426bp for 11R-P53 and mGM-CSF fragments; D: Elisa assay showed effective expression of mGM-CSF and 11R-P53 of SG655-mGMP and SG655-mGMPC clones (SG655-mGMP: 1226.01, 820.36, 1197.63, 1358.33 ng/mL for clone 1-4; SG655-GMPC: 1506.68, 2341.83, 2008.64 ng/mL for clone 1-3, respectively); E: Western blot assay showed effective expression of 11R-P53 and P53 in 293 cell clones after transfection with SG655-mGMP and SG655-mGMPC.
11R augments the expression of target genes in cancer stem cells
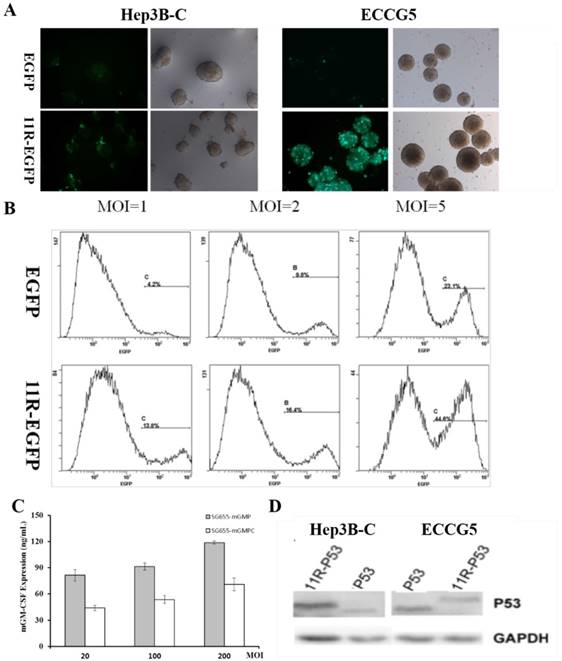
In order to test the impact of 11R on target gene expression, the expression of EGFP was firstly examined in hepatocellular carcinoma stem cells Hep3B-C and teratoma stem cells ECCG5. Compared to uncoupled EGFP, 11R-EGFP showed enhanced green fluorescence intensity in both Hep3B-C and ECCG5 cells post transfection with OVA SG655-mGMP (Fig 2A). And the improving effect of 11R on the expression of EGFP in Hep3B-C cells was further verified by FCM assay after transfection with OAV at different MOI via showing higher percent of EGFP positive cells (with V.S. without 11R: 13.8% V.S. 4.2%, 16.4% V.S. 9.8%, 44.6% V.S. 23.1%, MOI=1, 2, 5) (Fig 2B).
After the confirmation of the improving effect of 11R on target gene expression, Elisa assay was applied to detect the secretion of GM-CSF in ECCG5 cell culture supernatants post transfection with OAV SG655-mGMP and SG655-mGMPC at different MOI (20, 100, 200). Results showed that mGM-CSF was efficiently expressed in ECCG5 cells, and the expression was positively associated with the MOI (MOI=20: 81.35 and 43.91 ng/mL, MOI=100: 91.23 and 53.59 ng/mL, MOI=200: 118.79 and 70.90 ng/mL for SG655-mGMP and SG655-mGMPC transfection respectively) (Fig 2C). The expression of 11-P53 was tested by Western blot through using anti-P53 antibody. Results showed that 11R-P53 was efficiently expressed in both Hep3B-C and ECCG5 cells with slight heavier weight than P53 (Fig 2D).
The augmenting effect of 11R within the construct. A: The expression of EGFP of Hep3B-C and ECCG5 cells post transfection was augmented by 11R via showing enhanced green fluorescence intensity; B: The improving effect of 11R on the expression of EGFP in Hep3B-C cells was further verified by FCM assay after transfection with OAV at different MOI through showing higher percent of EGFP positive cells (with V.S. without 11R: 13.8% V.S. 4.2%, 16.4% V.S. 9.8%, 44.6% V.S. 23.1%, MOI=1, 2, 5); C: Elisa assay was applied to detect the secretion of GM-CSF in ECCG5 cell culture supernatants post transfection with OAV SG655-mGMP and SG655-mGMPC at different MOI, and showed effective expression (MOI=20: 81.35 and 43.91 ng/mL, MOI=100: 91.23 and 53.59 ng/mL, MOI=200: 118.79 and 70.90 ng/mL for SG655-mGMP and SG655-mGMPC transfection respectively). D: The expression of 11-P53 was tested by Western blot through using anti-P53 antibody, and showed that 11R-P53 was efficiently expressed in both Hep3B-C and ECCG5 cells with slight heavier weight than P53.
SG655-mGMP targets cancer stem cells through growth inhibition and apoptosis induction in vitro
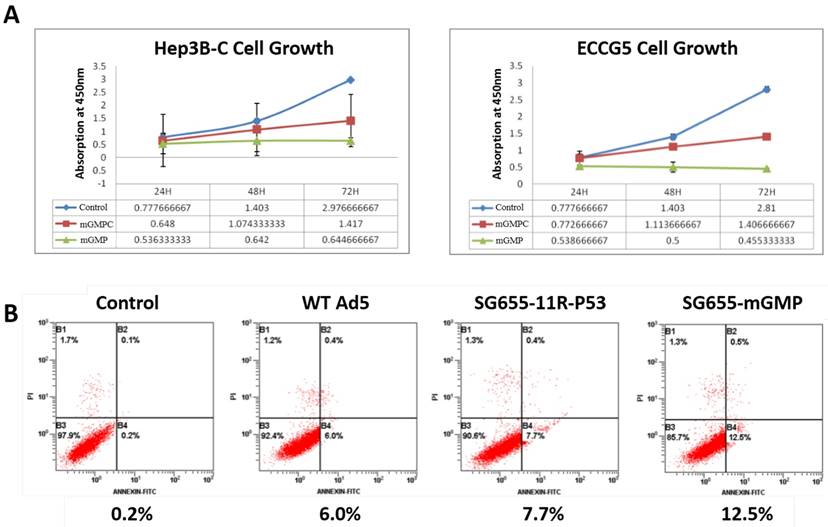
After confirmation on the expression of 11R-P53 and GM-CSF, CCK-8 assay was applied to test the effect of OAV SG655-mGMP on the growth of Hep3B-C and ECCG5 cells in vitro. Results showed that compared to the control virus, uncoupled P53 expressing OAV SG655-mGMPC inhibited the growth of both Hep3B-C and ECCG5 cells slightly, while the 11R-P53 expressing OAV SG655-mGMP exhibited more remarkable inhibitory effect on the growth of Hep3B-C and ECCG5 cells (Fig 3A). FCM assay was then used to test the effect of OAV on Hep3B-C cells. Results showed that wild type OAV and 11R-P53 expressing OAV SG7605-11R-p53 induced higher percent of apoptotic cells (6.0% and 7.7%, respectively) than the control (0.2%), and OAV SG655-mGMP induced the highest percent of apoptotic cells from Hep3B-C cells (12.5%) (Fig 3B). These results indicated that the OAV SG655-mGMP exhibited enhanced activity than the 11R-P53 expressing OAV SG7605-11R-p53 and the P53 expressing OAV SG655-mGMPC toward both Hep3B-C and ECCG5 cells through augmenting the growth inhibitory effect and apoptosis induction.
SG655-mGMP exerts synergetic effect toward cancer stem cells in vivo
Then, the in vivo therapeutic effect of OAV SG655-mGMP on ECCG5 cells was examined after the establishment of C57BL/6 mouse models by subcutaneously transplanting ECCG5 cells (Fig 4A). The treatment was carried out with control PBS, 11R-P53 expressing OAV SG655-11R-P53, mGM-CSF expressing OV SG655-mGM-CSF and SG655-mGMP when the diameter of xenografts reached 7-8mm. Since the treatment starting D0 day, the volumes were measured every two days within a month. SG655-11R-P53 and SG655-mGM-CSF treatments showed significant inhibition on the growth of xenografts in an undistinguishable pattern with PBS treatment. Though, the SG655-mGMP treatment exhibited most powerful inhibition on the growth of xenografts (Fig 4B).
The growth inhibition and apoptosis induction of SG655-mGMP toward cancer stem cells. A: CCK-8 assay was applied to test the effect of OAV SG655-mGMP on the growth of Hep3B-C and ECCG5 cells, and showed that uncoupled P53 expressing OAV SG655-mGMPC inhibited the growth of both Hep3B-C and ECCG5 cells slightly, when compared to the control virus, while the 11R-P53 expressing OAV SG655-mGMP exhibited more remarkable inhibitory effect on the growth of Hep3B-C and ECCG5 cells; B: FCM assay was then used to test the effect of OAV on Hep3B-C cells, and showed that wild type OAV and 11R-P53 expressing OAV SG7605-11R-p53 induced higher percent of apoptotic cells (6.0% and 7.7% respectively) than the control (0.2%), and OAV SG655-mGMP induced the highest percent of apoptotic cells from Hep3B-C cells (12.5%).
SG655-mGMP exerted synergetic effect toward cancer stem cells in vivo. A: The mouse models were established; B: SG655-11R-P53 and SG655-mGM-CSF treatments reduced the volume of treated xenografts in an undistinguishable pattern with the control PBS treatment, while the SG655-mGMP treatment exhibited most powerful inhibition on the growth of xenografts by reducing the volume to lowest level; C: The IHC assay showed that OAV SG655-11R-P53 and SG655-mGM-CSF treatments improved the expression of inflammation factor AIF1 slightly compared to the control PBS treatment, while the OAV SG655-mGMP treatment significantly improved the expression of AIF1. D: The enhancing effect on immune cells infiltration of OAV SG655-11R-P53, SG655-mGM-CSF and SG655-mGMP treatments were examined by FCM through comparing to PBS treatment, and showed that OAV SG655-11R-P53 and SG655-mGM-CSF enhanced the infiltration of immune cells with modest level, while the OVA SG655-mGMP exhibited most powerful function (NK cell: 0.6%, 2.5%, 1.8% and 3.2%; Macrophage: 0.9%, 6.0%, 5.7% and 9.5%; Granulocyte: 2.2%, 6.1%, 6.8% and 9.0%; DC: 1.1%, 5.5%, 5.4% and 6.1% for PBS, SG655-11R-P53, SG655-mGM-CSF and SG655-GMP, respectively).
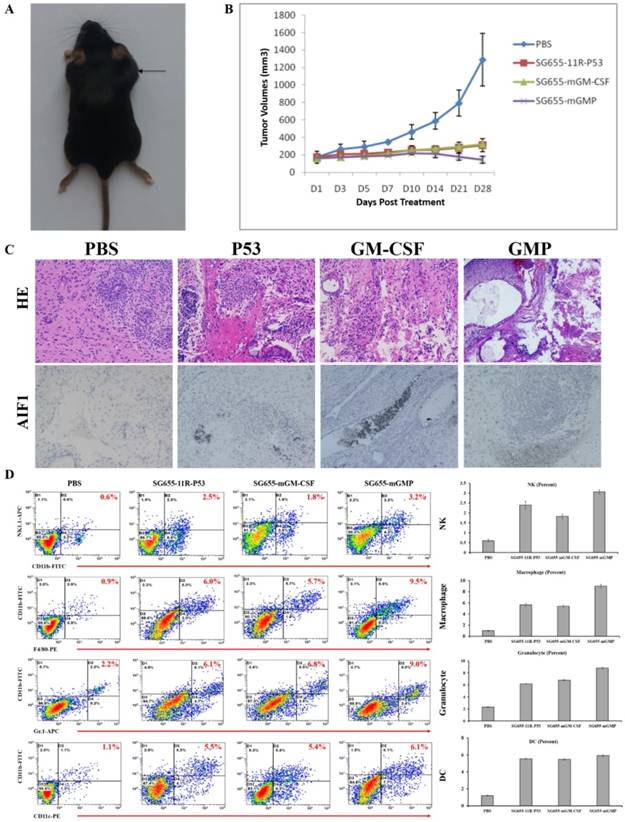
Afterward, all mice were euthanized 4 weeks post treatment; xenografts were exposed and saved for IHC assay or digested for FCM assay. The IHC assay showed that OAV SG655-11R-P53 and SG655-mGM-CSF treatments slightly improved the expression of inflammation factor AIF1 compared to the control PBS treatment, and the OAV SG655-mGMP treatment significantly improved the secretion of AIF1 when compared to the other three treatments (Fig 4C). In addition to the improvement on the secretion of AIF1, the enhancements on the infiltration of immune cells, including granulocyte, dendritic cell, macrophage and NK cell were also induced by OAV SG655-11R-P53 and SG655-mGM-CSF treatments with modest level, the OAV SG655-mGMP treatment induced the infiltration of immune cells with highest level (Fig 4D). These results indicated that OAV SG655-mGMP treatment exhibited significant inhibition on the growth of xenografts by improving the expression of AIF and infiltration of immune cells.
Discussion
Based on our previous studies, that the tumor suppressive effect of P53 can be enhanced by 11R [15, 25, 27]. 11R-P53 was further combined with the immune cell recruiting factor GM-CSF as well as the anti-tumor activity of OAV in SG655-mGMP in this study. Our results showed effective function of 11R in our OAV system by enhancing the expression of EGFP in both Hep3B-C and ECCG5 cells. And then the expression of GM-CSF and 11-P53 were detected in both Hep3B-C and ECCG5 cells, which resulted in augmented tumor growth inhibition and apoptosis induction of the OAV SG655-mGMP compared to the 11R-P53 expressing OAV SG655-11R-P53 and the mGM-CSF expressing OV SG655-mGM-CSF in vitro and enhanced synergistic anti-tumor effects in vivo.
In our previous study, we concluded that the anti-tumor activity of P53 expressing OAV was augmented by 11R against gallbladder cancer cells [27]. We here showed that this augmented anti-tumor activity can be further combined with one of the most important immune factors, GM-CSF. More importantly, CSCs, which have been ubiquitously considered as a major cause for drug resistance and cancer recurrence, were chosen to test the anti-tumor activity of the OAV SG655-mGMP, and results showed synergistic effects of the OAV SG655-mGMP toward CSCs. Thus, the OAV SG655-mGMP is a powerful weapon to treat malignancies by targeting CSCs. However, further studies are needed to investigate the mechanism on the function of the OAV SG655-mGMP, whether memory effectors or other immune effectors are involved in, and can it be used to target other type of CSCs.
The combination of P53 and immune factor GM-CSF has been intensively studied in last few years, and concluded that it effectively inhibit the growth of glioma cells in vitro and in mouse model and laryngeal cancer cells when combined with B7-1 gene [28]. However, one major problem for cancer treatment with tumor suppressor genes is the penetration of the encoded protein. Based on our previous finding, that 11R, which has been exclusively proved with enhancing effect on target genes, was effective in augmenting the anti-tumor activity of P53 carrying OAV against gallbladder cells in vitro and in vivo. We used 11R to facilitate the penetration of P53, thus to better suppress tumor growth. Our system has the potential to serve as a novel universal platform for cancer treatment with OAV based on the combination of tumor suppressors and immune factors. Nevertheless, our system still has to be tested with other combination of tumor suppressors and immune factors.
In conclusion, the OAV SG655-mGMP in this study provides a novel platform to combine tumor suppressors and immune factors for targeting hepatocellular cancer stem cells and teratoma stem cells, and possesses great potential for targeting other type of CSCs as well as clinical cancer treatment.
Acknowledgements
This work was supported by the Capacity Building Project of Shanghai Engineering Research Center (16DZ2281000), the Chinese Key Project for Infectious Diseases (No 2012ZX0002-014-005, 2013ZX10002-010-007) and the National High Technology Research and Development Program of China (863 Program no. 2012AA020806).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zheng R, Zeng H, Zhang S, Chen T, Chen W. National estimates of cancer prevalence in China, 2011. Cancer letters. 2016;370:33-8
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5-29
3. Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL. et al. Cancer stem cells-perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer research. 2006;66:9339-44
4. Kise K, Kinugasa-Katayama Y, Takakura N. Tumor microenvironment for cancer stem cells. Advanced drug delivery reviews. 2016;99:197-205
5. Schatton T, Frank NY, Frank MH. Identification and targeting of cancer stem cells. BioEssays: news and reviews in molecular, cellular and developmental biology. 2009;31:1038-49
6. Wang T, Shigdar S, Gantier MP, Hou Y, Wang L, Li Y. et al. Cancer stem cell targeted therapy: progress amid controversies. Oncotarget. 2015;6:44191-206
7. Ciurea ME, Georgescu AM, Purcaru SO, Artene SA, Emami GH, Boldeanu MV. et al. Cancer stem cells: biological functions and therapeutically targeting. International journal of molecular sciences. 2014;15:8169-85
8. Matchett KB, Lappin TR. Concise reviews: cancer stem cells: from concept to cure. Stem Cells. 2014;32:2563-70
9. Kaiser J. The cancer stem cell gamble. Science. 2015;347:226-9
10. Rycaj K, Tang DG. Cell-of-Origin of Cancer versus Cancer Stem Cells: Assays and Interpretations. Cancer research. 2015;75:4003-11
11. Yoshida GJ, Saya H. Therapeutic strategies targeting cancer stem cells. Cancer science. 2016;107:5-11
12. Alvarez-Breckenridge C, Kaur B, Chiocca EA. Pharmacologic and chemical adjuvants in tumor virotherapy. Chemical reviews. 2009;109:3125-40
13. Uchida H, Marzulli M, Nakano K, Goins WF, Chan J, Hong CS. et al. Effective treatment of an orthotopic xenograft model of human glioblastoma using an EGFR-retargeted oncolytic herpes simplex virus. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:561-9
14. Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y. et al. Oncolytic viruses as therapeutic cancer vaccines. Molecular cancer. 2013;12:103
15. Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J. et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:2780-8
16. Garber K. China approves world's first oncolytic virus therapy for cancer treatment. Journal of the National Cancer Institute. 2006;98:298-300
17. Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nature reviews Drug discovery. 2015;14:642-62
18. Nishio N, Diaconu I, Liu H, Cerullo V, Caruana I, Hoyos V. et al. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer research. 2014;74:5195-205
19. Marcato P, Dean CA, Giacomantonio CA, Lee PW. Oncolytic reovirus effectively targets breast cancer stem cells. Molecular therapy: the journal of the American Society of Gene Therapy. 2009;17:972-9
20. Cripe TP, Wang PY, Marcato P, Mahller YY, Lee PW. Targeting cancer-initiating cells with oncolytic viruses. Molecular therapy: the journal of the American Society of Gene Therapy. 2009;17:1677-82
21. Friedman GK, Cassady KA, Beierle EA, Markert JM, Gillespie GY. Targeting pediatric cancer stem cells with oncolytic virotherapy. Pediatric research. 2012;71:500-10
22. Cheema TA, Wakimoto H, Fecci PE, Ning J, Kuroda T, Jeyaretna DS. et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12006-11
23. Smith TT, Roth JC, Friedman GK, Gillespie GY. Oncolytic viral therapy: targeting cancer stem cells. Oncolytic virotherapy. 2014;2014:21-33
24. Chiocca EA, Blair D, Mufson RA. Oncolytic viruses targeting tumor stem cells. Cancer research. 2014;74:3396-8
25. Li J, Yu Y, Wang J, Yan Z, Liu H, Wang Y. et al. Establishment of a novel system for the culture and expansion of hepatic stem-like cancer cells. Cancer letters. 2015;360:177-86
26. Liu T, Wang Y, Peng X, Zhang L, Cheng J, Jin H. et al. Establishment of mouse teratocarcinomas stem cells line and screening genes responsible for malignancy. PloS one. 2012;7:e43955
27. Wang J, Yu Y, Yan Z, Hu Z, Li L, Li J. et al. Anticancer activity of oncolytic adenoviruses carrying p53 is augmented by 11R in gallbladder cancer cell lines in vitro and in vivo. Oncology reports. 2013;30:833-41
28. Ye ZL, Huang Y, Li LF, Zhu HL, Gao HX, Liu H. et al. Argonaute 2 promotes angiogenesis via the PTEN/VEGF signaling pathway in human hepatocellular carcinoma. Acta pharmacologica Sinica. 2015;36:1237-45
Author contact
![]() Corresponding authors: Qi-Jun Qian, Tel: + 86-21-81875371; Fax: + 86-21-65580677; E-mail: qianqjcn; Hua-Jun Jin, Tel: + 86-21-81875372; E-mail: hj-jincom.
Corresponding authors: Qi-Jun Qian, Tel: + 86-21-81875371; Fax: + 86-21-65580677; E-mail: qianqjcn; Hua-Jun Jin, Tel: + 86-21-81875372; E-mail: hj-jincom.

 Global reach, higher impact
Global reach, higher impact