Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(2):258-265. doi:10.7150/jca.16525 This issue Cite
Research Paper
Preoperative Albumin to Globulin Ratio (AGR) as Prognostic Factor in Renal Cell Carcinoma
1. Department of Urology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
2. Department of Medical Oncology, the Fifth Affiliated Hospital of Sun Yat-Sen University, Zhuhai, China
3. Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
4. Medicine school of Sun Yat-Sen University, Guangzhou, China
5. Department of Biochemistry and Molecular Medicine, University of California Davis, Sacramento, California, United States of America
* These authors contributed equally to this work.
Received 2016-6-18; Accepted 2016-10-15; Published 2017-1-15
Abstract
Background: Malnutrition and systemic inflammatory response are frequently associated with prognosis in patients with several types of cancer, including renal cell carcinoma (RCC). The study is aimed to investigate the ability of preoperative serum albumin to globulin ratio (AGR) to predict the long-term mortality of RCC patients.
Methods: The study is a retrospective study of an unselected cohort of 895 RCC patients who underwent a curative radical or partial nephrectomy at the Department of Urology in the Sun Yat-Sen University Cancer Center between January 2000 and December 2012 and had documented preoperative serum total protein and albumin (ALB) levels. The preoperative AGR was calculated as the ratio of ALB to (total protein-ALB) and its association with other clinical indices was assessed using survival analysis.
Results: Low preoperative AGR was associated with older population, lower hemoglobin, higher total protein, lower ALB, lower body mass index and advanced stage. The univariate and multivariate Cox analyses demonstrated that preoperative AGR was an independent prognostic indicator of overall survival (OS) (hazard ratio (HR): 0.63, 95% confidence interval (CI): 0.43 to 0.93, P=0.022). In addition, patients with low preoperative AGR at pT1-2, pT3-4, pN0, pN1, pM0 and pM1 stages had significantly shorter OS than patients with high preoperative AGR.
Conclusion: Preoperative AGR is a proven objective, reproducible, inexpensive survival predictor of RCC patients following surgical resection and should be considered for routine clinical use.
Keywords: Renal Cell Carcinoma, Albumin, Total Protein, Albumin to Globulin ratio.
Introduction
Renal cell carcinoma (RCC) is the most common malignant tumor of the kidney, with 66,800 and 62,700 estimated new cases occurring in China each year 1 and the United State in 2016 2, respectively. Incidental and early stage tumors have been detected more frequently because of increased use of imaging techniques including ultrasound and computed tomography (CT) in recent years 3-4. Although more RCC patients are diagnosed at early stage tumors, its mortality is still rising. Approximately 20% to 30% of patients with localized tumors after radical or partial nephrectomy will later develop metastatic disease 5. As renal tumors are insensitive to radiotherapy and chemotherapy, majority of metastatic patients die. Although the TNM system proposed by UICC and AJCC 6 and Fuhrman's Nuclear Grading system 7 are commonly used for prognosis, they are not entirely reliable 8. Other well-known prognostic factors are lymphocytic infiltration and histological subtype 8. Due to the insufficiency of these prognostic factors, new factors including clinical and laboratory indicators are being considered.
Increasing evidence supports the involvement of systemic nutritional status and inflammation in cancer progression 9-11. Albumin (ALB) and globulin (GLB) are the two major components of serum proteins. Hypoalbuminemia in cancer patients not only is an indicator of poor nutritional status but also relates to chronic inflammation 12-13. Furthermore, increased level of GLB could serve as a marker of chronic inflammation response and reflect a cumulative exposure of various pro-inflammatory cytokines 14. Recent studies have demonstrated that ALB to GLB ratio (AGR), which is calculated as AGR= ALB/(total protein-ALB), is an independent prognostic factor for breast cancer, lung cancer, nasopharyngeal carcinoma, colorectal cancer and so on 15-18. AGR level not only reflects the nutritional status but also represents the systemic inflammation. Thus, discriminating AGR might be a potential independent risk factor for RCC. However, to our best knowledge, the prognostic significance of AGR in RCC has not been reported. The aim of our research was to assess the prognostic significance of preoperative AGR in long-term survival of RCC patients and to evaluate whether it could provide additional prognostic information to well-established clinicopathological parameters.
Material and Methods
Patients
The subjects of the retrospective study were a cohort of 912 consecutive RCC patients who underwent a curative radical or partial nephrectomy at the Department of Urology in Sun Yat-Sen University Cancer Center between January 2000 and December 2012. Among these patients, 17 patients (1.86%) had incomplete laboratory data. Thus, 895 patients were included in the analysis. The study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center and performed in accordance with the ethical standards of the World Medical Association Declaration of Helsinki 19. All included patients provided written informed consent and their information were recorded and registered in our cancer registry system.
Follow-up
Follow-up schedules were established and applied referring to the NCCN Clinical Practice Guidelines. RCC patients at early stage who need to be closely monitored after partial or radical nephrectomy (pT1a and pT1b) were subjected to follow-up evaluations every six months for the first 2 years and once a year thereafter. RCC patients at advanced stage were subjected to follow-up evaluations every 3-6 months for the first three years and once a year thereafter. The follow-up evaluations included all routine clinical, laboratory and radiological examinations. In addition, all patients were also followed up via telephone interviews. The last follow-up was completed in November 01, 2015. Patients who were still alive at the last follow-up were censored.
Clinical and laboratory parameters
All clinicopathological data including demographic parameters, Fuhrman grade, tumor histology, tumor stage and laboratory data were retrieved from the electronic medical records at our hospital. The AJCC/UICC TNM staging system (the 7th edition) was applied to classify the tumor stage. The laboratory data, including the levels of ALB, total proteins, hemoglobin (HGB), alkaline phosphatase (ALP), serum creatinine (Scr) and uric acid (UA), were measured one day before surgical intervention. Serum ALP >135 U/L, Scr > 130 μmol/L and UA > 420 μmol/L were defined as elevated. Preoperative AGR was calculated using the equation: AGR = ALB / (total protein- ALB).
Statistical analysis
The end point of the study was overall survival (OS), which was defined as the time interval between the date of surgery and the date of death of individual patient. The optimal cut-off value of preoperative AGR was determined by using the receiver operating curve (ROC) analysis, as previously described 20-21. The threshold that best discriminated the differences between the survived and deceased cases (in terms of sensitivity and specificity) was used, allowing us to treat AGR as a dichotomous variable.
The continuous and categorical variables were presented as means ± standard deviations (SD) as well as frequencies and percentages, respectively. Continuous data were analyzed using the Mann-Whitney U-test and categorical data were analyzed using the chi-square test. Patients' clinical end points were calculated using the Kaplan-Meier method and compared by the log-rank test. Univariate analysis was performed to determine the significance of variables and Cox regression model was performed for OS. Subsequently, the variables with P<0.05 were further analyzed using multivariate Cox proportion analysis to determine independent prognostic factors. Hazard ratios (HRs) estimated from the Cox analysis were reported as relative risks with corresponding 95% confidence intervals (CIs). All Statistical analyses were performed using Empower (R) (www.empowerstats.com, X&Y solutions, Inc., Boston, MA), R (http://www.R-project.org) and Statistical Package for Social Sciences (SPSS) 21.0 software (IBM, Armonk, NY). A two-sided P<0.05 was considered statistically significant.
Baseline characteristics of all patients.
| Characteristics | Cases (n=895) | Percentage (%) |
|---|---|---|
| Age, year (mean±SD) | 51.44±13.44 | |
| Hemoglobin, g/L, (mean±SD) | 134.21±20.47 | |
| Total protein, g/L (mean±SD) | 72.22±6.61 | |
| Serum albumin, g/L (mean±SD) | 43.10±4.80 | |
| Body mass index, kg/m2 (mean±SD) | 23.41±3.58 | |
| AGR, (mean±SD) | 1.54±0.34 | |
| Gender | ||
| Male | 600 | 67.00 |
| Female | 295 | 33.00 |
| Pathological types | ||
| Clear cell carcinoma | 696 | 77.80 |
| Multilocular cystic renal cell carcinoma | 64 | 7.20 |
| Chromophobe renal carcinoma | 32 | 3.60 |
| Others | 103 | 11.50 |
| Fuhrman-grade | ||
| Ⅰ | 205 | 22.90 |
| Ⅱ | 329 | 36.80 |
| Ⅲ | 85 | 9.50 |
| Ⅳ | 13 | 1.50 |
| Unknown | 263 | 29.40 |
| pT status | ||
| T1 | 613 | 68.50 |
| T2 | 164 | 18.30 |
| T3 | 88 | 9.80 |
| T4 | 30 | 3.40 |
| pN status | ||
| N0 | 829 | 92.60 |
| N1 | 66 | 7.40 |
| pM status | ||
| M0 | 852 | 95.20 |
| M1 | 43 | 4.80 |
| pTNM stage | ||
| Ⅰ | 597 | 66.70 |
| Ⅱ | 143 | 16.00 |
| Ⅲ | 96 | 10.70 |
| Ⅳ | 59 | 6.60 |
| Alkaline phosphatase, U/L | ||
| Normal | 858 | 95.90 |
| Elevated | 37 | 4.10 |
| Serum creatinine, μmol/L | ||
| Normal | 859 | 96.00 |
| Elevated | 36 | 4.00 |
| Uric acid, μmol/L | ||
| Normal | 705 | 78.80 |
| Elevated | 190 | 21.20 |
| AGR group | ||
| Low AGR group (AGR≤1.47) | 371 | 41.50 |
| High AGR group (AGR>1.47) | 524 | 58.50 |
Abbreviation: pTNM: pathologic tumor-node-metastasis; AGR: Albumin to Globulin ratio.
Results
Clinicopathological Features
The 895 enrolled patients were 51.44±13.44 years old. Of them, 600 (67.00%) were male and 295 (33.00%) were female. 597, 143, 96 and 59 patients were at stage I, II, III and IV, respectively. The median follow-up time from diagnosis was 69.68 months (95%CI: 65.73-73.63). 171 patients died during the follow-up. Table 1 summarizes patients' characteristics.
Identification of the optimal cut-off value for AGR
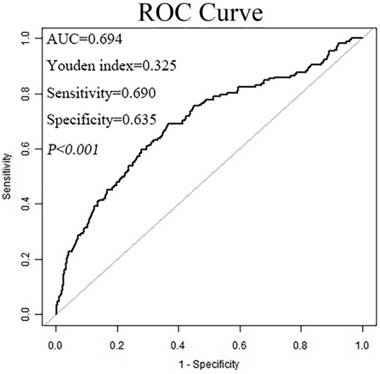
Serum ALB and serum total proteins levels were 43.10±4.80 g/L and 72.22±6.61 g/L, respectively. The calculated AGR was 1.54±0.34. ROC curves in Figure 1 showed that when analyzed as a dichotomous variable, AGR=1.47 as the cut-off value provided the strongest prognostic point for the OS. Therefore, this level was chosen to stratify patients. 371 patients with AGR ≤ 1.47 were classified into low AGR group, whereas 524 with AGR> 1.47 were classified into high AGR group.
The receiver operating characteristic (ROC) curve for the preoperative albumin to globulin ratio (AGR).
Association of AGR with the clinicopathological features of RCC patients
Patients in different AGR groups showed significant differences in pT-stage (P<0.001), pN-stage (P<0.001), pM-stage (P<0.001), pTNM-stage (P<0.001) and ALP group (P<0.001) (Table 2). Additionally, patients in low AGR group were significantly older (P<0.001), and had lower HGB (P<0.001), higher TP (P<0.001), lower ALB (P<0.001) and lower BMI (P<0.001) than patients in high AGR group (Table 2).
Characteristics of the 895 patients with RCC.
| Characteristics | Low AGR group | High AGR group | P value |
|---|---|---|---|
| Age, year (mean±SD) | 55.20 ± 13.33 | 48.78 ± 12.88 | <0.001 a |
| Hemoglobin, g/L, (mean±SD) | 126.30 ± 21.40 | 139.81 ± 17.79 | <0.001 a |
| Total protein, g/L (mean±SD) | 73.97 ± 6.67 | 70.98 ± 6.29 | <0.001 a |
| Serum albumin, g/L (mean±SD) | 40.46 ± 5.14 | 44.97 ± 3.49 | <0.001 a |
| Body mass index, kg/m2 (mean±SD) | 22.94 ±3.48 | 23.74 ±3.61 | 0.001 a |
| Gender | <0.001 b | ||
| Male | 220 (59.30%) | 380 (72.50%) | |
| Female | 151 (40.70%) | 144 (27.50%) | |
| Pathological types | 0.075 b | ||
| Clear cell carcinoma | 283 (76.30%) | 413 (78.80%) | |
| Multilocular cystic renal cell carcinoma | 29 (7.80%) | 35 (6.70%) | |
| Chromophobe renal carcinoma | 8 (2.20%) | 24 (4.60%) | |
| Others | 51 (13.70%) | 52 (9.90%) | |
| Fuhrman-grade | 0.066 b | ||
| Ⅰ | 80 (21.60%) | 125 (23.90%) | |
| Ⅱ | 125 (33.70%) | 204 (38.90%) | |
| Ⅲ | 46 (12.40%) | 39 (7.40%) | |
| Ⅳ | 7 (1.90%) | 6 (1.10%) | |
| Unknown | 113 (30.50%) | 150 (28.60%) | |
| pT status | <0.001 b | ||
| T1 | 203 (54.70%) | 410 (78.20%) | |
| T2 | 85 (22.90%) | 79 (15.10%) | |
| T3 | 60 (16.20%) | 28 (5.30%) | |
| T4 | 23 (6.20%) | 7 (1.30%) | |
| pN status | <0.001 b | ||
| N0 | 324 (87.30%) | 505 (96.40%) | |
| N1 | 47 (12.70%) | 19 (3.60%) | |
| pM status | <0.001 b | ||
| M0 | 339 (91.40%) | 513 (97.90%) | |
| M1 | 32 (8.60%) | 11 (2.10%) | |
| pTNM stage | <0.001 b | ||
| Ⅰ | 196 (52.80%) | 401 (76.50%) | |
| Ⅱ | 67 (18.10%) | 76 (14.50%) | |
| Ⅲ | 65 (17.50%) | 31 (5.90%) | |
| Ⅳ | 43 (11.60%) | 16 (3.10%) | |
| Alkaline phosphatase, U/L | <0.001 b | ||
| Normal | 343 (92.50%) | 515 (98.30%) | |
| Elevated | 28 (7.50%) | 9 (1.70%) | |
| Serum creatinine, μmol/L | 0.159 b | ||
| Normal | 352 (94.90%) | 507 (96.80%) | |
| Elevated | 19 (5.10%) | 17 (3.20%) | |
| Uric acid, μmol/L | 0.339 b | ||
| Normal | 298 (80.30%) | 407 (77.70%) | |
| Elevated | 73 (19.70%) | 117 (22.30%) |
Abbreviation: pTNM: pathologic tumor-node-metastasis; AGR: Albumin to Globulin ratio.
a Mann-Whitney U-test.
b Chi-square test.
Association of preoperative AGR with OS
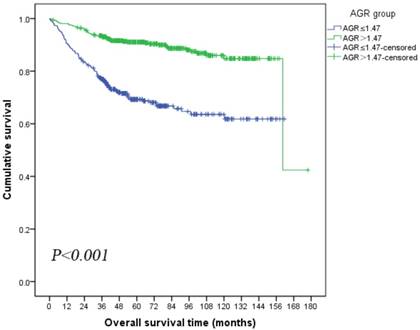
Kaplan-Meier survival analysis showed that patients in low AGR group had significantly poorer OS than patients in high AGR group (mean OS: low AGR vs high AGR, 114.01 vs 151.51 months, log-rank P<0.001; Figure 2). The univariate analysis revealed that preoperative high AGR was associated with decreased risk of death (HR: 0.29, 95% CI: 0.21-0.40, P<0.001, Table 3). Therefore, a multivariate analysis using Cox proportional hazards model for OS was performed to determine various prognostic indicators including age, HGB, BMI, pathology, Fuhrman grade, pT-stage, pN-stage, pM-stage, ALP and AGR. The results showed that preoperative AGR was an independent prognostic indicator of OS (HR: 0.63, 95%CI: 0.43-0.93, P=0.022, Table 3). In addition, serum ALB, pT-stage, pN-stage and pM-stage were also identified as independent prognostic indicators for OS.
Association of preoperative AGR with subgroups
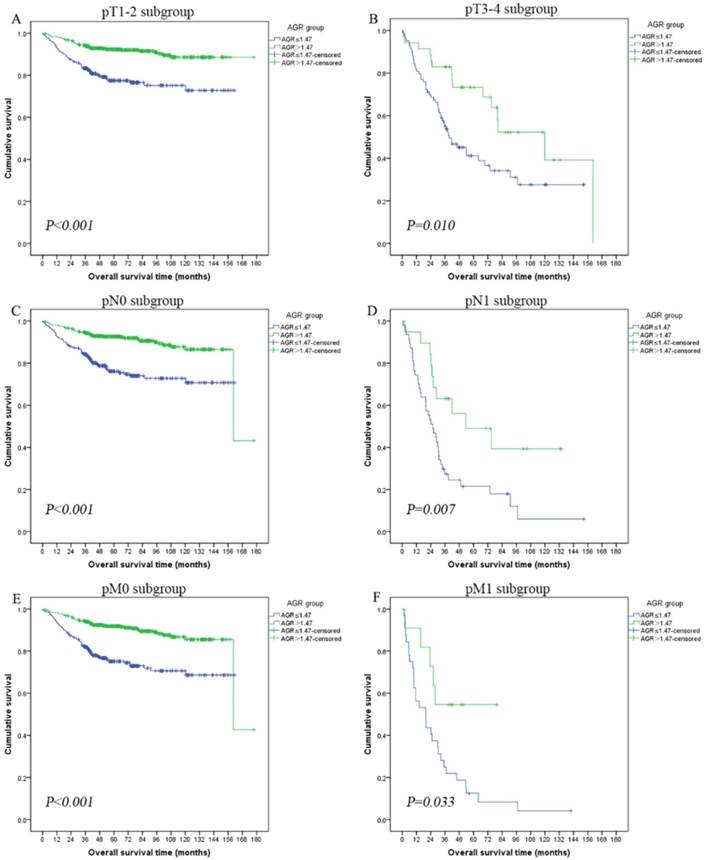
The prognostic influence of preoperative AGR in subgroups of pT-stage, pN-stage and pM-stage were further investigated. Patients with low AGR level had a significantly shorter OS than patients with high AGR level in pT1-2 subgroup (mean OS: low AGR vs high AGR, 127.67 vs 162.92 months, log-rank P<0.001; Figure 3A), pT3-4 subgroup (mean OS: low AGR vs high AGR, 67.29 vs 101.12 months, log-rank P=0.010; Figure 3B), pN0 subgroup (mean OS: low AGR vs high AGR, 125.45 vs 153.87 months, log-rank P<0.001; Figure 3C), pN1 subgroup (mean OS: low AGR vs high AGR, 38.80 vs 74.23 months, log-rank P=0.007; Figure 3D), pM0 subgroup (mean OS: low AGR vs high AGR, 123.11 vs 152.67 months, log-rank P<0.001; Figure 3E) and pM1 subgroup (mean OS: low AGR vs high AGR, 29.25 vs 52.02 months, log-rank P=0.033; Figure 3F).
Kaplan-Meier curves depicting overall survival (OS) according to the preoperative optimal value of albumin to globulin ratio (AGR) in patients with renal cell carcinoma.
Kaplan-Meier curves showing overall survival (OS) according to the preoperative optimal value of albumin to globulin ratio (AGR) in all patients with renal cell carcinoma. Patients were stratified according to the pT-status, pN-status, and pM-status. (A) Kaplan-Meier analysis of OS in pT1-2 subgroup. (B) Kaplan-Meier analysis of OS in pT3-4 subgroup. (C) Kaplan-Meier analysis of OS in pN0 subgroup. (D) Kaplan-Meier analysis of OS in pN1 subgroup. (E) Kaplan-Meier analysis of OS in pM0 subgroup. (F) Kaplan-Meier analysis of OS in pM1 subgroup.
Correlation of basic characteristics in all patients to the overall survival (OS) by Cox regression analyses.
| Predictors | univariate analyses | multivariate analyses | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.02 | 1.01 to1.03 | 0.003 a | 1.00 | 0.99 to 1.02 | 0.138 b |
| Hemoglobin | 0.98 | 0.98 to 0.99 | <0.001 a | 0.99 | 0.98 to 1.00 | 0.063 b |
| Total protein | 1.00 | 0.98 to 1.02 | 0.854 a | |||
| Serum albumin | 0.92 | 0.90 to 0.94 | <0.001 a | 0.95 | 0.92 to 0.99 | 0.015 b |
| Body mass index | 0.89 | 0.85 to 0.93 | <0.001 a | 0.95 | 0.90 to 1.00 | 0.054 b |
| Gender | ||||||
| Male | 1.00 (ref.) | |||||
| Female | 0.90 | 0.65 to 1.25 | 0.529 a | |||
| Pathological types | ||||||
| Clear cell carcinoma | 1.00 (ref.) | 1.00 (ref.) | ||||
| Multilocular cystic renal cell carcinoma | 2.48 | 1.58 to 3.87 | <0.001 a | 1.58 | 0.93 to 2.69 | 0.089 b |
| Chromophobe renal carcinoma | 0.19 | 0.03 to 1.40 | 0.103 a | 0.22 | 0.03 to 1.67 | 0.146 b |
| Others | 1.63 | 1.08 to 2.46 | 0.020 a | 0.97 | 0.61 to 1.54 | 0.918 b |
| Fuhrman-grade | ||||||
| Ⅰ | 1.00 (ref.) | 1.00 (ref.) | ||||
| Ⅱ | 1.16 | 0.73 to 1.86 | 0.525 a | 1.16 | 0.72 to 1.88 | 0.529 b |
| Ⅲ | 2.76 | 1.61 to 4.73 | <0.001 a | 1.66 | 0.94 to 2.93 | 0.079 b |
| Ⅳ | 4.75 | 1.96 to 11.48 | <0.001 a | 2.23 | 0.87 to 5.70 | 0.092 b |
| Unknown | 1.94 | 1.25 to 3.00 | 0.003 a | 1.60 | 1.00 to 2.56 | 0.047 b |
| pT status | ||||||
| T1 | 1.00 (ref.) | 1.00 (ref.) | ||||
| T2 | 2.33 | 1.57 to 3.46 | <0.001 a | 1.52 | 0.99 to 2.33 | 0.053 b |
| T3 | 5.39 | 3.66 to 7.93 | <0.001 a | 2.34 | 1.51 to 3.62 | <0.001 b |
| T4 | 11.60 | 7.20 to 18.67 | <0.001 a | 1.75 | 0.91 to 3.36 | 0.091 b |
| pN status | ||||||
| N0 | 1.00 (ref.) | 1.00 (ref.) | ||||
| N1 | 8.21 | 5.87 to 11.50 | <0.001 a | 3.05 | 1.97 to 4.74 | <0.001 b |
| pM status | ||||||
| M0 | 1.00 (ref.) | 1.00 (ref.) | ||||
| M1 | 9.84 | 6.74 to 14.36 | <0.001 a | 3.49 | 2.19 to 5.57 | <0.001 b |
| pTNM stage | ||||||
| Ⅰ | 1.00 (ref.) | |||||
| Ⅱ | 1.91 | 1.19 to 3.07 | 0.007 a | |||
| Ⅲ | 6.25 | 4.21 to 9.26 | <0.001 a | |||
| Ⅳ | 15.62 | 10.46 to 23.31 | <0.001 a | |||
| Alkaline phosphatase | ||||||
| Normal | 1.00 (ref.) | 1.00 (ref.) | ||||
| Elevated | 2.33 | 1.36 to 3.97 | 0.001 a | 0.54 | 0.29 to 1.00 | 0.050 b |
| Serum creatinine | ||||||
| Normal | 1.00 (ref.) | |||||
| Elevated | 1.66 | 0.92 to 2.99 | 0.092 a | |||
| Uric acid | ||||||
| Normal | 1.00 (ref.) | |||||
| Elevated | 1.15 | 0.80 to 1.65 | 0.455 a | |||
| AGR group | ||||||
| Low AGR group | 1.00 (ref.) | 1.00 (ref.) | ||||
| High AGR group | 0.29 | 0.21 to 0.40 | <0.001 a | 0.63 | 0.43 to 0.93 | 0.022 b |
Abbreviation: HR, hazard ratio; CI, confidence interval; pTNM: pathologic tumor-node-metastasis; AGR: Albumin to Globulin ratio.
a univariate cox regression analyses;
b multivariate cox regression analyses.
Discussion
In our study, we demonstrated that preoperative AGR could be used as a prognostic factor of OS for RCC patients. Although several studies have shown a relationship between AGR and prognosis of patients with various types of cancers 15-18, 21, to our best knowledge, this is the first study to assess the value of preoperative AGR as a prognostic maker for predicting OS of patients with RCC.
Serum ALB and GLB are two main constituents of serum total protein and can be easily and cost-effectively measured, thus providing a simple means of estimating visceral protein function. Serum ALB is a hepatic protein with a half-life of 14-20 days. It functions as a carrier molecule for various minerals, hormones and fatty acids and also helps to maintain oncotic pressure in capillaries 22. In addition, Serum ALB is characterized as a negative acute-phase protein, and its pool is affected by a number of inflammatory conditions 23. Besides, a previous research revealed that serum ALB inhibited the proliferation of human breast cancer cell lines by modulating the activities of autocrine growth regulatory factors in vitro 24. Moreover, albumin synthesis might be suppressed by malnutrition and systematic inflammation, resulting in hypoalbuminemia, which could weaken human immune defense mechanisms. Recent studies have revealed that hypoalbuminemia was more attributed to systemic inflammation than malnutrition in the dialysis patients 25. As part of cancer-related systemic inflammation, activation of proinflammatory cytokines such as IL-6 and tumor necrotic factor-α could lead to low serum ALB concentration 26. In addition, elevated accumulation of acute phase proteins, immunoglobulins as well as other serum proteins may increase serum GLB level. These changes were reflective of an inflammatory state. Some researchers have demonstrated that serum GLB is associated with the prognosis of cancer patients 27-28.
Taken together, we hypothesized the AGR is a more accurate prognostic indicator than serum ALB or GLB alone. A large retrospective cohort study demonstrated that low AGR level was associated with an increased risk for cancer incidence and mortality in both short- and long-term follow-up in generally healthy population 29. Although cancer types are implicated, low AGR level is widely accepted to be linked to tumor progression 30. Based on observations that cancer often rises at the site of chronic inflammation and inflammatory cells are present in tumor sites 31, this phenomenon may be caused by chronic inflammation. We believe that nutritional status and systemic inflammatory response play major roles in the progression of RCC and preoperative AGR could be a prognostic indicator for RCC patients.
In this large retrospective cohort study, a cut-off preoperative AGR value of 1.47 was used for predicting the OS of RCC patients. The results showed that preoperative AGR ≤1.47 was associated with older age population, low HGB, low BMI, low ALB and advanced RCC stage. The reason is that low preoperative AGR was associated with malnutrition and inflammatory response, which was consistent with that weight loss and ongoing inflammation-like response contribute to the subsequent death of RCC patients, especially in advanced stages. According to the Kaplan-Meier curves and log-rank test, patients with low preoperative AGR level had significantly shorter OS than patients with high AGR level. Furthermore, the univariate and multivariate Cox regression models revealed that AGR was a significant independent predictor for OS of RCC patients subjected to surgery. Besides, subgroup analysis of patients at pT1-2, pT3-4, pN0, pN1, pM0 and pM1 stages showed that patients with low preoperative AGR had significantly shorter OS than patients with high preoperative AGR. For these reasons, we believe that preoperative AGR is an independent prognostic factor for RCC patients.
The study has several limitations. Firstly, some specific inflammatory markers such as C-reactive protein and cytokine levels, which were also important factors of inflammation, were not measured because they were not routinely measured at our hospital prior to 2006. Secondly, preoperative AGR was only assessed at a single time point before surgery and in a single center. Further large-scale population-based prospective studies are needed to fully consolidate the results.
Despite the aforementioned limitations, our study had some clinical implications. First of all, we are the first to show that low preoperative AGR may be a clinical indicator associated with shorter OS of RCC patients. Secondly, AGR is routinely measured at low cost in clinical practice, thus it has potential as a simple, convenient predictive and stratification factor to assist with clinical decision-making for RCC patients. Thirdly, at least in theory, AGR is a predictive factor superior to other nutritional or inflammatory indicators. Nutritional assessment and support as well as anti-inflammatory therapy may be suitable treatment choices for patients with AGR ≤1.47. Although Algra et al. have demonstrated the ability of anti-inflammatory therapy (e.g., aspirin and other non-steroidal anti-inflammatory drugs) to prevent and/or treat some cancers32, the value of anti-inflammatory therapy for RCC patients needs to be further studied. At least, AGR can provide a useful tool for future clinical trials and patient management.
In conclusion, preoperative AGR is a proven objective, reproducible and inexpensive predictor of survival of RCC patients subjected to surgical resection and consideration should be given for its routine clinical use.
Implications for Practice
The serum albumin to globulin ratio (AGR) not only reflects patients' nutritional status but also represents their systemic inflammation. This prospective cohort study of 895 patients with renal cell carcinoma (RCC) showed that preoperative AGR was an independent prognostic indicator of overall survival (OS) of RCC patients.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (Grant No. 81302224 and 81202013), China Postdoctoral Science Foundation (Grant No. 2016M592582), Natural Science Foundation of Guangdong Province (Grant No. 2016A030610219) and Medical Scientific Research Foundation of Guangdong Province, China (Grant No. B2012131). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Conception and Design: YK, HXB, GSJ.
Provision of Study Material or Patients: YK, HXB, GSJ, CD, HH.
Collection and Assembly of Data: YK, HXB, GSJ, CD, CX, ZYJ, HQM, XYF,
Data Analysis and Interpretation: YK, HXB, GSJ, YGW, QZK, LZW, ZM, LRW, ZFJ.
Manuscript Writing: YK, HXB, GSJ, CD, LRW, ZFJ, HH.
Final Approval of Manuscript: All the authors.
Conflicts of interest
All the authors state they have no conflicts of interest.
References
1. Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30
3. Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. UROLOGY. 1998;51:203-205
4. Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982-1997). UROLOGY. 2000;56:58-62
5. Eggener SE, Yossepowitch O, Pettus JA. et al. Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J CLIN ONCOL. 2006;24:3101-3106
6. Kim SP, Alt AL, Weight CJ. et al. Independent validation of the 2010 American Joint Committee on Cancer TNM classification for renal cell carcinoma: results from a large, single institution cohort. J Urol. 2011;185:2035-2039
7. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. AM J SURG PATHOL. 1982;6:655-663
8. Volpe A, Patard JJ. Prognostic factors in renal cell carcinoma. WORLD J UROL. 2010;28:319-327
9. Delmore G. Assessment of nutritional status in cancer patients: widely neglected? SUPPORT CARE CANCER. 1997;5:376-380
10. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223-226
11. Coussens LM, Werb Z. Inflammation and cancer. NATURE. 2002;420:860-867
12. McMillan DC, Watson WS, O'Gorman P. et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. NUTR CANCER. 2001;39:210-213
13. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. NUTR J. 2010;9:69
14. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448-454
15. Azab BN, Bhatt VR, Vonfrolio S. et al. Value of the pretreatment albumin to globulin ratio in predicting long-term mortality in breast cancer patients. AM J SURG. 2013;206:764-770
16. Zhou T, He X, Fang W. et al. Pretreatment Albumin/Globulin Ratio Predicts the Prognosis for Small-Cell Lung Cancer. Medicine (Baltimore). 2016;95:e3097
17. Du XJ, Tang LL, Mao YP. et al. The pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma. PLOS ONE. 2014;9:e94473
18. Azab B, Kedia S, Shah N. et al. The value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancer. INT J COLORECTAL DIS. 2013;28:1629-1636
19. World Medical Association Declaration of Helsinki. ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14-18
20. Absenger G, Szkandera J, Pichler M. et al. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer. 2013;109:395-400
21. Zhang B, Yu W, Zhou LQ. et al. Prognostic Significance of Preoperative Albumin-Globulin Ratio in Patients with Upper Tract Urothelial Carcinoma. PLOS ONE. 2015;10:e144961
22. Doweiko JP, Nompleggi DJ. The role of albumin in human physiology and pathophysiology, Part III: Albumin and disease states. JPEN J Parenter Enteral Nutr. 1991;15:476-483
23. Bharadwaj S, Ginoya S, Tandon P. et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf). 2016
24. Laursen I, Briand P, Lykkesfeldt AE. Serum albumin as a modulator on growth of the human breast cancer cell line, MCF-7. ANTICANCER RES. 1990;10:343-351
25. Kaysen GA. Serum albumin concentration in dialysis patients: why does it remain resistant to therapy? KIDNEY INT SUPPL. 2003:S92-S98
26. Seaton K. Albumin concentration controls cancer. J NATL MED ASSOC. 2001;93:490-493
27. Adly L, Hill D, Sherman ME. et al. Serum concentrations of estrogens, sex hormone-binding globulin, and androgens and risk of breast cancer in postmenopausal women. INT J CANCER. 2006;119:2402-2407
28. Guthrie GJ, Roxburgh CS, Farhan-Alanie OM. et al. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer. 2013;109:24-28
29. Suh B, Park S, Shin DW. et al. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. ANN ONCOL. 2014;25:2260-2266
30. Coussens LM, Werb Z. Inflammation and cancer. NATURE. 2002;420:860-867
31. Mantovani A, Allavena P, Sica A. et al. Cancer-related inflammation. NATURE. 2008;454:436-444
32. Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. LANCET ONCOL. 2012;13:518-527
Author contact
![]() Corresponding author: Kai Yao, Department of Urology, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, Guangdong 510060, P.R. China. Email: yaokaiorg.cn.
Corresponding author: Kai Yao, Department of Urology, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, Guangdong 510060, P.R. China. Email: yaokaiorg.cn.

 Global reach, higher impact
Global reach, higher impact