Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(2):314-322. doi:10.7150/jca.16526 This issue Cite
Research Paper
β3GnT8 Promotes Gastric Cancer Invasion by Regulating the Glycosylation of CD147
1. Department of Clinical Oncology, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei, China
2. Department of Biochemistry and Molecular Biology, Soochow University, Suzhou 215123, Jiangsu, China
3. Department of pharmacology, School of Basic Medicine, Hubei University of Medicine, Shiyan 442000, Hubei, China
4. Institute of Cancer Research, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei, China
Received 2016-6-18; Accepted 2016-10-17; Published 2017-2-5
Abstract
β1, 3-N-acetylglucosminyltransferase 8(β3GnT8) synthesizes a unique cabohydrate structure known as polylactosamine, and plays a vital role in progression of various human cancer types. However, its involvement in gastric cancer remains unclear. In this study, we analyzed the expression and clinical significance of β3GnT8 by Western blot in 6 paired fresh gastric cancer tissues, noncancerous tissues and immunohistochemistry on 110 paraffin-embedded slices. β3GnT8 was found to be over-expressed in gastric cancer tissues, which correlated with lymph node metastasis and TNM stage. Forced the expression of β3GnT8 promoted migration and invasion of gastric cancer cells, whereas β3GnT8 knockdown led to the opposite results. Further studies showed that the regulated β3GnT8 could convert the heterogeneous N-glycosylated forms of CD147 and change the polylactosamine structures carried on CD147. In addition, our data suggested the annexin A2 (ANXA2) to be an essential interaction partner of β3GnT8 during the process of CD147 glycosylation. Collectively, these results provide a novel molecular mechanism for β3GnT8 in promotion of gastric cancer invasion and metastasis. Targeting β3GnT8 could serve as a new strategy for future gastric cancer therapy.
Keywords: β3GnT8, CD147, invasion, gastric cancer, glycosylation
Introduction
Gastric cancer is one of the most common malignancies worldwide, particularly in Eastern Asia (Korea, Mongolia, Japan, and China) [1]. It is difficult to cure unless it is found at an early stage [2]. Owing to the lack of early detection markers and effective therapeutic strategies, it has often reached an advanced stage [3]. Most of gastric cancer patients die due to tumor recurrence and metastasis, and the 5-year survival rate is only 20-40%[4]. Thus, it is urgently needed for an improved understanding of the molecular mechanisms contributing to gastric cancer invasion and metastasis.
β1, 3-N-acetylglucosminyltransferase 8(β3GnT8) exerts its activity on tetraantennary N-glycans and elongates polylactosamine chains [5]. Initially, it had been reported that β3GnT8 was cloned as β3GalT7 from a human lung cDNA library [6]. Subsequently, it was named β3GnT8 based on its position in a phylogenetic tree and enzymatic activity [5]. Hence, both β3GalT7 and β3GnT8 are authorized for this gene. It was indicated that β3GnT8 expression in the cervix tumor tissues was obviously higher than that in normal tissues [7]. A correlation between β3GnT8 and metastatic potential has been proposed base on its markedly enhanced expression in tumors such as colorectal cancer [8] and gliomas [9]. In a human gastric cancer cell line AGS, the expression of matrix metalloproteinase-2 (MMP-2) could be regulated by β3GnT8 [10]. In addition, the ability of β3GnT8 to mediate CD147 signal transduction pathway has been confirmed in a gastric cancer cell line SGC-7901[11]. However, the regulatory mechanisms and biochemical properties of β3GnT8 in gastric cancer invasion need further exploration.
In the present study, we demonstrated that β3GnT8 was not only increased in gastric cancer tissues, but also was associated with clinical features, such as lymph node metastasis and TNM stage. We also confirmed that β3GnT8 promoted gastric cancer cell invasion by regulating the N-glycosylation of CD147. Our findings provide novel insights into the crucial role of β3GnT8 in gastric cancer progression and suggest β3GnT8 as a potential target for the prevention of gastric cancer metastasis.
Materials and Methods
Tissue samples
110 human gastric cancer and 45 adjacent non-tumor tissues were collected from patients who underwent surgical resection between 2007 and 2014 at the Taihe Hospital, Hubei University of Medicine. These tissues were stored at -80°C immediately after surgical removal. 6 paired fresh gastric cancer tissues and noncancerous tissues were also obtained from the Taihe Hospital and stored in liquid nitrogen until use. None of the patients had received radiotherapy or chemotherapy before surgery. A written informed consent was obtained from each patient involved in this study and the study protocol was approved by the ethics committee of Hubei University of Medicine. All the clinicopathological parameters including age, gender, differentiation status, lymph node invasion and TNM stage were retrieved from patients' medical records. Investigations involving humans have been performed in accordance with the principles of Declaration of Helsinki.
Western blot analysis
Total proteins were extracted from the gastric cancer tissues and cells using the standard methods [9]. Proteins were quantified using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Then samples were separated by 10% SDS-PAGE and transferred to PVDF membranes. The primary antibodies were anti-β3GnT8 (1:1000), anti-CD147 (1:500; Santa Cruz, CA, USA), anti-annexin A2 (ANXA2) (1:1000; Santa Cruz), and anti-GAPDH (1:1000; Santa Cruz). The specificity of β3GnT8 antibody has been verified in our previous study [7]. Bands on the membranes were visualized using an ECL detection kit obtained from Beyotime Institute of Biotechnology (Jiangsu, China).
Immunohistochemical (IHC) staining
Formalin-fixed, paraffin-embedded tissue specimens were cut into 4-μm sections. Then sections were dewaxed in xylene and rehydrated through graded ethanols. To block endogenous peroxidase, 3% hydrogen peroxide in methanol was used. Sections were then incubated with the anti-β3GnT8 antibody (1:100) or 4µg/ml biotinylated Lycopersicon esculentum agglutinin (LEL) (Sigma, St. Louis,MO, USA). After washing in phosphate-buffered saline (PBS), tissues were incubated with Horseradish peroxidase (HPR)-labeled goat anti-rabbit secondary antibody (Santa Cruz) or HRP-conjugated streptavidin (Sigma). Finally, the sections were visualized using diaminobenzidine and counterstain with hematoxylin (Beyotime Institute of Biotechnology). Sections were photographed on an Olympus photomicroscope (Inha, Japan). The degree of IHC staining was evaluated by two independent pathologists. Staining intensity was graded as “0” (negative), “1” (weak), “2” (moderate) and “3” (strong); staining percentage was graded as “0” (<5%), “1” (5-25%), “2” (25-50%), “3” (50-75%) or “4” (>75%)[12]. A final immunoreactivity scores (IRS) was calculated by multiplying the values of the staining intensity and staining percentage. The IRS value>4 was defined as high expression and IRS value ≤4 as low expression. ImagePro Plus (Media Cybernetics, Silver Spring, MD, USA) was used to quantitatively score the tissue sections.
Cell lines and transfection
Human gastric cancer cell lines AGS and NCI‑N87 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). SGC-7901 cell line was purchased from the Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 (GIBCO BRL, Carlsbad, MD, USA) containing 10% fetal bovine serum (FBS) (HyClone, Waltham, UT, USA) in a humidified atmosphere with 5% CO2 at 37˚C. The pEGFP-C1 (Mock), pEGFP-C1-β3GnT8 (T8S), pSilencircle-β3GnT8Scr (T8Scr), and pSilencircle-β3GnT8 (T8Si) plasmids were constructed by our laboratory [9]. Transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Cells were collected 48 h after transfection for other assays.
RNA extraction and quantitative real-time PCR
Total RNA from cultured cells was extracted using TRIzol reagent (Invitrogen). Reverse transcription of RNA was carried out using the reverse transcription kit (Invitrogen). Quantitative PCR was conducted using SYBR-Green Real-Time PCR Master Mix kit (Toyobo, Osaka, Japan) and an ABI detection system (Applied Biosystems, Foster City, CA, USA). The PCR primers were designed for β3GnT8 were 5′-GTCGCTACAGTGACCTGCTG-3′ (forward) and 5′-GTCTTTGAGCGTCTGGTTGA-3′ (reverse); for GAPDH were 5′-CCAACCGCGAGAAGATGA-3′ (forward) and 5′-CCAGAGGCGTACAGGGATAG-3′ (reverse). GAPDH was used as an internal control. The data were collected and analyzed using the comparative Ct (threshold cycle) method.
Lectin flow cytometry
Briefly, 5 × 105 cells were harvested with 0.25% trypsin, and permeabilized by 0.1% triton X-100 for 5 minutes at room temperature. Biotin-labeled LEL was added to a final concentration of 20 μg/ml and incubated at 37˚C for 2 h. Then phycoerythrin-conjugated streptavidin (Sigma) of 10 μg/ml was used, and incubated for 1 h at 37˚C in the dark. Fluorescent analyses were performed using a FACScan flow cytometer (Becton-Dickinson, Mountain View, CA, USA). Only live cells were established as gates.
Lectin blot
The protein samples were extracted, quantified and subjected to 10% SDS-PAGE as described for western blot analysis. After blocking with Carbo-Free Blocking Solution (Vector Labs, Burlingame, CA, USA), the membranes were incubated with 2 μg/ml of biotinylated LEL for 1 h. Then HRP-conjugated streptavidin (Sigma) was used as a secondary antibody. The blots were developed using an ECL substrate solution.
Cell migration and invasion assays
Wound-healing assay was performed to examine the migration rates of cultured cells. Briefly, the cell layer that reached confluence was scratched by a 20 μl pipette tip. Then photographs were taken immediately (time zero) and 24 h after wounding. Cell migration was evaluated by measuring the difference in wound width. For cell invasion assay, cells in serum-free medium were plated on 24 well Transwell inserts (Corning Life Sciences, Tewksbury, MA, USA) precoated with Matrigel. Medium containing 10% FBS was added to the lower chamber. After 24 h culture, the cells that have moved through the pores to the lower side were stained with eosin staining solution (Beyotime Institute of Biotechnology).Invasion was evaluated by counting the number of the invaded cells.
Immunoprecipitation(IP)
Total cell lysates were prepared with IP lysis buffer (10 mM Tris, 1% triton-X-100, 1 mM EDTA, 100 mM NaCl and a mixture of protease inhibitors) as previously described [13]. IP of indicated protein was performed with a 1:80 dilution of antibody overnight at 4 °C with constant rotation. Antibody-protein conjugates were pulled down by incubating samples with 10 μl of Sepharose A beads (Thermo Fisher, Rockford, IL, USA) for 4 h at 4 °C. Finally, the beads were washed, boiled, centrifuged and the recovered samples were run on 10% SDS-PAGE for western blot or lectin blot as described above.
LC-MS/MS analysis
Coomassie brilliant blue R-250 (Sigma) staining was performed to visually detect specific immunoprecipitated bands of β3GnT8 antibody. The bands were subjected to in gel digestion by trypsin(Sigma). Digested peptides were analyzed by nano-HPLC (Ultimate 3000, Dionex) coupled to a linear quadrupole ion trap-Orbitrap (LTQ Orbitrap XL)mass spectrometer (Thermo Fisher) equipped with a nano-ESI source[14]. A nonlinear gradient using 2% ACN in 0.1% formic acid in water and 0.1% formic acid in 98% acrylonitrile was used with a flowrate of 250 nl/min[14]. All measurements were performed in the positive-ion reflective mode at an accelerating voltage of 18 kV and delayed-pulsed ion extraction [15]. All MS/MS data were analyzed using Mascot (Matrix Science, London, UK; version 2.3.01) and searched against the latest NCBI nr protein database [16].
Statistical analysis
Data from the statistical analysis represent the mean ± standard deviation (SD) from at least three independent experiments. Statistical differences between the two groups were examined by Student's t-test. Analyses of β3GnT8 and polylactosamines expression with clinicopathologic parameters were performed using Fisher's exact test. P < 0.05 was considered statistically significant. All tests were preformed by SPSS 16.0 software (SPSS Inc, Chicago, IL, USA).
Results
β3GnT8 is highly expressed in gastric cancer tissues
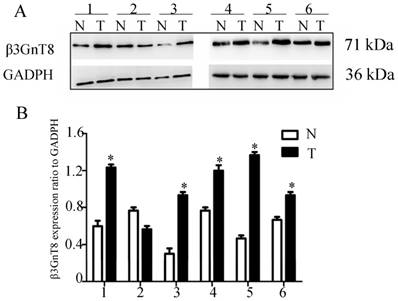
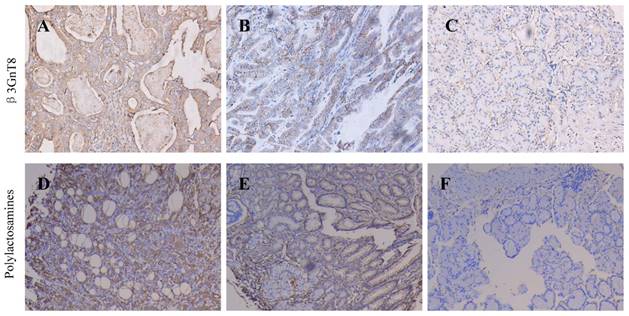
We fist examined the expression of β3GnT8 using Western blot in 6 pairs of gastric cancer tissues and their corresponding nontumorous tissues. Gastric cancer tissues showed marked higher levels than did corresponding non-tumor tissues (P<0.05, Figure 1). In order to investigate the clinical significance of β3GnT8 in gastric cancer progression, immunohistochemical (IHC) staining was used to observe the expression of β3GnT8 in 110 gastric cancer tissues. As expected, β3GnT8 was mainly localized in the cytoplasm of gastric cancer cells, and its expression was much more dominant in tumor tissues than that in neighboring non-tumor tissues (Figure 2). β3GnT8 is involved in the biosynthesis of polylactosamine chains. Using LEL staining, differential expression profiles of polylactosamines could be observed [17]. Polylactosamines were predominantly expressed in the membrane and cytoplasm of gastric cancer cells, and their expression was clearly higher in gastric cancer tissues (Figure 2).
β3GnT8 expression in human gastric cancer by western blot analysis. (A) The protein level of β3GnT8 was high in 6 representative paired samples of gastric cancer tissue (T) compared with nontumorous adjacent tissues (N). GAPDH was used as a loading control. (B) The bar chart showed the ratio of β3GnT8 protein to GADPH. Bars represented mean ± SD of three independent tests. (*P<0.05 compared with nontumorous adjacent tissues).
Then the relationship between β3GnT8, polylactosamine chains expression and clinicopathological features of gastric cancer was analyzed. As shown in Table 1, expression of β3GnT8 was correlated with the increased clinical stage (P<0.05), depth of invasion (P<0.05) and lymph lode metastasis (P<0.05), but not statistically related to tumor differentiation, size, lauren classification, location or patients' gender and age. Polylactosamines were also closely related with lymph nodes metastasis (P<0.05), depth of invasion (P<0.05) and TNM staging (P<0.05), whereas no correlation with other clinicopathological factors. Furthermore, we found that β3GnT8 expression was positively correlated with polylactosamines expression in gastric cancer tissues (P=0.003). These results suggest that β3GnT8 may be of diagnostic and/or prognostic value as a biomarker in gastric cancer.
Relationship between β3GnT8, polylactosamines expression and clinicopathological features of gastric cancer patients
| Clinicopathological features | n | β3GnT8 | P | polylactosamines | P | ||
|---|---|---|---|---|---|---|---|
| High | Low | High | Low | ||||
| Age | |||||||
| <60 | 51 | 27 | 24 | 0.578 | 22 | 29 | 0.442 |
| ≥60 | 59 | 32 | 27 | 28 | 31 | ||
| Gender | |||||||
| Male | 57 | 28 | 29 | 0.616 | 25 | 32 | 0.756 |
| Female | 53 | 31 | 22 | 26 | 27 | ||
| Tumor Size | |||||||
| <5cm | 45 | 15 | 30 | 0.341 | 25 | 20 | 0.139 |
| ≥5cm | 65 | 44 | 21 | 32 | 33 | ||
| Lauren classification | |||||||
| Intestinal | 62 | 30 | 32 | 0.445 | 27 | 35 | 0.217 |
| Diffuse | 48 | 29 | 19 | 24 | 24 | ||
| Tumor location | |||||||
| Proximal | 8 | 4 | 4 | 0.329 | 3 | 5 | 0.221 |
| Middle | 52 | 25 | 27 | 23 | 29 | ||
| Distal | 50 | 30 | 20 | 26 | 24 | ||
| Differentiation | |||||||
| Moderate-High | 69 | 39 | 30 | 0.188 | 33 | 36 | 0.168 |
| Low | 41 | 18 | 23 | 20 | 21 | ||
| TNM stage | |||||||
| I+II | 47 | 28 | 19 | 0.013* | 25 | 22 | 0.024* |
| III +IV | 63 | 50 | 13 | 48 | 15 | ||
| Lymph node metastasis | |||||||
| Positive | 90 | 72 | 18 | 0.002* | 65 | 25 | 0.011* |
| Negative | 20 | 17 | 3 | 15 | 5 | ||
| Invasion depth | |||||||
| T1+T2 | 23 | 19 | 4 | 0.036* | 15 | 8 | 0.024* |
| T3+T4 | 87 | 68 | 19 | 61 | 26 | ||
*P < 0.05
β3GnT8 promotes the migration and invasion of gastric cancer cells
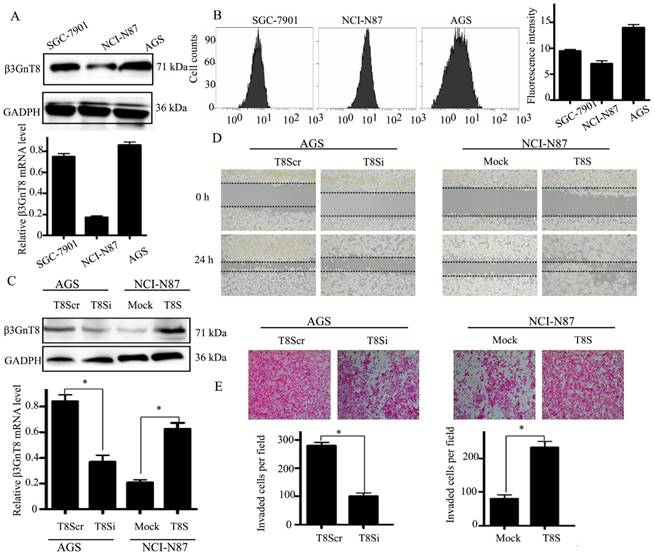
To further explore the role of β3GnT8 in gastric cancer, we screened three gastric cancer cell lines, AGS, SGC-7901 and NCI-N87 for its expression. Among the cell lines, AGS had the highest expression of β3GnT8 at both the mRNA and protein levels (Figure 3A). The expression of polylactosamines was also detected by flow cytometry with LEL (Figure 3B). Fluorescent intensity was computed as the median value of each staining. NCI-N87 cells showed lowest expression of polylactosamines, whereas AGS cells exhibited highest levels of polylactosamines. We therefore selected these two cell lines in the following experiments.
Then NCI-N87 cell line was transfected with a pEGFP-C1-β3GnT8 plasmid to over-express β3GnT8. AGS cell line was treated with a pSilencircle-β3GnT8 plasmid to knockdown endogenous β3GnT8 expression. The effect of ectopic expression and knockdown of β3GnT8 in cells was confirmed by quantitative RT-PCR and western blot (Figure 3C).Compared with that of control cells, the migration and invasion abilities were markedly stimulated in NCI-N87 cells that over-expressing β3GnT8 as indicated by wound-healing and transwell assays(P<0.05, Figure 3D and 3E). Likewise, knockdown of β3GnT8 could apparently repress the migration and invasion abilities of AGS cells (P < 0.05, Figure 3D and 3E). Taken together, these results suggest that the dynamic changes of β3GnT8 may be common events during the progression of gastric cancer.
Immunohistochemical staining for the expression of β3GnT8 and polylactosamines in gastric cancer and adjacent non-tumor tissues (×200). (A) High positive β3GnT8 expression in gastric cancer. (B)Low positive β3GnT8 expression in gastric cancer. (C) Negative β3GnT8 expression in adjacent non-tumor tissues. (D) High positive polylactosamines expression in gastric cancer. (E)Low positive polylactosamines expression in gastric cancer. (F) Negative polylactosamines expression in adjacent non-tumor tissues.
β3GnT8 promotes gastric cancer cell migration and invasion. (A) Quantitative RT-PCR and western blot analysis of β3GnT8 expression in three gastric cancer cell lines. (B) Lectin flow cytometry analysis of polylactosamine expression in three gastric cancer cell lines. (C) The mRNA and protein levels of β3GnT8 were detected by quantitative RT-PCR and western blot in NCI-N87 and AGS cells transiently transfected with pEGFP-C1 (Mock), pEGFP-C1-β3GnT8 (T8S), or pSilencircle-β3GnT8Scr (T8Scr), and pSilencircle-β3GnT8 (T8Si) vectors, respectively. (D) Wound healing assay with gastric cancer cells. Microscopic observations were recorded after scratching the cell surface (200×). (E) Transwell assay with gastric cancer cells (200×). Histograms showed the numbers of invasion cells. Bars represented mean ± SD of three independent tests, all *P < 0.05.
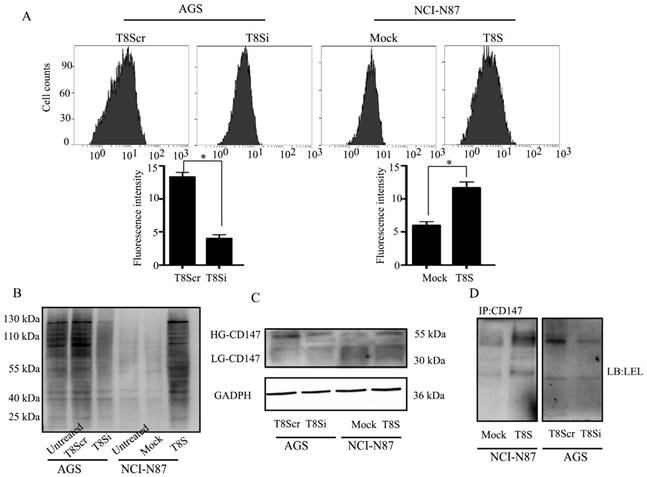
β3GnT8 regulates the N-glycosylated forms of CD147 in gastric cancer cells. (A) Lectin flow cytometry analysis of polylactosamine expression in NCI-N87 and AGS cells transiently transfected with pEGFP-C1 (Mock), pEGFP-C1-β3GnT8 (T8S), or pSilencircle-β3GnT8Scr (T8Scr), and pSilencircle-β3GnT8 (T8Si) vectors, respectively. (B) Lectin blot assay with gastric cancer cells. (C)Western blot analysis of CD147 glycosylation in gastric cancer cells. (D)Immunoprecipitation plus lectin blot was performed. Bars represented mean ± SD of three independent tests, all *P < 0.05. WCL, whole cell lysate.
Glycosylation of CD147 is involved in β3GnT8 mediated gastric cancer cell invasion
CD147 is a glycoprotein that carries polylactosamine sugars on its N-glycosylation sites [18]. To further investigate if β3GnT8 could affect CD147 glycosylation in gastric cancer cells, firstly, we analyzed the effect of β3GnT8 on the synthesis of total polylactosamine chains. The results showed that knockdown of β3GnT8 in AGS cells dramatically decreased total polylactosamine levels (Figure 4A). Conversely, polylactosamines were increased after ectopic expression of β3GnT8 in NCI-N87 cells. Lectin blot analysis revealed that the polylactosamine units on glycoproteins were also altered by different β3GnT8 levels (Figure 4B). Lectin blot analysis showed the consistent alteration of glycan structures with flow cytometry assay.
Furthermore, we observed that high glycosylated (HG)-CD147 was markedly increased by over-expression of β3GnT8 in NCI-N87 cells (Figure 4C). Inversely, the expression of HG-CD147 was reduced when β3GnT8 was down-regulated in AGS cells. After the IP with anti-CD147 antibody, lectin blot assay from total cell lysate protein revealed the similar results (Figure 4D).These data indicated that the regulated β3GnT8 converted the heterogeneous N-glycosylated forms of CD147 in gastric cancer cells, and significantly changed the polylactosamine structures on CD147.
Identification of interaction partners of β3GnT8 in gastric cancer cells by proteomic analysis
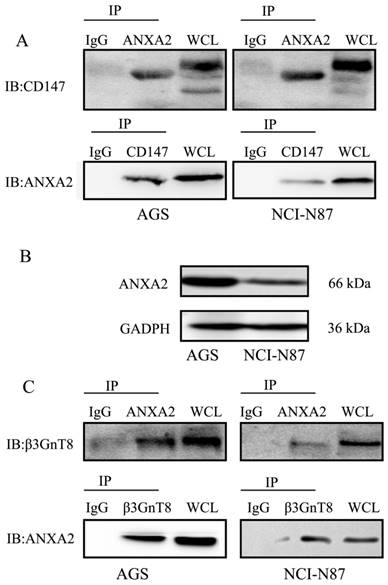
To explore the mechanism underlying β3GnT8-mediated glycosylation of CD147 in gastric cancer cells, a proteomic approach by IP of β3GnT8 followed by Nano-HPLC and LC-MS/MS analysis was applied. And a negative control using serum IgG was used to distinguish non-specifically bound proteins. We thereby identified total of 9 proteins from the analyses, which were immunoprecipitated together with β3GnT8 but not with the control. Table 2 shows the most abundant hits also indicating the number of biological repeats in AGS and NCI-N87 cells. A careful review of the literature revealed that there exist interaction effects between ANXA2 and CD147 [19]. As expected, we found that ANXA2 specifically bound to CD147 in gastric cancer cells (Figure 5A). We therefore selected ANXA2 as a candidate molecule.
In order to validate the interaction between ANXA2 and β3GnT8, we undertook co-IP assay. Protein expression of ANXA2 in AGS and NCI-N87 cells was detected by western blot. As shown in Figure 5B, the expression of ANXA2 was much higher in AGS cells than that in NCI-N87 cells. ANXA2 was then found to co-immunoprecipitate with β3GnT8 in AGS and NCI-N87, indicating that ANXA2 and β3GnT8 interact in their native form in gastric cancer cells (Figure 5C). However, directly binding between the CD147 and β3GnT8 was not found in this study. ANXA2 and β3GnT8 may act as a functional complex, which plays an important role in CD147 glycosylation.
Co-immunoprecipitation of β3GnT8 and ANXA2 in gastric cancer cells. (A) CD147 immunoprecipitated with ANXA2 in NCI-N87 and AGS cells. (B) The protein expression of ANXA2 was detected by western blot in NCI-N87 and AGS cells. (C) β3GnT8 immunoprecipitated with ANXA2 in NCI-N87 and AGS cells. Serum IgG was used as a negative control.
Discussion
Glycosylation controls diverse protein functions and regulates various cellular phenotypes. Alterations in cell surface glycosylation are believed to play crucial roles in tumor progression, metastasis, and therapeutics[20]. Recent studies reveal that decreased core-fucosylation of N-glycans [21, 22] and reduced gland mucin-specific O-glycans [23] contribute to malignancy in gastric cancer. Identifying these biomarkers could provide new insights for understanding the mechanisms of gastric cancer invasion, and designing better therapeutic strategies. β3GnT8 transfers GlcNAc to the non-reducing terminus of the Galβ1-4GlcNAc of tetra-antennary N-glycan to form polylactosamine structures [5]. As β3GnT8 expression was associated with aggressive tumor phenotype, we identified and functionally characterized β3GnT8 as an important regulator in gastric cancer invasion. In addition, it provides a potential therapeutic target for the future treatment of gastric cancer.
Identification of potential interaction partners of β3GnT8 by LC-MS/MS analysis in different gastric cancer cells
| Protein | Description | Uniprot accession number | AGS a | NCI-N87 a |
|---|---|---|---|---|
| ACTB | Actin, cytoplasmic 1 | P60709 | 18 | 19 |
| JUP | Junction plakoglobin | P14923 | 23 | 13 |
| DCD | Dermcidin | P81605 | 12 | 16 |
| ANXA2 | Annexin A2 | P07355 | 22 | 29 |
| PIP | Prolactin-inducible protein | P12273 | 21 | 27 |
| HRNR | Hornerin | Q86YZ3 | 8 | 5 |
| TET1 | Methylcytosine dioxygenase TET1 | Q8NFU7 | 17 | 14 |
| AGPAT1 | 1-acyl-sn-glycerol-3-phosphate acyltransferase alpha | Q99943 | 4 | 2 |
| HSPD1 | Heat shock 60kDa protein 1 | P10809 | 2 | 3 |
a Number of unique peptides identified.
Polylactosamine, which can be incorporated into both N- and O-linked glycans, is a unique glycan composed of repeating N-acetyllactosamine units[24]. This polymers carried on N-glycans are believed to aid metastasis by making the cells more invasive [25-27]. Inhibition of polylactosamines using different strategies always results in the loss of the metastatic ability. Conversely, induction of their expression has been shown to result in acquisition of the metastatic phenotype. Lectin staining with LEL, which recognizes polylactosamines, was undertaken to obtain expression profiles of these structures[28, 29]. In the present study, we showed that polylactosamine chains were significantly up-regulated in gastric cancer tissues compared with the adjacent non-cancerous tissues. Moreover, polylactosamine levels were significantly correlated with aggressive tumor characteristics (lymph node metastasis and TNM stage). We also confirmed that the expression levels of β3GnT8 were positively correlated with polylactosamines expression in gastric cancer tissues and cell lines. Over-expression and knockdown of β3GnT8 could markedly increase and reduce polylactosamines in gastric cancer cells. To our knowledge, this is the first report on the role of polylactosamines in gastric cancer progression. However, to further confirm the functions of β3GnT8 and polylactosamines, a larger number of gastric cancer samples than available would be required.
In addition, we explored the mechanisms behind the association between β3GnT8 and gastric cancer metastasis and identified downstream effectors contributing to this process. CD147, a cell surface transmembrane glycoprotein, has been reported to be correlated with gastric cancer aggressiveness. For example, CD147 expression was associated with gastric cancer invasion, metastasis and TNM stage [30]. Up-regulated CD147 could enhance gastric cancer cell invasion and angiogenesis [31]. Because of the heterogeneous N-glycosylation, CD147 shows both high glycosylated (HG)-CD147 (~40-60 kDa), and a low glycosylated (LG) -CD147 (~32 kDa) form[32]. Elevated glycosylation of CD147, yielding HG-CD147, is attributable to high polylactosamine content [33]. Our results showed that knockdown of β3GnT8 could efficiently inhibit HG-CD147 in AGS cells. In contrast, up-regulation of β3GnT8 significantly enhanced HG-CD147 expression in NCI-N87 cells. β3GnT8 associates with HG-CD147 and enhances the biosynthetic conversion of LG-CD147 to HG-CD147. This is in line with our previous study that β3GnT8 could regulate the metastatic potential of colorectal cancer cells by altering the glycosylation of CD147[8]. It is so far not well understood how these processes are regulated and which interaction partners are involved.
We performed a proteomic screen for investigation of the interactome of β3GnT8 in gastric cancer cells. We thereby identified a number of interaction proteins. We confirmed ANXA2 as a general binding partner for β3GnT8 by co-IP assay. ANXA2 is a calcium-dependent phospholipid binding protein that is mainly located on the cell membrane[34]. Its aberrant expression enhanced the malignant properties of cancer cells[35]. Up-regulated ANXA2 was associated with lymph node metastasis, advanced TNM stage in patients with gastric cancer[36, 37]. Here, we found that AGS cells expressed more ANXA2 protein than NCI-N87 cells. Changes in the expression of ANXA2 were closely associated with the invasion phenotype of different gastric cancer cell. β3GnT8 and ANXA2 may act as a functional complex during the invasion and migration of gastric cancer cells.
Recent studies have revealed that ANXA2 and CD147 interact with each other in the same signal transduction pathway[19]. ANXA2 was found to be co-localized and co-immunoprecipitated with CD147 in hepatocellular carcinoma cells[38, 39]. In this study, the two molecules were also found to co-immunoprecipitate with each other in gastric cancer cells. According to our data, we suggest that ANXA2 might act as a linker between CD147 and β3GnT8, and then regulate β3GnT8-induced glycosylation of CD147.
In conclusion, our data highlight the molecular etiology and clinical significance of β3GnT8 in gastric cancer. β3GnT8 promotes gastric cancer cell migration and invasion by functionally converting the polylactosamine chains carried on CD147. ANXA2 is an essential interaction partner of β3GnT8 during CD147 glycosylation. Thus, targeting β3GnT8 may represent a new therapeutic strategy for the treatment of gastric cancer patients.
Acknowledgements
The present study was supported by the Natural Science Foundation of Hubei Provincial Department of Education (Q20162115), Innovative Research Team of Hubei University of Medicine (2014CXG02), and the Scientific and Technological Project of Shiyan City of Hubei Province (15K65).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87-108
2. Wang J, Yu JC, Kang WM. et al. Treatment strategy for early gastric cancer. Surg Oncol. 2012;21:119-23
3. Yan W, Wu K, Herman JG. et al. Epigenetic silencing of DACH1 induces the invasion and metastasis of gastric cancer by activating TGF-beta signalling. Journal of cellular and molecular medicine. 2014;18:2499-511
4. Hu L, Duan YT, Li JF. et al. Biglycan enhances gastric cancer invasion by activating FAK signaling pathway. Oncotarget. 2014;5:1885-96
5. Ishida H, Togayachi A, Sakai T. et al. A novel beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8), which synthesizes poly-N-acetyllactosamine, is dramatically upregulated in colon cancer. FEBS Lett. 2005;579:71-8
6. Huang C, Zhou J, Wu S. et al. Cloning and tissue distribution of the human B3GALT7 gene, a member of the beta1,3-Glycosyltransferase family. Glycoconjugate journal. 2004;21:267-73
7. Jiang Z, Ge Y, Zhou J. et al. Subcellular localization and tumor distribution of human beta3-galactosyltransferase by beta3GalT7 antiserum. Hybridoma (Larchmt). 2010;29:141-6
8. Ni J, Jiang Z, Shen L. et al. beta3GnT8 regulates the metastatic potential of colorectal carcinoma cells by altering the glycosylation of CD147. Oncol Rep. 2014;31:1795-801
9. Liu J, Shen L, Yang L. et al. High expression of beta3GnT8 is associated with the metastatic potential of human glioma. Int J Mol Med. 2014;33:1459-68
10. Shen L, Liu Z, Tu Y. et al. Regulation of MMP-2 expression and activity by beta-1,3-N-acetylglucosaminyltransferase-8 in AGS gastric cancer cells. Mol Biol Rep. 2011;38:1541-50
11. Jiang Z, Hu S, Hua D. et al. beta3GnT8 plays an important role in CD147 signal transduction as an upstream modulator of MMP production in tumor cells. Oncol Rep. 2014;32:1156-62
12. Yang GZ, Hu L, Cai J. et al. Prognostic value of carbonic anhydrase VII expression in colorectal carcinoma. BMC Cancer. 2015;15:209
13. Chavez JD, Schweppe DK, Eng JK. et al. Quantitative interactome analysis reveals a chemoresistant edgotype. Nature communications. 2015;6:7928
14. Han Y, Yu G, Sarioglu H. et al. Proteomic investigation of the interactome of FMNL1 in hematopoietic cells unveils a role in calcium-dependent membrane plasticity. Journal of proteomics. 2013;78:72-82
15. Hofmann BT, Schluter L, Lange P. et al. COSMC knockdown mediated aberrant O-glycosylation promotes oncogenic properties in pancreatic cancer. Mol Cancer. 2015;14:109
16. Blount AL, Schmidt K, Justice NJ. et al. FoxL2 and Smad3 coordinately regulate follistatin gene transcription. The Journal of biological chemistry. 2009;284:7631-45
17. Seko A, Yamashita K. Activation of beta1,3-N-acetylglucosaminyltransferase-2 (beta3Gn-T2) by beta3Gn-T8. Possible involvement of beta3Gn-T8 in increasing poly-N-acetyllactosamine chains in differentiated HL-60 cells. The Journal of biological chemistry. 2008;283:33094-100
18. Huang W, Luo WJ, Zhu P. et al. Modulation of CD147-induced matrix metalloproteinase activity: role of CD147 N-glycosylation. The Biochemical journal. 2013;449:437-48
19. Zhao P, Zhang W, Tang J. et al. Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer science. 2010;101:387-95
20. Taniguchi N, Kizuka Y. Glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Advances in cancer research. 2015;126:11-51
21. Zhao YP, Xu XY, Fang M. et al. Decreased core-fucosylation contributes to malignancy in gastric cancer. PLoS ONE. 2014;9:e94536
22. Liu L, Yan B, Huang J. et al. The identification and characterization of novel N-glycan-based biomarkers in gastric cancer. PLoS ONE. 2013;8:e77821
23. Shiratsu K, Higuchi K, Nakayama J. Loss of gastric gland mucin-specific O-glycan is associated with progression of differentiated-type adenocarcinoma of the stomach. Cancer science. 2014;105:126-33
24. Henion TR, Schwarting GA. N-linked polylactosamine glycan synthesis is regulated by co-expression of beta3GnT2 and GCNT2. J Cell Physiol. 2014;229:471-8
25. Saitoh O, Wang WC, Lotan R. et al. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. The Journal of biological chemistry. 1992;267:5700-11
26. Srinivasan N, Bane SM, Ahire SD. et al. Poly N-acetyllactosamine substitutions on N- and not O-oligosaccharides or Thomsen-Friedenreich antigen facilitate lung specific metastasis of melanoma cells via galectin-3. Glycoconjugate journal. 2009;26:445-56
27. Krishnan V, Bane SM, Kawle PD. et al. Altered melanoma cell surface glycosylation mediates organ specific adhesion and metastasis via lectin receptors on the lung vascular endothelium. Clinical & experimental metastasis. 2005;22:11-24
28. Shen L, Yu M, Xu X. et al. Knockdown of beta3GnT8 reverses 5-fluorouracil resistance in human colorectal cancer cells via inhibition the biosynthesis of polylactosamine-type N-glycans. International journal of oncology. 2014;45:2560-8
29. Togayachi A, Kozono Y, Ishida H. et al. Polylactosamine on glycoproteins influences basal levels of lymphocyte and macrophage activation. Proc Natl Acad Sci U S A. 2007;104:15829-34
30. Chu D, Zhu S, Li J. et al. CD147 expression in human gastric cancer is associated with tumor recurrence and prognosis. PloS one. 2014;9:e101027
31. Zheng HC, Takahashi H, Murai Y. et al. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. British journal of cancer. 2006;95:1371-8
32. Fan J, Wang S, Yu S. et al. N-acetylglucosaminyltransferase IVa regulates metastatic potential of mouse hepatocarcinoma cells through glycosylation of CD147. Glycoconjugate journal. 2012;29:323-34
33. Tang W, Chang SB, Hemler ME. Links between CD147 function, glycosylation, and caveolin-1. Molecular biology of the cell. 2004;15:4043-50
34. Zhuang H, Tan M, Liu J. et al. Human epididymis protein 4 in association with Annexin II promotes invasion and metastasis of ovarian cancer cells. Molecular cancer. 2014;13:243
35. Xing R, He H, He Y. et al. ANXA2 remodels the microstructures of caco2 cells. Cell Mol Biol (Noisy-le-grand). 2013;59(Suppl):OL1848-54
36. Han Y, Ye J, Dong Y. et al. Expression and significance of annexin A2 in patients with gastric adenocarcinoma and the association with E-cadherin. Experimental and therapeutic medicine. 2015;10:549-54
37. Emoto K, Sawada H, Yamada Y. et al. Annexin II overexpression is correlated with poor prognosis in human gastric carcinoma. Anticancer research. 2001;21:1339-45
38. Zhang W, Zhao P, Xu XL. et al. Annexin A2 promotes the migration and invasion of human hepatocellular carcinoma cells in vitro by regulating the shedding of CD147-harboring microvesicles from tumor cells. PloS one. 2013;8:e67268
39. Zhao P, Zhang W, Wang SJ. et al. HAb18G/CD147 promotes cell motility by regulating annexin II-activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology. 2011;54:2012-24
Author contact
![]() Corresponding authors: Dr Shiliang Wu, Department of Biochemistry and Molecular Biology, Soochow University, No.1 Shizi Street, Suzhou 215123, Jiangsu, China; E-mail: shiliang_wucom. Dr Zhiguo Luo, Department of Clinical Oncology, Taihe Hospital, Hubei University of Medicine, 30 South Renmin Road, Shiyan 442000, Hubei, China; E‑mail: zhiguo_luocom.
Corresponding authors: Dr Shiliang Wu, Department of Biochemistry and Molecular Biology, Soochow University, No.1 Shizi Street, Suzhou 215123, Jiangsu, China; E-mail: shiliang_wucom. Dr Zhiguo Luo, Department of Clinical Oncology, Taihe Hospital, Hubei University of Medicine, 30 South Renmin Road, Shiyan 442000, Hubei, China; E‑mail: zhiguo_luocom.

 Global reach, higher impact
Global reach, higher impact